Abstract
Soil organic matter (SOM) is the main adsorbent for polycyclic aromatic hydrocarbons (PAHs) and the principal aggregating agent for soil aggregation that can affect PAH bioavailability and bioaccessibility in soils. The objective of this study was to analyze the relationship between PAH dissipation and variation in soil aggregate–size distribution in two field-contaminated soils with different soil organic C (SOC) content (Anthrosols, 1.41% SOC; Phaeozems, 8.51% SOC) in phytoremediation with alfalfa. The results showed that there were significant reductions of 10.2 and 15.4% of the total PAHs in unplanted and planted treatments, respectively, for Anthrosols. However, there was no significant reduction of total PAHs in either unplanted or planted treatment for Phaeozems. For Anthrosols, mass percentages of coarse sand and fine sand were significantly reduced while coarse silt and fine silt were significantly increased for the planted soil compared to the initial soil (p < 0.05). For Phaeozems, there was no significant variation in aggregate–size distribution among different treatments except that coarse silt in planted and unplanted soil was slightly reduced. The main reason for the dissipation of PAHs in Anthrosols could be that macroaggregates were broken into microaggregates, which made some trapped PAHs become bioaccessible to soil microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are by-products of incomplete combustion of organic materials and are ubiquitous in the environment (Wilcke 2007). Some of them are known or suspected carcinogens or mutagens (Duan et al. 2015). Soil is a mainly terrestrial reservoir of PAHs. Some of the high-molecular-weight PAHs have been found to persist in soil for several decades (Shuttleworth and Cerniglia 1995).
It is well known that dissipation of PAHs is more difficult in long-term field-contaminated soils than in laboratory-spiked soils (Ahn et al. 2005; Smith et al. 2011). The main difference between spiked and field-contaminated soils is the aging effect that decreases extractability and bioavailability of PAHs to soil microorganisms and other biological receptors with increasing PAHs–soil interaction time (Hatzinger and Alexander 1995; White and Alexander 1996). Several mechanisms have been suggested and explained for the aging of chemicals in soils, including partitioning into or onto humic substances (Chiou et al. 1979, 1983), or diffusion into three-dimensional micropores of soil particles (Steinberg et al. 1987). Many studies have reported that the partition to soil organic matter (SOM) was the chief mechanism for organic chemicals, particularly for hydrophobic organic chemicals (HOCs) to be sequestered in soil (Luthy et al. 1997). However, very little attention has been devoted to the physical protection of HOCs in soil aggregates. SOM is not only the most important sorbent for HOCs in soils but also the major agent of soil aggregate formation and stabilization in soils (Tisdall and Oades 1982; Balesdent et al. 2000). Soil aggregate formation can conversely protect SOM by developing physical barriers between microorganisms and enzymes and their substrates (Elliott and Coleman 1988). Then, the PAHs combined with SOM can also be protected within soil aggregates. Hence, the changes of soil structure might affect the bioavailability of PAHs in them. Steinberg et al. (1987) reported that the release of residual 1,2-dibromoethane in agricultural topsoils up to 19 years was greatly improved by pulverization of the soil, and Nam et al. (2003) reported that soil aggregation might be another important determine factor in the decreased biodegradation of aged phenanthrene (Phe) except for soil organic carbon (SOC) content. The apparent sequestration of HOCs by geosorbents is not well understood, because there is no direct observation of the HOCs’ molecular distributed within natural geosorbents (Luthy et al. 1997).
Particle–size fractionation of soils has been widely applied to separate SOM pools with different quality and turnover rates (Christensen 2001). For example, the SOM in sand–size fraction is largely comprised of fresh or slightly degraded plant material, the silt–size fraction contains partially decomposed residues, and the SOM in clay–size fraction is dominated by highly processed organic matter with aromatic and aliphatic structures (Kiem et al. 2002; Chen and Chiu 2003). Because SOM is the main adsorbent for PAHs in soils, many studies have also been carried on the distribution of PAHs in particle–size aggregates of field-contaminated soils (Müller et al. 2000; Doick et al. 2005; Ni et al. 2008) and the bioavailability of PAHs associated with different particle–size aggregates (Amellal et al. 2001). The results of Shor et al. (2003) showed that intraaggregate mass transport limitations, and not the inherent bacterial PAH utilization ability, were most important in controlling biodegradation rate of PAHs in sediments.
Phytoremediation is a green technology that uses plants to decontaminate soils of organic and inorganic compounds. Plants may promote PAH dissipation through a mixture of mechanisms, such as plant uptake and accumulation, increasing soil microbial activity, and alteration of soil physical and chemical properties in the rhizosphere (Harvey et al. 2002; Parrish et al. 2005; Wenzel 2009). In our previous study, we have compared the dissipation of PAHs in freshly spiked and two long-term field-contaminated soils in phytoremediation and found that PAHs are easily dissipated in freshly spiked soils than in field-contaminated soils (Wei et al. 2017). Moreover, the dissipations of PAHs in the two field-contaminated soils were also different and we explained that that was due to the different SOM contents in soils. In our another previous study, however, we found that the percentage removals of total PAHs from field-contaminated soils in phytoremediation had a positive correlation with the percentage distributions of total PAHs in coarse sand fraction (r = 0.813, p < 0.10) and had a significantly negative correlation with those in fine silt fraction (r = 0.898, p < 0.05) (Ni et al. 2013). Hence, we suppose that the different dissipation of PAHs in the two field-contaminated soils might be related with soil aggregate structure, not only determined by the content of SOM. To our knowledge, there is little information on the variation of soil aggregate–size distribution in phytoremediation and its relationship with the dissipation of PAHs in soils. In this study, therefore, we further reported the variations of soil aggregates in the two field-contaminated soils in phytoremediation with alfalfa (Medicago sativa L.) in our previous study (Wei et al. 2017) and analyzed the relationship between variation in soil aggregate–size distribution and PAH dissipation.
Materials and methods
Chemicals
PAH mixture standard (47940-U) and EPA 610 PAH Mix (4S8743) were purchased from Sigma-Aldrich. Acetonitrile (HPLC grade) was obtained from Fisher Scientific. Dichloromethane was bought from Sinopharm Chemical Reagent Co., Ltd. and redistilled before use.
Soils
Two PAH field-contaminated soil samples were used in this study. One is Anthrosols, the other is Phaeozems. According to the international soil classification system, the texture of Anthrosols and Phaeozems is silt sandy loam (39.5% sand, 48.6% silt, and 11.9% clay) and loam (51.1% sand, 36.7% silt, and 12.2% clay), respectively. All soil samples were air dried and sieved through a 2-mm mesh screen and thoroughly mixed. Selected soil properties and concentrations of 15 USEPA priority PAHs (except acenaphthylene) of the two soils are listed in Tables 1 and 2, respectively.
Pot experiments
The procedure of pot experiments was same as presented in our previous study (Wei et al. 2017). There were two treatments with three replicates, an unplanted control and soil planted with alfalfa. At the end of pot experiments, plants were removed and soil samples were freeze dried. Dried soil samples were sieved through a 2-mm mesh screen for aggregate–size fractionation and PAH analysis.
Soil aggregate–size fractionation
Soil samples of initial, unplanted, and planted treatments were fractionated into coarse sand (2000–105 μm), fine sand (105–53 μm), coarse silt (53–20 μm), fine silt (20–2 μm), and clay (< 2 μm) according to the method of Ni et al. (2008). All particle–size aggregates were freeze dried and weighed for further analysis.
Sample extraction
PAHs in bulk soils and aggregates were extracted with a Soxhlet extractor. The detailed procedure was presented in our previous study (Wei et al. 2017).
PAH analysis
The 15 USEPA priority PAHs were analyzed in this study, including naphthalene (Nap), acenaphthene (Ace), fluorene (Flu), Phe, anthracene (Ant), fluoranthene (FluA), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chry), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenz[a,h]anthracene (DBA), benzo[g,h,i]perylene (BghiP), and indenol[1,2,3-cd]pyrene (IP). Acenaphthylene was excluded due to its low fluorescence. The PAHs were analyzed using a Waters® ACQUITY UPLC system equipped with a fluorescence detector and a reversed phase column ACQUITY UPLC® BEH Shield RP18 (150 mm × 2.1 mm, 1.7 μm). The programs of gradient mobile phase and wavelength were listed in Tables S1 and S2, respectively.
Quality control
The detection limit of the 15 PAHs ranged from 0.03 to 1.53 μg L−1. Method blanks (solvent) and spiked samples (EPA 610 PAH Mix (4S8743), Sigma-Aldrich, USA, spiked into soil) were extracted and analyzed using the same procedure as the samples. The recoveries of PAHs ranged from 77.8 ± 3.1 to 97.2 ± 0.5%, except that the Nap recovery was 28.7 ± 5.7% (Table S3). Results of blanks extracted were below detection limits. The results of samples are presented without recovery ratio correction.
Statistical analysis
Statistical analysis of the data was performed in replicates by one-way analysis of variance (ANOVA) using Tukey’s test (p < 0.05).
Results
PAH dissipation in soils
Table 2 shows the concentrations of PAHs detected in Anthrosols and Phaeozems before and after a 10-month plant cultivation. For the Anthrosols, the total PAH concentration significantly decreased for both of the unplanted and planted soils compared with the initial soil (p < 0.05); the reduction was 10.2 and 15.4% in unplanted and planted soils, respectively. The largest reduction was BghiP, which dissipated about 50% for both of unplanted and planted treatments. For the Phaeozems, there were no significant changes of total PAH concentrations in both the unplanted and the planted soils relative to the initial soil.
Variation in soil aggregate–size distribution
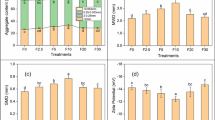
Soil mass recoveries of the aggregate–size fractionation ranged from 96.7 to 99.5% for Anthrosols and from 96.1 to 98.4% for Phaeozems. As shown in Fig. 1, for Anthrosols, mass percentages of coarse sand and fine sand were significantly reduced while coarse silt and fine silt were significantly increased for planted soil when comparing with initial and unplanted soils (except for fine silt fraction) (p < 0.05) (Fig. 1a). Comparing with initial soil, mass percentages of coarse sand were significantly reduced and coarse silt was significantly increased in unplanted soil (p < 0.05) (Fig. 1a). For Phaeozems, there were no significant variation of aggregate–size distribution among different treatments except that the mass percentage of coarse silt was significantly decreased for planted and unplanted soil when comparing with initial soil (p < 0.05) (Fig. 1b).
Distribution of PAHs in soil aggregates
Figure 2 is the distribution of PAHs in soil particle–size aggregates before and after the 10-month plant cultivation. The recoveries of PAHs in the sum of particle–size aggregates to total PAHs in bulk soils ranged from 88.2 to 107.9% for Anthrosols and from 99.7 to 112.9% for Phaeozems. For Anthrosols, the percentages of PAHs were significantly decreased in coarse sand while significantly increased in coarse silt in planted soil when comparing with initial and unplanted soils (p < 0.05) (Fig. 2a). There were no significantly differences of percentages of PAHs in particle–size aggregates between initial and unplanted soils (Fig. 2a). For Phaeozems, there were no significantly differences of percentages of PAHs in particle–size aggregates among different treatments (p < 0.05) (Fig. 2b).
Discussions
PAH dissipation in soils
For the Anthrosols, the total PAH concentration significantly decreased for both of the unplanted and planted soils compared with the initial soil (p < 0.05) and the largest reduction was BghiP (Table 2). The common understanding about the microbial degradation of high-molecular-weight PAHs is the cooxidation or cometabolism (Keck et al. 1989; Kanaly and Harayama 2000). Binet et al. (2000) also reported that ryegrass was able to accelerate the dissipation of a range of PAHs, including DBA and BghiP. For the Phaeozems, there were no significant changes of total PAH concentrations in both the unplanted and the planted soils compared with the initial soil, which might attribute to the high SOC content that limits the bioavailability of PAHs. It has been reported that SOM is the predominant sorbent for HOCs (Pignatello 1998). However, our results further indicated that the influences of SOM on the dissipation of PAHs are not only reflected in the SOM quantity but also in the stability of soil aggregates affected by SOM (See the following sections for details.).
Variation in soil aggregate–size distribution
SOM is one of the most principal adsorbent that can affect the bioavailability of HOCs in soils (Luthy et al. 1997). Moreover, Nam et al. (2003) reported that soil aggregation might be another important determining factor in the decreased biodegradation of aged Phe except for SOC content. Hence, variations in soil aggregate–size distribution before and after the 10-month phytoremediation were analyzed (Fig. 1). The different variations of particle–size aggregates in Anthrosols and Phaeozems might be due to the different SOM content, because that organic matter is believed one of the primary aggregating agents in soils (Tisdall and Oades 1982; Annabi et al. 2011). Some components of SOM such as polysaccharides, humic substances, root material, and fungal hyphae have a vital role in soil structural stabilization. Whitbread (1995) reported that the stability of soil macroaggregates increased at higher organic matter contents, probably due to organic bonding mechanisms. The SOC content in Phaeozems (8.51%) is far greater than that in Anthrosols (1.41%); the stability of macroagrregates in Phaeozems is therefore greater than that in Anthrosols.
Soil aggregation is affected by many factors such as changes in SOM, moisture content and microbial community, crop type, root growth, and tillage application (Denef et al. 2002; Verchot et al. 2011). Some of these factors can interact with and mutually affect each other. For example, soil water holding capacity (WHC) usually increases with increasing SOM content (Hudson 1994). In this study, variation of soil aggregate–size distribution was greater in Anthrosols than Phaeozems (Fig. 1) which also might be due to the different WHC of the two soils. WHC of Anthrosols (44.7%) is less than that of Phaeozems (63.9%) (Table 1), which might cause relatively more drying–wetting cycles in Anthrosols than Phaeozems, because the amount of water for watering unplanted and planted treatments was almost same at each time for both soils. Jager and Bruins (1975) reported that soils become structureless after repeated drying–wetting cycles, which is primarily ascribed to breakdown of air-dry aggregates by fast rewetting. With rapid wetting, air pressure develops inside aggregates, due to the water fast entering the soil pores, which will result in slaking of unstable aggregates. For Anthrosols (Fig. 1a), the significant difference of the distribution of soil particle–size aggregates between planted and unplanted treatments might be due to the root growth. In the short term, root growth may stimulate aggregate breakdown and soil disintegration by creating zones of collapse, thereby inducing soil loosening (Materechera et al. 1994). Reid et al. (1982) suggested that reduced stability of aggregates following the short-term growth of corn could be ascribed to a destruction of the linkages between organic matter, iron or aluminum, and mineral particles by the roots.
Relationship between variation in soil aggregates and PAH dissipation
Combining the variations of soil particle–size aggregates (Fig. 1) and the distribution of PAHs in particle–size aggregates (Fig. 2), it can be inferred that the different dissipation of PAHs between Anthrosols and Phaeozems in phytoremediation might be related to the variations of their particle–size aggregates. In Fig. 1a, mass percentages of coarse sand and fine sand fractions were significantly reduced while coarse silt and fine silt were significantly increased in planted and unplanted treatments of Anthrosols, which indicates that some of the soil macroaggregates (the coarse sand and fine sand fractions) were broken up into microaggregates (coarse silt and fine silt) in Anthrosols. As soil macroaggregates were broken up, some of PAHs trapped within aggregates became exposed to soil microorganisms and plants and were dissipated, and some were still trapped and distributed into smaller aggregates. The variations of the distribution of PAHs in particle–size aggregates of Anthrosols (Fig. 2a) also back up this point. The results of the Pearson correlation (one-tailed test) showed that the percentages of total PAHs dissipated in Anthrosols were significantly positively correlated with the decreased mass percentage of coarse sand (r = 0.786, p = 0.032, n = 6) and the increased mass percentage of coarse silt (r = 0.823, p = 0.022, n = 6). Nam et al. (2003) reported that soil aggregation might be another important determine factor in the reduced biodegradation of aged phenanthrene except for SOC content. Pee et al. (2015) also reported that ultrasonic irradiation increased the bioaccessibility of PAHs in sediment by breaking up large particles into small particles.
There was no significant change of particle–size aggregates in Phaeozems (Fig. 1b), and there was almost no reduction of soil PAH concentrations (Table 2), which was mainly due to the sequestration of PAHs in soil micropores and the inaccessibility of PAHs to microorganisms (LeBoeuf and Weber 1997; Xing and Pignatello 1997). SOM content is considered one of the most important factors that affects the bioavailability of PAHs in soils; however, Fismes et al. (2002) reported that PAH concentrations in vegetables increased with PAH concentrations in soils, although the SOM content was also proportionally increased with PAH concentrations in soils. Hence, the effect of SOM on the bioavailability of PAHs in soils is reflected not only in the quantity of SOM but also in other factors such as the soil aggregate stability influenced by SOM. Therefore, almost no reduction of PAHs in Phaeozems might be due to the higher stability of particle–size aggregates which caused the inaccessibility of PAHs sequestered in them. One may think that no reduction of total PAHs in Phaeozems might due to the lack of degradation microorganisms. However, the results of Shor et al. (2003) showed that intraaggregate mass transport limitations, and not the inherent bacterial PAH utilization ability, were most important in controlling biodegradation rate of PAHs in sediments. Hence, we think that the different dissipation of PAHs between Anthrosols and Phaeozems was mainly due to the different variations of soil aggregates.
It is well known that long-term tillage causes an alteration of soil dry aggregate–size distribution (Yang and Wander 1998; Eynard et al. 2004) and water-stable aggregation (Unger et al. 1998; Álvaro-Fuentes et al. 2008). Drying–wetting cycles and root growth can also have effects on aggregate formation in soils (Denef et al. 2002). Soil management systems that promote aggregate destruction, primarily of macroaggregates, can increase SOM degradation rates due to the exposure of the organic matter that was previously sequestered in the aggregates (Six et al. 1998; Lobe et al. 2011). SOM is the main adsorbent for PAHs in soils. Thus, the PAHs associated with SOM can be accessed by soil microorganisms in these processes. In this study, the percentages of total PAHs distributed in macroaggregates (coarse sand and fine sand) are 65.4 and 80.1% for the initial Anthrosols and Phaeozems, respectively. Other research results also showed that PAHs are mainly distributed in soil macroaggregates (Ni et al. 2008; Liao et al. 2013). Therefore, soil management systems, due to their effect on aggregate transformation rates, can also influence PAH degradation. Hence, proper soil managements such as traditional tillage and wetting and drying cycles can be chosen to enhance bioaccessibility of PAHs in long-term polluted soils in phytoremediation and natural attenuation by breaking soil macroaggregates into smaller aggregates.
Conclusions
In this study, the dissipation of PAHs was greater in Anthrosols than in Phaeozems, which was attributed to the lower SOC content of the Anthrosols. Moreover, the variation in soil aggregate–size distribution and the distributions of PAHs in particle–size aggregates of Anthrosols suggest that lower SOC contents leads to easier breaking up of soil macroaggregates into smaller size aggregates in Anthrosols, which made some trapped PAHs bioaccessible and easier to be bioremediated. Thus, proper soil management that breaks up macroaggregates (e.g., traditional tillage and wetting and drying cycles) can be chosen to enhance bioaccessibility of PAHs in bioremediation of long-term polluted soils. The results in this study also provide a new idea and method for the remediation of PAHs in field-contaminated soils in situ and ex situ.
References
Ahn S, Werner D, Luthy RG (2005) Physicochemical characterization of coke-plant soil for the assessment of polycyclic aromatic hydrocarbon availability and the feasibility of phytoremediation. Environ Toxicol Chem 24:2185–2195
Álvaro-Fuentes J, Arrúe JL, Gracia R, López MV (2008) Tillage and cropping intensification effects on soil aggregation: temporal dynamics and controlling factors under semiarid conditions. Geoderma 145:390–396
Amellal N, Portal JM, Berthelin J (2001) Effect of soil structure on the bioavailability of polycyclic aromatic hydrocarbons within aggregates of a contaminated soil. Appl Geochem 16:1611–1619
Annabi M, Le Bissonnais Y, Le Villio-Poitrenaud M, Houot S (2011) Improvement of soil aggregate stability by repeated applications of organic amendments to a cultivated silty loam soil. Agric Ecosyst Environ 144:382–389
Balesdent J, Chenu C, Balabane M (2000) Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillages Res 53:215–230
Binet P, Portal JM, Leyval C (2000) Dissipation of 3–6-ring polycyclic aromatic hydrocarbons in the rhizosphere of ryegrass. Soil Biol Biochem 32:2011–2017
Chen JS, Chiu CY (2003) Characterization of soil organic matter in different particle-size fractions in humid subalpine soils by CP/MAS 13C NMR. Geoderma 117:129–141
Chiou CT, Peters LJ, Freed VH (1979) A physical concept of soil-water equilibria for nonionic organic compounds. Science 206:831–832
Chiou CT, Porter PE, Schmedding DW (1983) Partition equilibria of nonionic organic compounds between soil organic matter and water. Environ Sci Technol 17:227–231
Christensen BT (2001) Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci 52:345–353
Denef K, Six J, Merckx R, Paustian K (2002) Short-term effects of biological and physical forces on aggregate formation in soils with different clay mineralogy. Plant Soil 246:185–200
Doick KJ, Burauel P, Jones KC, Semple KT (2005) Distribution of aged 14C-PCB and 14C-PAH residues in particle-size and humic fractions of an agricultural soil. Environ Sci Technol 39:6575–6583
Duan L, Naidu R, Thavamani P, Meaklim J, Megharaj M (2015) Managing long-term polycyclic aromatic hydrocarbon contaminated soils: a risk-based approach. Environ Sci Pollut Res 22:8927–8941
Elliott ET, Coleman DC (1988) Let the soil work for us. Ecol Bull 39:23–32
Eynard A, Schumacher TE, Lindstrom MJ, Malo DD (2004) Aggregate sizes and stability in cultivated South Dakota prairie Ustolls and Usterts. Soil Sci Soc Am J 68:1360–1365
Fismes J, Perrin-Ganier C, Empereur-Bissonnet P, Morel JL (2002) Soil-to-root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J Environ Qual 31:1649–1656
Harvey PJ, Campanella BF, Castro PM, Harms H, Lichtfouse E, Schäffner AR, Smrcek S, Werck-Reichhart D (2002) Phytoremediation of polyaromatic hydrocarbons, anilines and phenols. Environ Sci Pollut Res 9:29–47
Hatzinger PB, Alexander M (1995) Effect of aging of chemicals in soil on their biodegradability and extractability. Environ Sci Technol 29:537–545
Hudson BD (1994) Soil organic matter and available water capacity. J Soil Water Conserv 49:189–194
Jager G, Bruins EH (1975) Effect of repeated drying at different temperatures on soil organic matter decomposition and characteristics, and on the soil microflora. Soil Biol Biochem 7:153–159
Kanaly RA, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067
Keck J, Sims RC, Coover M, Park K, Symons B (1989) Evidence for cooxidation of polynuclear aromatic hydrocarbons in soil. Water Res 23:1467–1476
Kiem R, Knicker H, Kögel-Knabner I (2002) Refractory organic carbon in particle-size fractions of arable soils I: distribution of refractory carbon between the size fractions. Org Geochem 33:1683–1697
LeBoeuf EJ, Weber WJ (1997) A distributed reactivity model for sorption by soils and sediments. 8. Sorbent organic domains: discovery of a humic acid glass transition and an argument for a polymer-based model. Environ Sci Technol 31:1697–1702
Liao X, Ma D, Yan X, Yang L (2013) Distribution pattern of polycyclic aromatic hydrocarbons in particle-size fractions of coking plant soils from different depth. Environ Geochem Health 35:271–282
Lobe I, Sandhage-Hofmann A, Brodowski S, Du Preez CC, Amelung W (2011) Aggregate dynamics and associated soil organic matter contents as influenced by prolonged arable cropping in the South African Highveld. Geoderma 162:251–259
Luthy RG, Aiken GR, Brusseau ML, Cunningham SD, Gschwend PM, Pignatello JJ, Reinhand M, Traina SJ, Weber WJ Jr, Westall JC (1997) Sequestration of hydrophobic organic contaminants by geosorbents. Environ Sci Technol 31:3341–3347
Materechera SA, Kirby JM, Alston AM, Dexter AR (1994) Modification of soil aggregation by watering regime and roots growing through beds of large aggregates. Plant Soil 160:57–66
Müller S, Wilcke W, Kanchanakool N, Zech W (2000) Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in particle-size separates of urban soils in Bangkok, Thailand. Soil Sci 165:412–419
Nam K, Kim JY, Oh DI (2003) Effect of soil aggregation on the biodegradation of phenanthrene aged in soil. Environ Pollut 121:147–151
Ni JZ, Luo YM, Wei R, Li XH (2008) Distribution of polycyclic aromatic hydrocarbons in particle-size separates and density fractions of typical agricultural soils in the Yangtze River Delta, east China. Eur J Soil Sci 59:1020–1026
Ni JZ, Lin JW, Wei R, Yang HY, Yang YS (2013) Does the distribution of polycyclic aromatic hydrocarbons in soil particle-size separates affect their dissipation during phytoremediation of contaminated soils? In: Xu JM, Wu JJ, He Y (eds) Functions of natural organic matter in changing environment. Springer, Netherlands, pp 669–672
Parrish ZD, Banks MK, Schwab AP (2005) Effect of root death and decay on dissipation of polycyclic aromatic hydrocarbons in the rhizosphere of yellow sweet clover and tall fescue. J Environ Qual 34:207–216
Pee GY, Na S, Wei Z, Weavers LK (2015) Increasing the bioaccessibility of polycyclic aromatic hydrocarbons in sediment using ultrasound. Chemosphere 122:265–272
Pignatello JJ (1998) Soil organic matter as a nanoporous sorbent of organic pollutants. Adv Colloid Interf Sci 76:445–467
Reid JB, Goss MJ, Robertson PD (1982) Relationship between the decreases in soil stability effected by the growth of maize roots and changes in organically bound iron and aluminum. J Soil Sci 33:397–410
Shor LM, Liang W, Rockne KJ, Young LY, Taghon GL, Kosson DS (2003) Intra-aggregate mass transport-limited bioavailability of polycyclic aromatic hydrocarbons to Mycobacterium strain PC01. Environ Sci Technol 37:1545–1552
Shuttleworth KL, Cerniglia CE (1995) Environmental aspects of PAH biodegradation. Appl Biochem Biotechnol 54:291–302
Six J, Elliot ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci Soc Am J 62:1367–1377
Smith MJ, Flowers TH, Duncan HJ, Saito H (2011) Study of PAH dissipation and phytoremediation in soils: comparing freshly spiked with weathered soil from a former coking works. J Hazard Mater 192:1219–1225
Steinberg SM, Pignatello JJ, Sawhney BL (1987) Persistence of 1, 2-dibromoethane in soils: entrapment in intraparticle micropores. Environ Sci Technol 21:1201–1208
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Unger PW, Jones OR, McClenagan JD, Stewart BA (1998) Aggregation of soil cropped to dryland wheat and grain sorghum. Soil Sci Soc Am J 62:1659–1666
Verchot LV, Dutaur L, Shepherd KD, Albrecht A (2011) Organic matter stabilization in soil aggregates: understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 161:182–193
Wei R, Ni JZ, Li XY, Chen WF, Yang YS (2017) Dissipation and phytoremediation of polycyclic aromatic hydrocarbons in freshly spiked and long-term field-contaminated soils. Environ Sci Pollut Res 24:7994–8003
Wenzel WW (2009) Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 321:385–408
Whitbread AM (1995) Soil organic matter: its fractionation and role in soil structure. In: Lefroy RDB, Blair GJ, Craswell ET (eds) Soil organic matter management for sustainable agriculture. ACIAR Proceedings 56:124–130
White JC, Alexander M (1996) Reduced biodegradability of desorption-resistant fractions of polycyclic aromatic hydrocarbons in soil and aquifer solids. Environ Toxicol Chem 15:1973–1978
Wilcke W (2007) Global patterns of polycyclic aromatic hydrocarbons (PAHs) in soil. Geoderma 141:157–166
Xing B, Pignatello JJ (1997) Dual-mode sorption of low-polarity compounds in glassy poly (vinyl chloride) and soil organic matter. Environ Sci Technol 31:792–799
Yang XM, Wander MM (1998) Temporal changes in dry aggregate size and stability: tillage and crop effects on a silty loam Mollisol in Illinois. Soil Tillage Res 49:173–183
Acknowledgements
This research was funded by the National Natural Science Foundation of China (41671326), the Basic Scientific Research Special Project for Research Institutes of Fujian Province (2015R1034), and the Program for Innovative Research Team in Fujian Normal University (IRTL1205).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Electronic supplementary material
ESM 1
(DOC 64 kb)
Rights and permissions
About this article
Cite this article
Wei, R., Ni, J., Chen, W. et al. Variation in soil aggregate–size distribution affects the dissipation of polycyclic aromatic hydrocarbons in long-term field-contaminated soils. Environ Sci Pollut Res 24, 22332–22339 (2017). https://doi.org/10.1007/s11356-017-9919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9919-z