Abstract
Waste activated sludge in China are mostly subjected to dewatering process before final disposal without stabilization. This study investigated the feasibility of organics degradation and H2 production from non-stabilized dewatered sludge (DS) by microbial electrolysis cells (MECs). Alkaline pretreatment was used to disintegrate sludge matrix and solubilize organic matters in DS. Then, the treatment performance of DS supernatant in a single-chamber MEC at various applied voltages was investigated. The COD (chemical oxygen demand) removal rate increased with increasing voltage, which ranged from 26.35 to 44.92% at 0.5–0.9 V. The average coulombic efficiency was 75.6%, while the cathodic hydrogen recovery was not satisfied (15.56–20.05%) with H2 production rates of 0.027–0.038 m3 H2/(m3 day). The reasons could be ascribed to the complexity of the substrate, H2 loss, and the confinement of configuration in scale-up. The organic matter degradation was influenced by the composition of DS. The carbohydrates could be readily used; meanwhile, the major component of the DS supernatant, i.e. proteins, was difficult to be utilized, which resulted from the low biodegradability of the transphilic fractions during the MEC operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing amount of sewage sludge was generated by wastewater treatment plants (WWTPs) to be handled. From consideration of sustainable development, sewage sludge is regarded as a “biosolid” rather than a “waste,” which can be explored as raw material to produce energy. Although anaerobic digestion is efficient in stabilizing sludge and producing biogas, alternative technologies are demanded to recover energy from biosolids.
MEC (microbial electrolysis cell) is a bioelectrochemistry process which could degrade organic matter in wastewater by microorganisms and at the meantime produce hydrogen/methane gas (Logan et al. 2008). To date, most MEC studies used acetate and other low-molecular-weight organics as substrates (Kadier et al. 2014). However, the organics in sludge mainly existed as extracellular polymeric substances (EPS) and/or intracellular biopolymer. These materials showed a slow hydrolysis rate and cannot be readily degraded by microorganisms, which limited the biological treatment performance including MEC. Therefore, it is necessary to apply disintegration techniques. These techniques, including alkaline treatment, sonication, ozonation, and microwave treatment, were used to accelerate the hydrolysis rate of waste activated sludge (WAS) in order to enhance anaerobic digestion and dewatering efficiency (Tunçal 2011; Kim et al. 2009; Cano et al. 2015; Zhen et al. 2017). Among them, alkaline treatment was extensively applied because of simpleness, effectiveness, and low energy consumption (Weemaes and Verstraete 1998). In the study of MEC process, alkaline pretreatment has been proved to increase H2 production rate from 5.67 ± 0.61 mg/g VSS (volatile suspended solids) to 15.08 ± 1.41 mg/g VSS for WAS (Lu et al. 2012a).
In developing countries, such as China, most WWTPs did not built or run anaerobic digesters for the technical and economic reasons. For these WWTPs, sewage sludge was only subjected to concentrating and dewatering treatment processes. The non-destabilized dewatered sludge (DS) posed a threat to the environment and human health during the subsequent sludge disposal. To solve this problem, one possible solution is via centralized sludge treatment plants. Like the centralized WWTPs which used pipelines to collect and transfer the wastewater sourced from different areas, a centralized sludge treatment plant collected non-destabilized DS from various WWTPs and safely disposed gathered DS in plant. Meanwhile, this method provides an opportunity to realize sludge recycling. The DS treatment process depends on its characteristics, which featured in high suspended solids (SS) in comparison with WAS. The high content of insolubilized organics in DS is more difficult to be used by microorganisms and therefore required a hydrolysis pretreatment to disrupt sludge matrix and extract the organics into soluble forms. Moreover, the high-solid sludge was unable to obtain uniform mixing and high rate of mass and heat transfer, which could be diluted during the biological treatment.
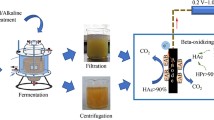
So far, the substrate of MEC was confined to either single substrate or biodegradable wastewater. The performance of composite substrate had been sparsely studied and the utilization of these mixed substrates from municipal and industrial sources represented the practical potential of MECs. Especially, no report was found to investigate the organic transformation characteristics of DS in MEC. In this study, a combined process of alkaline pre-hydrolysis and MEC was examined using DS as a substrate. Firstly, the alkaline pretreatment was adopted to solubilize DS organics into the supernatant as much as possible. Then, the DS supernatant was fed into a single membraneless MEC operated under various applied voltages. The electrochemical parameters, such as current and coulombic efficiency, effluent quality, and gas production were comprehensively tracked aiming at investigating the biodegradation ability of pretreated sludge. Finally, the variations of proteins and carbohydrates and the changes of various DOM (dissolved organic matter) fractions in the organic matters extracted from DS under a typical batch operation of MEC were determined for better interpretation of the involved mechanism.
Materials and methods
Materials
The DS was collected from an A/O (anoxic/oxic) WWTP with capacity of 64 × 104 m3/day in Nanjing, China. The sludge treatment process in the plant was gravity sedimentation followed by centrifugal dewatering. The moisture content of DS was 82.5% and the ratio of VSS/SS was 51.8%.
The sewage sludge with SS and VSS contents of 31,543 and 14,237 mg/L, respectively, was collected from the aeration tank in the same WWTP for the inoculation of MEC.
Alkaline pretreatment
DS of 100.0 g was mixed with NaOH solution (0.5 mol/L) of 400 mL. Then, the mixture was placed in a rotary shaker at a speed of 100 rpm and temperature of 25.0 °C for 24 h to perform the hydrolysis. Finally, the mixture was settled for 1 h and the supernatant was withdrawn and acidified to pH of 7.0 ± 0.2 to feed the MEC.
MEC startup and operation
MEC startup
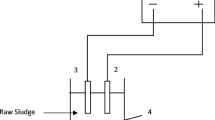
MEC anodes were enriched in two-chamber MFCs (microbial fuel cells) as follows: sewage sludge was fed into the anode chamber (269.3 mL) of MFC, which contained a carbon fiber brush (length of 50 mm, diameter of 50 mm, Toray T700 24K). The cathode chamber (269.3 mL) of MFC contained a carbon cloth (50 mm × 50 mm, WOS1002 CeTech, Taiwan) coated with 0.5 mg/cm2 of Pt (JM Hispec3000). The two chambers were separated by a proton exchange membrane. Sewage sludge was used as inoculum, and the volume ratio of sludge to culture medium was 1:2. The culture medium contained NaAc of 1.5 g/L, KH2PO4 of 2.4145 g/L, K2HPO4·3H2O of 7.3539 g/L, NH4Cl of 0.31 g/L, and KCl of 0.13 g/L. The phosphate buffer solution (PBS, 50 mM at pH 7.0) was dosed in the cathode chamber. A 1-kΩ external resistor was placed in series to determine the current by measuring the voltage drop. All experiments were carried out at 25 °C. The electrolyte in the chambers was replaced when the voltage of resistance reached maximum value and started to decline. When the maximum voltage of resistance was reproduced three times, the exoelectrogenic microbes were colonized on the anode surface. Then, this anode was moved to the MEC. The size of cuboid MEC was 60 mm × 70 mm × 110 mm with effective volume of 462 mL (Fig. 1). The anode chamber was carbon fiber brush taken from anode chamber of MFC. The cathode chamber was carbon cloth coated with Pt.
MEC operation
The supernatant derived from the pre-hydrolyzed DS was dosed with KH2PO4, NH4Cl, K2HPO4·3H2O, and KCl to the concentration of 2.4145, 7.3539, 0.31, and 0.13 g/L, respectively, and then fed into MEC (Fig. 1) with 3.6 mL of trace mineral solution and 1.5 mL of vitamin solution (Balch et al. 1979; Lovley et al. 1984). Resistance (10 Ω) and direct current power supply were applied between the cathode and anode. One typical batch operation lasted 10 days. The MECs were operated using duplicate reactors for over more than three cycles at room temperature (20–25 °C).
Analytical methods
The COD (chemical oxygen demand) content was measured using standard methods (APHA 1998). The concentration of protein was analyzed using coomassie blue staining G-250 (China EPA 2002). The total carbohydrate content was determined using anthrone-sulfuric acid colorimetric method (Laurentin and Edwards 2003). The biogas was collected using gas sampling bag (E-Switch, 200 mL), and the total volume was measured using a glass syringe.
The performance of the MEC was evaluated in terms of hydrogen production rate (Q, m3 H2/(m3 day)), current (I, A), coulombic efficiency (C E ), cathodic hydrogen recovery (rcat), and energy recovery relative to the electrical input (η E ) as described (Logan et al. 2008). It was found obviously in the study that these parameters presented a periodic change with the addition of the substrates due to the existence of biocathode. So, each reported curve was reproducible in the stable cycles.
Fractionation of organic matter from DS
Both the solution extracted from DS by NaOH which regarded as influent of MEC and the effluent from MEC was fractionated into five classes: hydrophobic acid (HPO-A), hydrophobic neutral (HPO-N), transphilic acid (TPI-A), transphilic neutral (TPI-N), and hydrophilic fraction (HPI), using XAD-8/XAD-4 resin chromatography following the established methods (Wei et al. 2011).
Three-dimensional excitation-emission matrix (EEM) fluorescence spectroscopy
EEM spectra of the components (DOM) of the DS supernatant and the effluent from MEC were measured using a fluorescence spectrometer (F7000, Hitachi). Filtrated samples were diluted to 1 mg/L of DOC (dissolved organic carbon) with 0.01 mol/L KCl and were acidified to pH 3 with HCl. A xenon excitation source was used in the spectrometer, and the excitation and emission slits were set to a 10-nm band-pass. The EEM spectra were obtained by scanning the sample over excitation wavelengths from 200 to 400 nm with 5 nm steps and emission wavelengths from 280 to 550 nm with 5 nm steps.
Results and discussion
Solubilization performance by alkaline pretreatment
The use of alkaline treatment for disintegration of microbial cells and solubilizing EPS from WAS is widely accepted at present (Liu et al. 2016). The distinguish between non-stabilized DS and WAS lies in the structure of the flocs, as the former shows a more condensed structure. Therefore, the effect of alkaline pretreatment on DS solubilization was examined.
The study by Everett (1974) has shown that during this process, the sludge is made swelling and the structure is loosed. The cells in sludge lose the viability and disrupt as they are unable to maintain an appropriate turgor pressure. Then, the added alkali reacts with the cell walls through several ways, including the saponification of lipids, which leads to the solubilization of cell membrane. Disruption of sludge cells results in the release of intracellular biopolymer out of the cell (Neyens et al. 2003). On the other hand, as stated (Katsiris and Kouzeli-Katsiri 1987), increment of sludge pH changed the bacterial surfaces to negative charge and consequently created high electrostatic repulsion to cause desorption of parts of EPS into the solution. These solubilized organics, including protein, humic acid, polysaccharides, lipids, and nucleic acid (Wilen et al. 2003; Dignac et al. 1998), can be used through MEC. At a liquid to DS ratio of 4 mL/g, the concentrations of solubilized COD, carbohydrates, and proteins were significantly improved (Table 1). Of these compounds, proteins (Table 1) and humic acid were the primary constituents, while the others were present in relatively low levels. The yellow appearance of sludge supernatant is a strong evidence of solubilization of humic acid. Because humic acid could be classified as yellow, brown, and black, all of which could be dissolved in NaOH (Li et al. 2009). This result was consistent with the finding by Li et al. (2009) and indicated the effectiveness of alkaline pretreatment to disrupt DS flocs and cells. The released intracellular organic molecules did not undergo further degradation by alkaline treatment (Liu et al. 2016). Thus, the supernatant containing solubilized organics was fed and degraded via the MEC.
Effect of applied voltage on COD removal
In MECs, organic matters are oxidized by anodic exoelectrogenic bacteria like Geobacter or Shewanella species capable of extracellular electron transfer. The produced electrons by the oxidation of biodegradable organic matters are transferred to the anode and consumed at the cathode to generate H2 (Logan and Regan 2006; Liu et al. 2006; Zhao et al. 2016). It has been reported that exoelectrogenic bacteria are utilizing various kinds of substrates as electron donors such as short-chain fatty acids (SCFAs), glucose, aromatic hydrocarbons, and proteins (Chaudhuri and Lovley 2003; Lovley et al. 2011). This implies the possibility of utilization of complex organic matters as substrates, such as WAS and DS, in the MECs. Therefore, in this study, the MECs were fed with sludge supernatant from alkali-treated DS as substrates. It has been proved that the kinetics of bioelectrochemical reaction depends considerably on the electrode potentials (Feng et al. 2015; Villano et al. 2010), which are determined by the applied voltage between the anode and cathode. So, the influence of applied voltages on the effluent COD was investigated and the results are shown in Fig. 2.
The activity of exoelectrogen in the MEC relies on the electrical potential of the electrodes (Ding et al. 2016). At room temperature (20–25 °C), the effluent COD decreased with an increase in applied voltage. At 0.5 V, MEC removed 26.35% COD. When the voltage increased to 0.7 V, the COD removal rate increased to 39.14%. However, the removal rate slowed down at 0.9 V, at which condition the COD removal rate was 44.92%. Increment of applied voltage resulted in augmentation of degradation of organic matter. The result was similar to that reported by Escapa et al. (2012). This phenomenon was more obvious at low applied voltage.
Effect of applied voltage on energy recovery
The changes of electrochemical parameters and gas production of MECs at various applied voltages are presented in Fig. 3 and Table 2.
Figure 3 shows the current changes reflecting the degree of electrochemical reaction on the electrode surface and accumulation of exoelectrogenic microbes during a cycle at various voltages/potentials. The current reached peak value quickly in 1–2 days, and then gradually decreased. The increment in current at first is attribute to the feeding which provides sufficient biodegradable organics for the microorganisms. The microorganisms were stimulated to degrade the newly added substrates. In the study conducted by Zhao et al. (2016), the hydrolysis of intracellular organic matters of WAS took 7 days, and until then, these organic matters could be utilized by anodic oxidation of MEC, which resulted in the current production. Owing to the effectiveness of pre-hydrolysis by alkaline treatment to release more organics into the solution, the current in this study generated rapidly.
The decreasing rate of current varied from a rapid drop to a slow drop. This result was owing to the utilization process of organic matters by biofilms. The biofilms on the anode of MECs readily degraded low-molecular-weight organics, such as acetic acid, at a high speed. The degradation rate slowed down as the consumption of low-molecular-weight organics, thus led to the current decrement. Then, the relatively high-molecular-weight organics, which were difficult to be biodegraded, such as protein, were utilized at a low speed. The relevant current value was low and became stable. The current was proportional to the biogas production as shown in Table 2. The same result was obtained in the earlier study (Lu et al. 2012a). When the applied voltage increased, the time for current to reach peak value advanced, which was a proof of stimulation effect of microorganisms.
A slight increase in C E from 71.91 to 78.89% can be seen in Table 2 as the voltage increased. The average C E was 75.60%, which suggested that the electrons from the substrates could be easily accessed by the microorganisms in MECs. Due to the anaerobic environment of MEC and the complexity of substrate, methanogens were unavoidably enriched on the anode than in raw WAS (Lu et al. 2012b). H2-oxidizing methanogens are expected to be the major pathway of H2 sink in this study (Lu et al. 2012b). Methane production in MEC consumes H2 directly or causes a loss of electron donors. The C E values were reported to range from 17 to 48% generated in single-chamber MEC at 0.5 V via complex substrates, such as domestic and swine wastewater (Ditzig et al. 2007; Wagner et al. 2009). The high C E in this study was partially ascribed to that most of the electron donors from DS supernatant were metabolized by exoelectrogens, which outcompeted fermentative bacteria. Another possible reason for the high C E was that the substrate adopted in this study was DS supernatant, which avoided accumulation of inert materials on electrodes caused by a mixture of solid and liquid, such as WAS. Furthermore, the NH3-N in MEC increased from initial concentration of 412 mg/L to effluent concentration of 471–485 mg/L (Fig. 4). In anaerobic bioreactor, the activity of methanogens decreased with increasing NH3-N (Chen et al. 2008). The high NH3-N concentration in MEC during the operation time inhibited the methanogen activity and therefore increased the accumulation of intermediate products to enhance the hydrogen production and resulted in a high C E .
However, the rcat value showed a declining trend. The disagreement of high C E with relatively low rcat was probably attributed to two reasons: one reason was that the hydrogen production rate was low and the hydrogen loss through the reactor to the environment was possible (Logan et al. 2008; Rozendal et al. 2006); another reason was likely the reactor configuration. Single-chamber MEC without membrane has attracted more attention as a result of high hydrogen recovery and simple structure (Call and Logan 2008). The major problem encountered in this configuration was the limitation of reactor scale-up. The large reactor volume in this study resulted in high internal resistance (Cusick et al. 2011). Besides, the relative area of carbon cloth (and carbon brush) was small compared with the whole reactor and the conversion of H2 to CH4, which also led to the descending performance.
The hydrogen production rate showed modest improvement at low voltage. The low hydrogen production rate even at high voltage of 0.9 V (Table 2) was as a result of characteristics of substrate and was relevant to the low rcat. DS was a complex biomass with high SS (17.5% in this study). At present, few studies were reported using DS directly as substrate in MEC. This study, however, adopted NaOH solution to solubilize the insoluble part of DS and then fed into the MEC. The sludge supernatant contained complex organics (Table 1). When feeding complex organics, such as swine and food wastewater, the hydrogen production rates in MEC were as low as 0.9–1.0 m3 H2/(m3 day) (Wagner et al. 2009) and 0.15 ± 0.03 m3 H2/(m3 day) (Tenca et al. 2013), respectively, much lower than the values obtained using SCFA (Call and Logan 2008; Jeremiasse et al. 2010). Besides, the high concentration of sludge supernatant (2481 mg COD/L) from this study could generate high resistance, resulting in overpotential and ohmic loss problems (Gajaraj et al. 2017) to minimal the difference in gas production at applied voltages.
The η E decreased when the voltage increased, which was partially as a result of the low rcat. The maximum η E was achieved at 0.5 V, which was much less than 100%. This indicated that more energy was required in the case of sludge supernatant than treating other substrates like sodium acetate and suggested an improvement in treatment efficiency to realize positive energy production in the further study.
Variations of proteins and carbohydrates in MECs
The treatment efficiency of MEC is affected by the influent COD (Sasaki et al. 2011; Sasaki et al. 2013). Using sodium acetate as substrate, the COD removal in a single-chamber MEC increased with the increasing influent COD (Teng et al. 2015). At influent COD of 1000–1350 mg/L and applied voltage of 0.5 V, the COD removal rate reached 72.5–75.3% (Teng et al. 2015). In our study, at influent COD of 2482 mg/L, the obtained COD removal rate was only 26%. The difference in the COD removal rates lied in the influent qualities. The DS supernatant contained complex organics, including macromolecular compounds. In the study by Lu et al. (2012a), the COD removal rate for WAS was 20–28%, which was comparable with our result. In order to determine the variations of macromolecular organic matters in MEC, the concentrations of proteins and carbohydrates during a typical operation cycle under different voltages were investigated. The results are shown in Figs. 5 and 6.
The MEC can easily utilize the carbohydrates in WAS to generate H2 through two pathways. One way is through metabolizing the carbohydrate directly by the exoelectrogens. The other way is through using the fermentative products of carbohydrates such as alcohols and VFAs (volatile fatty acids) to produce electrons by the exoelectrogens (Logan 2009). The removal of carbohydrates could be illustrated by the example of glucose. In the case of anaerobic degradation, glucose reduction results from several possible reactions via fermentation, acetoclastic methanogenesis, and hydrogenotrophic processes (Eqs. (1) and (2)). The produced hydrogen could be consumed by hydrogenotrophic methanogenesis (Eq. (3)), or acetogenesis to produce acetate (under higher hydrogen pressure, Eq. (4)), which can be eventually converted to methane through acetoclastic methanogenesis (Gajaraj et al. 2017; Zinder and Anguish 1992).
Whereas in the case of MEC, the fermentation by-products of glucose, mainly acetate (Selembo et al. 2009), are oxidized at the anode through Eq. (5).
Exoelectrogens at the cathode are capable of producing hydrogen too or even methane through direct electron transferring (Gajaraj et al. 2017), as shown in the following equations:
In Fig. 5, the concentration of carbohydrates decreased sharply at the initial period, and then declined slowly. At voltages of 0.5, 0.7, and 0.9 V, the removal rates of carbohydrates at the end of a cycle were 48.43, 58.85, and 64.27%, respectively, whereas these values reached 37.67, 37.67, and 55.86% at only half of the cycle. The results indicated that carbohydrates were preferred for degradation in MEC, which was proved by Catal (2016) and Lu et al. (2012a). These matters could be directly used by exoelectrogens to produce hydrogen. Besides, certain end-products of fermentation of these matters, such as VFAs and alcohols, could also be utilized (Catal 2016; Logan 2009). Consequently, most carbohydrates were removed in a short time.
The fate of proteins in MEC is presented in Fig. 6. Protein reduction was increased by using higher voltages. The result was consistent with the finding by Nam et al. (2014). Although the concentration dropped with time, the removal rate was relatively low. The maximum protein removal rate of 33.73% was obtained at voltage of 0.9 V, only half of the maximum carbohydrates removal rate. Because most known exoelectrogens prefer simple substrates (like organic acids, alcohol, monosaccharide) for extracellular electron transfer, the direct oxidization of polymeric and complex materials generally requires cooperation of exoelectrogens with polymer-degrading bacteria, often fermenters (Lu and Ren 2016). Besides, the intermediates formed during the hydrolysis of large molecular weight protein may not be a good proton source for hydrogen generation (Lu et al. 2010). The low performance of COD reduction is certain for the proteins in MECs. The reason for the observed differences of COD removal between proteins and carbohydrates may result from the biodegradability and electron loss to competing mechanisms.
In the study conducted by Wang et al. (2013), the MEC was fed with a synthetic dairy wastewater, which contained 10.6% protein, while the DS supernatant adopted in this study consisted of 27.3% protein. The COD removal rates were comparable in the two studies; however, the removal rate of protein in synthetic dairy wastewater was more than 70% at 0.8 V (Wang et al. 2013), which almost doubled the value achieved in our study. The reason was likely the complex compositions of influent and various bacteria on the anode. Although protein had been reported to be directly used to produce hydrogen in MEC (Lu et al. 2010), the complexity of proteins and the presence of degradation byproducts, such as VFAs, may result in different hydrogen production performance (Lu et al. 2012a; Sasaki et al. 2011). For example, the BSA (bovine serum albumin, a kind of pure protein)-fed MEC had a better performance than that those fed peptone (complex protein) in terms of hydrogen production in MEC.
In terms of WAS (Lu et al. 2012(a)), the removal rate of COD was comparable with our study, which proved the feasibility of DS in MEC. The performances of MECs under different types of influent were summed in Table 3. The observed differences of removal rates between carbohydrates and proteins at various applied voltages proved the above discussion on the decreasing trend of ηE. At high voltage, the reduction of η E had nothing to do with the activity of the microorganisms. Otherwise, the same decreasing trend of carbohydrates and proteins would be found.
Characterization of DS solution in MEC by EEM spectra
The five fractions from DS supernatant organic matter extracted by NaOH revealed fluorescence peaks in regions I and II, and region IV by Chen et al. (2003), referring to the redundancy of aromatic proteins and soluble microbial by-product-like materials, respectively (Fig. 7b–f). The fulvic acid-like components were detected at Ex/Em = 250/415 in the raw DS supernatant (DOM, Fig. 7a) extracted by NaOH, while the humic acid-like substances were not detected. The HPO-A fraction showed the highest fluorescent intensity in Regions I and II among the DOM fractions, followed by HPO-N, HPI, TPI-N, and that of the TPI-A was the lowest. Therefore, the NaOH-extracted DS supernatant was dominated by hydrophobic materials, followed by hydrophilic materials, with the lowest content for the transphilic materials. The fulvic acid-like components in the NaOH-extracted DS supernatant were mainly composed of HPO-N fraction.
The fluorescent intensity of DS supernatant (DOM) decreased after MEC treatment (Fig. 8a), which proved the COD reduction, although the aromatic proteins and soluble microbial by-product-like materials were still the principal components. Previous study showed that the fulvic acid-like components in the HPO-N and TPI-A fractions of WAS could be readily degraded by MFC treatment (Jiang et al. 2010), which was consistent with our study (Fig. 8d, e). Results showed that the MEC operation preferentially degraded HPI, HPO-A, and HPO-N fractions in the NaOH-extracted DS supernatant (Fig. 8). HPI is consisted of polar compounds of low molecular weights, presenting the most readily degradable compounds. While the HPO-A fraction generally has the least polarity and the highest molecular weight, which should have a more complex characteristic (Maurice et al. 2002; Namour and Müller 1998). The degradation of hydrophobic fractions (HPO-A and HPO-N) was likely through the anaerobic fermentation, during which generated transphilic fractions of TPI-A and TPI-N.
Conclusions
In this study, DS was successfully treated by a combined process of alkaline pre-hydrolysis and MEC. The effect of applied voltage on the performance of MEC was investigated. Results showed that the removal rate of COD increased with an increase in applied voltage. This increasing effect was more obvious at low voltage. At voltage of 0.9 V, the maximum COD removal rate for the pre-hydrolyzed DS supernatant attained 44.92%.
At various applied voltages, the current reached peak value in 1–2 days after feeding and then decreased. The biogas production increased with increment of the current. The average coulombic efficiency was 75.60% with average recovery rate of hydrogen conversion of 17.63%.
At voltages of 0.5, 0.7, and 0.9 V, the hydrogen production rates were 0.027 m3/(m3 day), 0.035 m3/(m3 day), and 0.038m3/(m3 day), respectively. The reason for the low rates was likely substrate types and low cathodic hydrogen recovery, which resulted in low energy recovery. This result implies the direction of further studies.
The removal rate of organic matter in MEC was influenced by the composition of DS. The carbohydrates could be readily used with removal rates of 48.43, 58.85, and 64.27% at voltages of 0.5, 0.7, and 0.9 V, respectively. However, the proteins were difficult to be utilized, which resulted in low COD removal rate. The TPI-A and TPI-N fractions of the proteins extracted from the DS showed low biodegradability during the MEC operation.
References
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC. https://www.standardmethods.org/
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43(2):260–296. https://www.researchgate.net/publication/230642230_Methanogens_A_Re-evaluation_of_a_unique_biological_group
Call D, Logan BE (2008) Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ Sci Technol 42(9):3401–3406. https://doi.org/10.1021/es8001822
Cano R, Pérezelvira SI, Fdzpolanco F (2015) Energy feasibility study of sludge pretreatments: a review. Appl Energy 149:176–185. https://doi.org/10.1016/j.apenergy.2015.03.132
Catal T (2016) Comparison of various carbohydrates for hydrogen production in microbial electrolysis cells. Biotechnol Biotechnol Equip 30(1):75–80. https://doi.org/10.1080/13102818.2015.1081078
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21(10):1229–1232. https://doi.org/10.1038/nbt867
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence excitation- emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol 37(24):5701–5710. https://doi.org/10.1021/es034354c
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99(10):4044–4064. https://doi.org/10.1016/j.biortech.2007.01.057
China EPA (2002) Water and wastewater monitoring and analyzing, 4th ed’, Chinese environmental. Science Press, Beijing
Cusick RD, Bryan B, Parker DS, Merrill MD, Mehanna M, Kiely PD, Liu G, Logan BE (2011) Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl Microbiol Biotechnol 89(6):2053–2063. https://doi.org/10.1007/s00253-011-3130-9
Dignac MF, Urbain V, Rybacki D et al (1998) Chemical description of extracellular polymers: implication on activated sludge floc structure. Water Sci Technol 38(8-9):45–53. https://doi.org/10.1016/S0273-1223(98)00676-3
Ding A, Yang Y, Sun G, Wu D (2016) Impact of applied voltage on methane generation and microbial activities in an anaerobic microbial electrolysis cell (MEC). Chem Eng J 283:260–265. https://doi.org/10.1016/j.cej.2015.07.054
Ditzig J, Liu H, Logan BE (2007) Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int J Hydrog Energy 32(13):2296–2304. https://doi.org/10.1016/j.ijhydene.2007.02.035
Escapa A, Gil-Carrera L, García V, Morán A (2012) Performance of a continuous flow microbial electrolysis cell (MEC) fed with domestic wastewater. Bioresour Technol 117:55–62. https://doi.org/10.1016/j.biortech.2012.04.060
Everett JG (1974) The effect of pH on the heat treatment of sewage sludges. Water Res 8(11):899–906. https://doi.org/10.1016/0043-1354(74)90104-3
Feng Y, Zhang Y, Chen S, Quan X (2015) Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron–graphite electrode. Chem Eng J 259:787–794. https://doi.org/10.1016/j.cej.2014.08.048
Gajaraj S, Huang Y, Zheng P, Hu Z (2017) Methane production improvement and associated methanogenic assemblages in bioelectrochemically assisted anaerobic digestion. Biochem Eng J 117:105–112. https://doi.org/10.1016/j.bej.2016.11.003
Jeremiasse AW, Hamelers HVM, Saakes M et al (2010) Ni foam cathode enables high volumetric H2 production in a microbial electrolysis cell. Int J Hydrog Energy 35(23SI):12716–12723. https://doi.org/10.1016/j.ijhydene.2010.08.131
Jiang J, Zhao Q, Wei L, Wang K (2010) Extracellular biological organic matters in microbial fuel cell using sewage sludge as fuel. Water Res 44(7):2163–2170. https://doi.org/10.1016/j.watres.2009.12.033
Kadier A, Simayi Y, Kalil MS, Abdeshahian P, Hamid AA (2014) A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew Energy 71:466–472. https://doi.org/10.1016/j.renene.2014.05.052
Katsiris N, Kouzeli-Katsiri A (1987) Bound water content of biological sludges in relation to filtration and dewatering. Water Res 21(11):1319–1327. https://doi.org/10.1016/0043-1354(87)90004-2
Kim TH, Nam YK, Park C, Lee M (2009) Carbon source recovery from waste activated sludge by alkaline hydrolysis and gamma-ray irradiation for biological denitrification. Bioresour Technol 100(23):5694–5699. https://doi.org/10.1016/j.biortech.2009.06.049
Laurentin A, Edwards CA (2003) A microtiter modification of the anthrone-sulfuric acid colorimetric assay for glucose-based carbohydrates. Anal Biochem 315(1):143–145. https://doi.org/10.1016/S0003-2697(02)00704-2
Li H, Jin Y, Nie Y (2009) Application of alkaline treatment for sludge decrement and humic acid recovery. Bioresour Technol 100(24):6278–6283. https://doi.org/10.1016/j.biortech.2009.07.022
Liu H, Ramnarayanan R, Logan BE (2006) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38(7):2281–2285. https://doi.org/10.1021/es034923g
Liu Y, Wang L, Ma J, Zhao X, Huang Z, Mahadevan GD, Qi J (2016) Mahadevan G.D., Qi J.Y. Improvement of settleability and dewaterability of sludge by newly prepared alkaline ferrate solution. Chem Eng J 287:11–18. https://doi.org/10.1016/j.cej.2015.11.037)
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7(5):375–381. https://doi.org/10.1038/nrmicro2113
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(12):512–518. https://doi.org/10.1016/j.tim.2006.10.003
Logan BE, Call D, Cheng S, Hamelers HVM, Sleutels THJA, Jeremiasse AW, Rozendal RA (2008) Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol 42(23):8630–8640. https://doi.org/10.1021/es801553z
Lovley DR, Greening RC, Ferry JG (1984) Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol 48(1):81–87. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC240316/pdf/aem00152-0089.pdf
Lovley DR, Ueki T, Zhang T et al (2011) Geobacter: the microbe electric's physiology, ecology, and practical applications. Adv Microb Physiol 59:1–100. https://doi.org/10.1016/B978-0-12-387661-4.00004-5)
Lu L, Ren ZJ (2016) Microbial electrolysis cells for waste biorefinery: a state of the art review. Bioresour Technol 215:254–264. https://doi.org/10.1016/j.biortech.2016.03.034
Lu L, Xing D, Xie T, Ren N, Logan BE (2010) Hydrogen production from proteins via electrohydrogenesis in microbial electrolysis cells. Biosens Bioelectron 25(12):2690–2695. https://doi.org/10.1016/j.bios.2010.05.003
Lu L, Xing D, Liu B et al (2012a) Enhanced hydrogen production from waste activated sludge by cascade utilization of organic matter in microbial electrolysis cells. Water Res 46(4):1015–1026. https://doi.org/10.1016/j.watres.2011.11.073
Lu L, Xing D, Ren N (2012b) Pyrosequencing reveals highly diverse microbial communities in microbial electrolysis cells involved in enhanced H2 production from waste activated sludge. Water Res 46:2425–2434. https://doi.org/10.1016/j.watres.2012.02.005
Maurice PA, Pullin MJ, Cabaniss SE (2002) A comparison of surface water natural organic matter in raw filtered water samples, XAD, and reverse osmosis isolates. Water Res 36(9):2357–2371. https://doi.org/10.1016/S0043-1354(01)00442-0
Nam JY, Yates MD, Zaybak Z, Logan BE (2014) Examination of protein degradation in continuous flow, microbial electrolysis cells treating fermentation wastewater. Bioresour Technol 171:182–186. https://doi.org/10.1016/j.biortech.2014.08.065
Namour P, Müller MC (1998) Fractionation of organic matter from wastewater treatment plants before and after a 21-day biodegradability test: a physical-chemical method for measurement of the refractory part of effluents. Water Res 32(7):2224–2231. https://doi.org/10.1016/S0043-1354(97)00428-4
Neyens E, Baeyens J, Creemers C (2003) Alkaline thermal sludge hydrolysis. J Hazard Mater B97(1-3):295–314. https://doi.org/10.1016/S0304-3894(02)00286-8
Rozendal RA, Hamelers HVM, Euverink GJW et al (2006) Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrog Energy 31(12):1632–1640. https://doi.org/10.1016/j.ijhydene.2005.12.006
Sasaki K, Morita M, Sasaki D, Hirano Si, Matsumoto N, Watanabe A, Ohmura N, Igarashi Y (2011) A bioelectrochemical reactor containing carbon fiber textiles enables efficient methane fermentation from garbage slurry. Bioresour Technol 102(13):6837–6842. https://doi.org/10.1016/j.biortech.2011.04.022
Sasaki D, Sasaki K, Watanabe A, Morita M, Matsumoto N, Igarashi Y, Ohmura N (2013) Operation of a cylindrical bioelectrochemical reactor containing carbon fiber fabric for efficient methane fermentation from thickened sewage sludge. Bioresour Technol 129:366–373. https://doi.org/10.1016/j.biortech.2012.11.048
Selembo PA, Perez JM, Lloyd WA, Logan BE (2009) High hydrogen production from glycerol or glucose by electrohydrogenesis using microbial electrolysis cells. Int J Hydrog Energy 34(13):5373–5381. https://doi.org/10.1016/j.ijhydene.2009.05.002
Standard Methods APHA (1998) For the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Tenca A, Cusick RD, Schieuano A et al (2013) Evaluation of low cost cathode materials for treatment of industrial and food processing wastewater using microbial electrolysis cells. Int J Hydrog Energy 38(4):1859–1865. https://doi.org/10.1016/j.ijhydene.2012.11.103
Teng WK, Liu GL, Luo HP et al (2015) Influence of substrate cod on methane production in single-chambered microbial electrolysis cell. Environ Sci 36(3):1021–1026. (in Chinese). https://doi.org/10.13227/j.hjkx.2015.03.035
Tunçal T (2011) Comparing alkaline and thermal disintegration characteristics for mechanically dewatered sludge. Environ Technol 32(14):1581–1588 (http://www.tandfonline.com/doi/abs/10.1080/09593330.2010.544677)
Villano M, Aulenta F, Ciucci C, Ferri T, Giuliano A, Majone M (2010) Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour Technol 101(9):3085–3090. https://doi.org/10.1016/j.biortech.2009.12.077
Wagner R, Regan J, Oh SE et al (2009) Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res 43(5):1480–1488. https://doi.org/10.1016/j.watres.2008.12.037
Wang W, Luo HP, Liu GL et al (2013) Exoelectrogens community analysis and hydrogen production in the microbial electrolysis cell using dairy wastewater. Microbiol China 40(11):2075–2082 (in Chinese) (http://journals.im.ac.cn/wswxtbcn/ch/reader/view_abstract.aspx?file_no=tb13112075
Weemaes MP, Verstraete J (1998) Evaluation of current wet sludge disintegration techniques. J Chem Technol Biotechnol 73:83–92. https://doi.org/10.1002/chin.199902318)
Wei LL, Zhao QL, Hu K, Lee DJ, Xie CM, Jiang JQ (2011) Extracellular biological organic matters in sewage sludge during mesophilic digestion at reduced hydraulic retention time. Water Res 45(3):1472–1480. https://doi.org/10.1016/j.watres.2010.11.003
Wilen BM, Jin B, Lant P (2003) The influence of key chemical constituents in activated sludge on surface and flocculating properties. Water Res 37(9):2127–2139. https://doi.org/10.1016/S0043-1354(02)00629-2
Zhao Z, Zhang Y, Yu Q, Ma W, Sun J, Quan X (2016) Enhanced decomposition of waste activated sludge via anodic oxidation for methane production and bioenergy recovery. Int Biodeterior Biodegrad 106:161–169. https://doi.org/10.1016/j.ibiod.2015.10.020
Zhen G, Lu X, Kato H, Zhao Y, Li YY (2017) Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: current advances, full-scale application and future perspectives. Renew Sust Energ Rev 69:559–577. https://doi.org/10.1016/j.rser.2016.11.187
Zinder SH, Anguish T (1992) Carbon monoxide, hydrogen, and formate metabolismduring methanogenesis from acetate by thermophilic cultures of Methanosarcina and Methanothrix strains. Appl Environ Microbiol 58(10):3323–3329. https://www.ncbi.nlm.nih.gov/pubmed/16348788
Acknowledgements
The authors gratefully acknowledge fundings from the National Natural Science Foundation of China (Grant No. 51408194), Ministry of Education Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Hohai University (Grant No. 2015002), the Fundamental Research Funds for the Central Universities (Grant No. 2017B16614), National Science and Technology Major Project (Grant No. 2016YFC0400800-04), Scientific and Technological Project of Henan Province (Grant No. 162102310057), and the Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Hu, K., Xu, L., Chen, W. et al. Degradation of organics extracted from dewatered sludge by alkaline pretreatment in microbial electrolysis cell. Environ Sci Pollut Res 25, 8715–8724 (2018). https://doi.org/10.1007/s11356-018-1213-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1213-1