Abstract
Due to the subtle occurrence of environmental polycyclic aromatic hydrocarbon (PAHs) pollution from incinerators, it is seldom considered a significant source of PAH pollution. However, considering the recent build-up of toxics in urban air, this may be a serious concern around the incinerator vicinity due to the potential consequences of PAHs on human health. Hence, this study determined 11 alkyl-naphthalene contributions from a hospital waste incinerator (HWI_0) into ambient air receptor points (HWI_1 to HWI_5) for a 1-year period: June 2014–May 2015. The HWI_0 and ambient gases were sampled using filter-sorbent sampling system and polyurethane foam (PUF) passive samplers, respectively, and all alkyl-naphthalenes were determined using GC-MS. Results showed that the source concentrations were in the range of 0–14.0 ng/m3 and generally higher than the receptor points. The receptor point concentration trends were mainly HWI_1 > HWI_2 ≥ HWI_3 ≥ HWI_5 ≥ HWI_4. Multivariate receptor model analysis suggested high correlations between source and the receptor points though there might be some significant contributions from other emission sources. The average monthly concentrations (∑alkyl-naphthalene) at HWI_0 and the receptors HWI_1, HWI_2, HWI_3, HWI_4 and HWI_5 were 67.4 ± 24.3, 57.9 ± 20.1, 42.8 ± 16.9, 39.7 ± 12.2, 36.5 ± 22.2 and 37.8 ± 15.4 ng/m3, respectively. Though these concentrations were lower than the estimated minimal risk level (MRL) for chronic inhalation exposure to naphthalene and its derivatives 0.003 mg/m3, continuous exposure to these pollutants might result in chronic effects. Finally, this study may be used to evaluate the environmental contribution of alkyl-naphthalenes from typical medical waste incinerator in Nigeria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollutants are ubiquitous in the air, water and soil, and their concentrations are rising (Olu-Owolabi et al. 2016; Diagboya et al. 2016). One class of environmental pollutants that are widespread and a major source of concern are the polycyclic aromatic hydrocarbons (PAHs) (ATSDR 2005; Olu-Owolabi et al. 2014; Liu et al. 2014). Due to the carcinogenicity of some of the PAHs, they have been classified as priority pollutants (ATSDR 1995; Olu-Owolabi et al. 2015; USEPA 2007). In order to evaluate the risks of our various anthropogenic actions and predict appropriate management practices for environmental PAHs, understanding source contributions to the environment may be vital (Park et al. 2011).
Several PAHs originating from the incomplete pyrolysis, at high temperature, of carbonaceous organic materials are common in the environment, and they are typically more concentrated near urban areas (Srogi 2007). Waste incinerators are one major environmental source of PAHs (Baraniecka et al. 2010; Liu et al. 2014; Srogi 2007; Wang et al. 2006; Anyakora et al. 2004). The PAHs from incinerators occur subtly, and their contribution is often underestimated. However, as PAH concentrations in the environment rise, incinerators’ contributions may become a grave concern around its vicinity due to their potential consequences on ecosystems and human health. This consideration is important because incineration is the preferred method of disposal of infectious and toxic hospital wastes in Nigeria. One major challenge in the country is that most hospitals use locally fabricated incinerators without air pollution control technologies. This with the attendant increase in medical wastes as human population grow in Africa’s sub-Sahara might lead to unprecedented amounts of PAHs in this region of the world.
One PAH of major concern is naphthalene. This is the simplest member of the PAH family containing two linearly fused benzene rings with molecular formula and weight of C10H8 and 128.2 g/mol, respectively. Naphthalene is a white crystalline solid with a characteristic detectable odour at a concentration as low as 0.08 ppm, and it evaporates easily. Fossil fuels naturally contain naphthalene and its derivatives, such as 1-methylnaphthalene and 2-methylnaphthalene (ATSDR 2005; Musat et al. 2009). These chemicals are used in making polyvinyl chloride (PVC) plastics, moth repellents, toilet deodorant, dyes, resins, leather tanning agents, vitamin K and the insecticide carbaryl. Naphthalene and its derivatives may be released into the environment at some hazardous waste sites, through industrial uses and from incomplete pyrolysis of carbonaceous organic materials (contributes about 70%) such as incineration, cigarette and wood smokes (ATSDR 2005). They can dissolve in water and may be present in drinking water, but they weakly bind to soil and can leach into underground water. Naphthalene can be metabolized to 1-naphthol and 2-naphthol which share some of the toxic properties of naphthalene. Though naphthalene and its derivatives have similar properties, substitution of methyl groups into its ring system can result in more reactivity and carcinogenity (ATSDR 2005; Musat et al. 2009; Dabestani and lvanov 1999). Naphthalene and its derivatives have been found in exposed cow milk, chicken eggs and some samples of fish and shellfish from polluted waters. The presence of these PAHs has been linked to haematological, hepatic, neurological, ocular and respiratory defects in human (ATSDR 2005; Landrum et al. 1987). Numerous studies concerning PAH emissions from various anthropogenic processes have been carried out with focus on the priority PAHs (Wang et al. 2012; Baraniecka et al. 2010; Srogi 2007; Zhao et al. 2008). However, there has been little focus on naphthalene and its derivatives. Hence, the objective of this study was to determine the monthly concentrations of 11 alkyl-naphthalene derivatives (1-methyl naphthalene, 2-methyl naphthalene, 1,3-dimethyl naphthalene, 1,5-dimethyl naphthalene, 1,6-dimethyl naphthalene, 1,7-dimethyl naphthalene, 2,7-dimethyl naphthalene, 2,6-dimethyl naphthalene, 1-ethyl naphthalene, 1,6,7-trimethyl naphthalene and 2,3,6-trimethyl naphthalene) within selected locations around a typical medical waste incinerator for a 1-year period: June 2014–May 2015.
Material and methods
Collection of samples, preparation and instrumental analysis
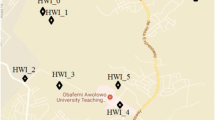
The Obafemi Awolowo University Teaching Hospital Waste Incinerator (HWI) is the source of the alkyl-naphthalene derivatives sampled. The HWI is a Rotary Kiln-type incinerator which uses electricity to generate the incinerating heat and equipped with flue gas scrubbers. The operating temperature range from 500 to 1100 °C and designed with the capacity to incinerate liquid and solid wastes. Table 1 shows the descriptions of the sampling locations. Air samples used in this study were collected from inside the stack (HWI_0) and within the vicinity of the HWI site (4° 55′ E; 7° 518′ N). Considering nearby residential areas and the prevailing meteorological conditions (wind speed and direction) around the HWI vicinity (Fig. 1), five sampling locations were selected.
Ambient air samples were collected using polyurethane foam (PUF) passive samplers (Gao et al. 2014). Prior to deployment, PUF disks was pre-cleaned with distilled water and then washed with acetone in a Soxhlet extractor for 24 h, followed by petroleum ether for another 24 h (Pozo et al. 2004). The disks were dried in a dessicator, covered in aluminium foil and transported to the sampling locations. The duration of each sampling was 28 days. The filters in the PUF samplers were replaced after every 28 days for a 1-year period (June 2014–May 2015). Stack gas samples were collected by adopting a modified Hoyos et al. (2008) method. Typically, it consists of a filter-sorbent sampling system having a sampling probe, a filter and a packed column of Amberlite XAD-2 resin (adsorbent material). Samples were iso-kinetically withdrawn from the gas stream of the HWI into the sample train.
The PAHs trapped in PUF disks and Amberlite XAD-2 resin were extracted with dichromethane using a Soxhlet extractor. Cleanup step was carried as described by Hoyos et al. (2008). The extracted PAHs were concentrated to 20 μL using a rotary evaporator under a gentle stream of nitrogen at 50 °C. Quantitative analysis of PAHs in the samples was carried out using gas chromatography/mass spectrophotometer (GC-MS). The GC (Agilent 7890) with mass detector (Agilent 5975) was operated in selected ion monitoring mode and using electron impact ionization. The chromatographic column dimension is 30-m × 0.25-mm internal diameter × 0.25-μm film thickness. Injection of 1 μL of each extracted sample was achieved in splitless mode using helium as a carrier gas at a constant flow of 1.2 mL min−1. The temperature program for the analysis was set as follows: the initial temperature (50 °C) was held for 2 min, ramped raised to 120 °C at 30 °C min−1 and then ramped again to 280 °C at 6 °C min−1 for 15 min. Determination of PAHs in both laboratory and field blanks was carried out. The internal standard method was used for both the calculation of the recovery (ranged between 70 and 120%) and quantification of the alkylated PAHs. All reagents used in this study were pesticide grade, and the method detection limit was calculated by multiplying the standard deviation of each PAH congener by the Student’s t values (at 99% confidence level). Statistical analysis was carried out using principal component analysis (PCA) and Pearson correlation available on the XLSTAT program.
Results and discussion
The compositions of various alkyl-naphthalenes in association with airborne particles were determined around the Obafemi Awolowo University Teaching Hospital Waste Incinerator (HWI) site (4° 55′ E; 7° 518′ N) for a 1-year period from the months of June 2014 through May 2015. The concentrations of alkyl-naphthalenes in the stack gas (HWI_0) were compared with those from selected distances (HWI_1–5) (Table 1). It has been assumed in this study that the concentrations of alkyl-naphthalenes in the selected sampling points are largely from the incinerated wastes (except otherwise indicated) because HWI uses electricity, rather than hydrocarbon fuel, to generate the incinerating heat. Hence, due to the differences in volatility and affinities for adsorption onto particulate matter (PM) in the atmosphere, as well as the prevailing wind speed and direction (Fig. 1), the concentrations of the alkyl-naphthalenes were expected to vary (and lower as well) from the HWI_0 (source) to the various sampled points (environment). Results from the study are shown in Fig. 2a–k.
Monthly concentrations of the alkyl-naphthalene from the various sampling points. a 1-Methyl naphthalene. b 2-Methyl naphthalene. c 1,3-Dimethyl naphthalene. d 1,5-Dimethyl naphthalene. e 1,6-Dimethyl naphthalene. f 1,7-Dimethyl naphthalene. g 2,7-Dimethyl naphthalene. h 2,6-Dimethyl naphthalene. i 1-Ethyl naphthalene. j 1,6,7-Trimethyl naphthalene. k 2,3,6-Trimethyl naphthalene. l Naphthalene. Note that the first 5 months of each plot are in 2015 while the last 7 months are in 2014
Spatial and temporal distributions of various alkyl-naphthalenes compared to HWI_0
Figure 2a shows the concentrations of 1-methyl naphthalene for the study period. It was observed that HWI_0 had the highest concentrations of 1-methyl naphthalene for all the months with the exception of June 2014. It is logical that HWI_0, which is the source, had the highest concentrations of all the alkyl-naphthalenes. The monthly HWI_0 concentrations of the 1-methyl naphthalene ranged from 1.5 to 9.7 ng/m3 with the highest concentration observed in March 2015. The monthly HWI_0 mean was 5.6 ± 3.1 ng/m3 . The peak months for 1-methyl naphthalene in the HWI_0 and ambient air samples were November and December 2014 and March to May 2015. The various sampling points had lower concentrations than the HWI_0; the trend was mainly HWI_1 (mean 4.8 ± 3.0 ng/m3) > HWI_2 (3.6 ± 2.4 ng/m3) > HWI_4 (3.2 ± 2.4 ng/m3) > HWI_5 (3.3 ± 2.3 ng/m3) > HWI_3 (3.1 ± 2.0 ng/m3). This trend was likely due to proximity of the sampling points to HWI_0 as well as differences in meteorological conditions (wind speed and direction) associated with the various sampling points. The exception to this trend was observed in June 2014; Fig. 2a suggests that at this time of the year (from June to October), lower amounts of 1-methyl naphthalene were formed in the incinerator resulting in the low concentrations (less than 4.0 and 1.0 ng/m3 in the HWI_0 and sampling points, respectively). Another implication of the June result is that 1-methyl naphthalene in the atmosphere around the HWI_0 has been supplemented by another source probably vehicular emission, gasoline power generating sets and burning of cooking fuels especially at the HWI_5—Teaching Hospital Staff Estate (Srogi 2007; Wang et al. 2012).
The results for 2-methyl naphthalene are shown in Fig. 2b. The figure showed that HWI_0 had the highest concentrations of 2-methyl naphthalene in all months except in August 2014. The monthly HWI_0 concentrations were higher than for 1-methyl naphthalene and ranged from 3.3 to 8.5 ng/m3 with the highest concentration observed in January 2015. The monthly mean was 6.0 ± 1.6 ng/m3 . The peak concentration months in the HWI_0 and ambient air samples were in December 2014 and January 2015. The sampling points had lower concentrations than the HWI_0 with a trend of HWI_1 (5.1 ± 1.5 ng/m3) > HWI_2 (3.7 ± 1.2 ng/m3) > HWI_3 (3.3 ± 1.2 ng/m3) > HWI_5 (3.0 ± 1.2 ng/m3) > HWI_4 (2.7 ± 2.1 ng/m3). This trend was attributed to proximity of the sampling points to HWI_0 and differences in meteorological conditions affecting the various sampling points. An exception to this trend was observed in June and August 2014, suggesting that in both months, 2-methyl naphthalene in the atmosphere might have been supplemented by another source (Wang et al. 2012).
The results for 1,3-dimethyl naphthalene (Fig. 2c) showed similar trends during the study period as both alkyl-naphthalenes above. Exceptions to this trend were observed in the months of June, July and August 2014: while in June and July, the HWI_0 had lower 1,3-dimethyl naphthalene concentrations than every other sampling points indicating significant supplementation from other sources; 1,3-dimethyl naphthalene was not detected in both the HWI_0 and sampling points in August.
Figure 2d shows the concentrations of 1,5-methyl naphthalene. With the exception of the result of June 2014, the results had similar trends as described above with the HWI_0 concentrations higher than those detected at the various sampling locations. However, June 2014 HWI_1 concentration was higher than the source (HWI_0); the value for HWI_1 (13.5 ng/m3) was also significantly higher than the 10 ng/m3 threshold for other PAHs, and this may be an indication of other significant contributors of 1,5-methyl naphthalene to the ambient PAH concentrations. It was also observed that June, July and August had higher concentrations above the usual threshold of 10 ng/m3 in the HWI_0. The monthly HWI_0 concentrations ranged from 6.1 to 13.5 ng/m3, with July 2015 being the highest, and the monthly mean was 8.6 ± 2.1 ng/m3. The trend for the sampling points was mainly HWI_1 (6.8 ± 2.7 ng/m3) > HWI_2 (5.0 ± 1.4 ng/m3) > HWI_3 (4.7 ± 2.1 ng/m3) > HWI_5 (4.4 ± 1.7 ng/m3) > HWI_4 (4.0 ± 1.7 ng/m3). These values were higher than for other derivatives, and Fig. 2d suggests that there may be a higher likelihood to produce this derivative (1,5-methyl naphthalene) in HWI_0 compared with other alkyl-naphthalenes.
The 1,6-dimethyl naphthalene results (Fig. 2e) were also similar to those described earlier with the exceptions of the June and July 2014 results where the HWI_3 (medical students’ hostel) and HWI_1 (close to HWI_0) location results were significantly higher than that of the HWI_0. The results for both months have been attributed to increased burning of cooking fuels in the students’ hostel and high 1,6-dimethyl naphthalene release from HWI_0 exhaust pipe, respectively. The monthly HWI_0 concentrations ranged from 1.3 to 9.6 ng/m3, January 2015 had the highest concentration, and the monthly mean was 5.3 ± 2.6 ng/m3 . The HWI_0 concentrations were higher than the sampling points, and the trend was HWI_1 (5.3 ± 2.6 ng/m3) > HWI_3 (3.9 ± 2.1 ng/m3) ≥ HWI_2 (3.8 ± 2.4 ng/m3) > HWI_5 (3.5 ± 1.9 ng/m3) ≥ HWI_4 (3.3 ± 2.4 ng/m3).
The results for 1,7-dimethyl naphthalene (Fig. 2f) and 2,7-dimethyl naphthalene (Fig. 2g) showed similar trends as in 1,6-dimethyl naphthalene with the exception of the months of June and August 2014 where the concentrations at the HWI_0 were lower than in some of the sampling points. The other results (Fig. 1h–k) were also similar in trend, but in the 2,6-dimethyl naphthalene (Fig. 2h) and 2,3,6-trimethyl naphthalene (Fig. 2k) results, the months of June to August 2014 had HWI_0 concentrations which were lower than some of the sampling points, while in the 1-ethyl naphthalene (Fig. 2i) and 1,6,7-trimethyl naphthalene (Fig. 2j) results, only the month of June had lower HWI_0 concentrations than the sampling points. These alkyl-naphthalene results when compared with the parent naphthalene results (Fig. 2i) showed that monthly naphthalene concentrations emitted from the HWI_0 and those found in the other sampling locations were in similar range as those of the alkyl-naphthalenes and there were no indications that naphthalene was the preferred emitted pollutant. The major reason for the fluctuation in the HWI_0 concentrations for some months (mainly June to August) may be attributed to low volume of medical waste incinerated during these months.
The average monthly concentrations of all alkyl-naphthalene (∑alkyl-naphthalene) at HWI_0, HWI_1, HWI_2, HWI_3, HWI_4 and HWI_5 were 67.4 ± 24.3, 57.9 ± 20.1, 42.8 ± 16.9, 39.7 ± 12.2, 36.5 ± 22.2 and 37.8 ± 15.4 ng/m3, respectively (Fig. 3). These average atmospheric concentrations when compared with estimated minimal risk level for chronic inhalation exposure to naphthalene and its derivatives, 0.003 mg/m3 (0.0007 ppm) (ATSDR 2005; Abdo et al. 2001; NTP 2000), were below the minimal risk level, but they were above the recommended values for ambient air in some countries, such as Germany and Italy (Srogi 2007). However, these values were lower that the total concentration of the more harmful polychlorinated naphthalenes (97.6 ng/m3) emitted from a similar waste incinerator (Hu et al. 2013). Though the risks associated with human exposure to atmospheric PAHs are highest at locations close to the HWI_0, continuous exposure to indoor PM2.5 (PM with aerodynamic diameter less than 2.5 μm) and particulate and gaseous phase PAHs have been recognized as significant health problems since most people spend approximately 90% of their time indoors (Srogi 2007). Considering the fact that these alkyl-naphthalenes were detected all year round in all sampled sites (especially indoor), the chronic effects of their presence are a health threat. Particularly, waste incineration plays a dominant role in medical waste disposal in Nigeria and this is unlikely to change anytime soon. The location of the studied HWI receives relatively little alkyl-naphthalene supplementation from other sources; however, as population increases in nearby vicinities and residences get closer to the HWI site, ambient concentrations of these alkyl-naphthalenes will increase accordingly resulting in higher chronic or even acute effects.
Correlation between the HWI_0 emissions and other HWI concentrations
Multivariate receptor model analysis (principal component analysis (PCA)) was used in this study for the source (HWI_0) identification and contributions to ambient air receptors (HWIs) of the various alkyl-naphthalene. For easy identification of each factor, gaseous phase data was employed in the analysis. In a typical PCA score plot, variables with similar source will be located close to each other, while those with divergent sources will be far apart (Jambu 1991; Lee et al. 2004). Figure 4 shows the PCA variable plot (with cumulative variance of 88.98%) in which factor 1 (F1) accounts for 71.35% of the total variance while factor 2 (F2) explains 17.63% of the total variance. Thus, most of the variability in the alkylated naphthalene concentrations could be explained. For variables that are close to each other but far from the centre, they may be said to be significantly positively correlated. However, if both variables are on the opposite side of the centre, then they are significantly negatively correlated, but for variables that are orthogonal, they are not correlated. From the proximity (Fig. 4) between the HWI_0 and the HWI_1, HWI_3, HWI_4 and HWI_5, it may be inferred that there was correlation between the stack emission and the ambient air at these sampling locations (Jambu 1991; Lee et al. 2004), implying that HWI_0 was the source of the alkyl-naphthalene at these points. However, Fig. 4 also shows that HWI_2 was located far away, suggesting that though HWI_0 may be a contributor to HWI_2, there may be other emission sources to this receptor. A similar result has been reported by Wang et al. (2005). Sampling point HWI_2 is the university staff estate entrance, and it is prone to PAH emission due to high vehicular traffic (El-Shahawi et al. 2010) from residents and visitors. Figure 5 shows the PCA observation plot. The figure indicates that the alkyl-naphthalene grouped in clusters close to the centre is from the HWI_0 source. However, 1,5-dimethyl naphthalene (1,5-MN) was distant from the cluster, suggesting that there may be other sources of emissions contributing 1,5-dimethyl naphthalene significantly to the receptor.
Observation plot of PCA indicating the positions of 1-methyl naphthalene (1-MN), 2-methyl naphthalene (2-MN), 1,3-dimethyl naphthalene (1,3-MN), 1,5-dimethyl naphthalene (1,5-MN), 1,6-dimethyl naphthalene (1,6-MN), 1,7-dimethyl naphthalene (1,7-MN), 2,7-dimethyl naphthalene (2,7-MN), 2,6-dimethyl naphthalene (2,6-MN), 1-ethyl naphthalene (1-EN), 1,6,7-trimethyl naphthalene (1,6,7-MN) and 2,3,6-trimethyl naphthalene (2,3,6-MN)
Conclusion
The study determined 11 alkyl-naphthalene (1-methyl naphthalene, 2-methyl naphthalene, 1,3-dimethyl naphthalene, 1,5-dimethyl naphthalene, 1,6-dimethyl naphthalene, 1,7-dimethyl naphthalene, 2,7-dimethyl naphthalene, 2,6-dimethyl naphthalene, 1-ethyl naphthalene, 1,6,7-trimethyl naphthalene and 2,3,6-trimethyl naphthalene) contributions from medical waste incinerator (HWI_0) by sampling stack gas and ambient air around incinerator vicinity for a 1-year period: June 2014–May 2015. Results showed that the HWI_0 concentrations were usually higher than the sampling points (receptors). The HWI_0 concentrations ranged from 0 to 14.0 ng/m3. The concentration trends for the various alkyl-naphthalene were mainly HWI_1 > HWI_2 ≥ HWI_3 ≥ HWI_5 ≥ HWI_4. Multivariate receptor model analysis using principal component analysis (PCA) suggested high correlations between the HWI_0 and the receptor points HWI_1, HWI_3, HWI_4 and HWI_5. However, receptor point 2 HWI_2 (university staff estate entrance) might have been significantly supplemented by other emission sources such as vehicular emission. The PCA also indicates that of all the alkyl-naphthalene, 1,5-dimethyl naphthalene might have been significantly contributed by other sources of emissions. Comparison of alkyl-naphthalene with the parent naphthalene indicated that neither the alkyl-naphthalene nor naphthalene was a preferred emitted pollutant by the HWI_0. The average monthly concentrations (∑alkyl-naphthalene) at HWI_0, HWI_1, HWI_2, HWI_3, HWI_4 and HWI_5 were 67.4 ± 24.3, 57.9 ± 20.1, 42.8 ± 16.9, 39.7 ± 12.2, 36.5 ± 22.2 and 37.8 ± 15.4 ng/m3, respectively. These concentrations were lower than the estimated minimal risk level (MRL) for chronic inhalation exposure to naphthalene and its derivatives—0.003 mg/m3. However, continuous exposure to these pollutants is a health threat because of possible chronic effects.
References
Abdo M, Grumbein S, Chou BJ, Herbert R (2001) Toxicity and carcinogenicity study in F344 rats following 2 years of whole-body exposure to naphthalene vapors. Inhal Toxicol 13:931–950
Anyakora CA, Ogbeche KA, Palmer P, Coker H, Ukpo G, Ogah C (2004) A screen for benzo(a)pyrene, a carcinogen, in the water samples from the Niger Delta region. Nig J Hosp Med 14:288–293
ATSDR (1995) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf
ATSDR (2005) Toxicological profile for naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. Agency for Toxic Substances and Disease Registry pp 1–347. https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=43
Baraniecka J, Pyrzynska K, Szewczynska M, Posniak M, Dobrzynska E (2010) Emission of polycyclic aromatic hydrocarbons from selected processes in steelworks. J Hazard Mater 183:111–115
Dabestani R, Lvanov IN (1999) A compilation of physical, spectroscopic and photophysical properties of polycyclic aromatic hydrocarbons. Photochem Photobiol 70:10–34
Diagboya PN, Olu-Owolabi BI, Adebowale KO (2016) Distribution and interactions of pentachlorophenol in soils: the roles of soil iron oxides and organic matter. J Contam Hydrol 191:99–106
El-Shahawi MS, Hamza A, Bashammakh AS, Al-Saggaf WT (2010) An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 80:1587–1597
Gao L, Zhang Q, Liu L, Li C, Wang Y (2014) Spatial and seasonal distributions of polychlorinated dibenzo-p-dioxins and dibenzofurans and polychlorinated biphenyls around a municipal solid waste incinerator, determined using polyurethane foam passive air samplers. Chemosphere 114:317–326
Hoyos A, Cobo M, Aristizabal B, Cordoba F, de Correa CM (2008) Total suspended particulate (TSP), polychlorinated dibenzodioxin (PCDD) and polychlorinated dibenzofuran (PCDF) emissions from medical waste incinerators in Antioquia, Colombia. Chemosphere 73:137–142
Hu J, Zheng M, Liu W, Li C, Nie Z, Liu G, Zhang B, Xiao K, Gao L (2013) Characterization of polychlorinated naphthalenes in stack gas emissions from waste incinerators. Environ Sci Pollut Res 20:2905–2911
Jambu M (1991) Exploratory and multivariate data analysis. Academic Press, Boston
Landrum PF, Giesy JP, Oris JT, Allred PM (1987) Photoinduced toxicity of polycyclic aromatic hydrocarbons to aquatic organisms. Oil in freshwater: chemistry, biology, countermeasure technology. Pergamon Press, Elmsford, p 304oe318
Lee WS, Chang-Chien GP, Wang LC, Lee WJ, Tsai PJ, Wu KY, Lin C (2004) Source identification of PCDD/fs for various atmospheric environments in a highly industrialized city. Environ Sci Technol 38:4937–4944
Liu G, Cai Z, Zheng M (2014) Sources of unintentionally produced polychlorinated naphthalenes. Chemosphere 94:1–12
Musat F, Galushko A, Jacob J, Widdel F, Kube M, Reinhardt R, Wilkes H, Schink B, Rabus R (2009) Anaerobic degradation of naphthalene and 2-methylnaphthalene by strains of marine sulfate-reducing bacteria. Environ Microbiol 11:209–219
NTP (2000) Toxicology and carcinogenesis studies of naphthalene (CAS No. 91-20-3) in F344/N rats (inhalation studies). National Toxicology Program. NTP TR 500, NIH Publ. No. 01-4434
Olu-Owolabi BI, Diagboya PN, Adebowale KO (2014) Evaluation of pyrene sorption-desorption on tropical soils. J Environ Manag 137:1–9
Olu-Owolabi BI, Diagboya PN, Adebowale KO (2015) Sorption and desorption of fluorene on five tropical soils from different climes. Geoderma 239-240:179–185
Olu-Owolabi BI, Diagboya PN, Okoli PC, Adebowale KO (2016) Sorption behaviour of pentachlorophenol in sub-Saharan tropical soils: soil types sorption dynamics. Environ Earth Sci 75:1494
Park S-U, Kim J-G, Jeong M-J, Song B-J (2011) Source identification of atmospheric polycyclic aromatic hydrocarbons in industrial complex using diagnostic ratios and multivariate factor analysis. Arch Environ Contam Toxicol 60:576–589
Pozo K, Harner T, Shoeib M, Urrutia R, Barra R, Parra O (2004) Passive-sampler derived air concentrations of persistent organic pollutants on a north–south transect in Chile. Environ Sci Technol 65:29–37
Srogi K (2007) Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: a review. Environ Chem Lett 5:169–195
USEPA (2007) Treatment technologies for site cleanup: Annual Status Report (ASR). Twelfth Edition. (EPA 542-R- 07-012)
Wang MS, Wang LC, Chang-Chien GP, Lin LF (2005) Characterization of polychlorinated dibenzo-p-dioxins and dibenzofurans in the stack flue gas of a municipal solid waste incinerator, in the ambient and in the banyan leaf. Aerosol Air Qual Res 37:171–184
Wang G, Huang L, Zhao X, Niu H, Dai Z (2006) Aliphatic and polycyclic aromatic hydrocarbons of atmospheric aerosols in five locations of Nanjing urban area, China. Atmos Res 81:54–66
Wang J, Chen S, Tian M, Zheng X, Gonzales L, Ohura T, Mai B, Simonich SLM (2012) Inhalation cancer risk associated with exposure to complex polycyclic aromatic hydrocarbon mixtures in an electronic waste and urban area in South China. Environ Sci Technol 46:9745–9752
Zhao L, Zhang F, Hao Z, Wang H (2008) Level of polycyclic aromatic hydrocarbon in different types of hospital waste incinerator ashes. Sci Total Environ 397:24–30
Acknowledgements
We acknowledge the technical support of the Departments of Chemical Engineering and Environmental Health, Obafemi Awolowo University. We also acknowledge the reviewers and critics for their excellent input in making the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Constantini Samara
Electronic supplementary material
ESM 1
(PDF 633 kb)
Rights and permissions
About this article
Cite this article
Adedayo Adesina, O., Ademola Sonibare, J., Diagboya, P.N. et al. Periodic characterization of alkyl-naphthalenes in stack gas and ambient air around a medical waste incinerator. Environ Sci Pollut Res 24, 21770–21777 (2017). https://doi.org/10.1007/s11356-017-9828-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9828-1