Abstract
Conventional air pollutants (PM10, CO, NOx) gradually increased from fall to winter during 2015 in Istanbul. Several air pollution episodes were observed during this period. This study was made in order to determine polycyclic aromatic hydrocarbon (PAH) levels, identify the sources of air pollution, and make toxicity assessment based on Benzo(a)pyrene equivalent concentrations. The sampling took 14 sequential days during winter. High-pressure weather conditions prevailed at the start of the sampling. The conditions were then changed to low-pressure condition towards the end of the sampling. Strong inversion was effective on the onset of the sampling. Strong inversion was effective at the onset of the sampling. A high-volume sampler was used to collect gas and particle phase samples. Total suspended particle concentrations were between 27 and 252 μg m−3. Sixteen PAH species were investigated. Total (gas + particle) PAH concentrations were between 76.4 and 1280.3 ng m−3, with an average of 301.4 ng m−3. Individual PAH concentrations were between not detected (n.d.) and 99.2 ng m−3 in the gaseous phase, and between n.d. and 11.5 ng m−3 in the particle phase. Phenanthrene had the highest share among 16 PAH compounds. Benzo(a)pyrene was not detected in 8 days. On the remaining days, its concentration ranged between 5.5 and 14.8 ng m−3 with an average of 3.7 ng m−3. Low-molecular-weight PAHs dominated gaseous phase; inversely, high-molecular-weight PAHs dominated particle phase. Possible sources were identified by diagnostic ratios. These ratios suggested that coal combustion and diesel vehicle exhaust emissions had a substantial impact on ambient air quality. Benzo(a)pyrene equivalencies were calculated for each PAH compound in order to make toxicity assessment. Total benzo(a)pyrene equivalencies ranged between 0.4 and 30.0 ng m−3 with an average of 7.2 ng m−3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is directly related to human health and it is a non-trivial problem especially in urban areas. Air pollution arises from complex sources in complicated settlements. Pollutant sources should be identified precisely before taking measures. After that, the management of the pollution issue could become easier. The main contributor to air pollution in urban areas is the combustion process. It may be used for power generation, domestic heating, transportation, etc. The combustion at high temperature results in the formation of the oxides of nitrogen (NOx). Thus, NOx is forensic of the combustion process. In a recent study of NASA, worldwide NOx trends, acquired from Aura Satellite, were evaluated between 2005 and 2014 (NASA 2015). NOx levels have decreased in US and Europe. On the contrary, an increasing trend in China and in some certain cities was also observed. A slight increase in NOx concentrations was observed in Istanbul in the last decade. However, NOx cannot address the source type alone. Due to the fact that there is a limited contribution of natural sources of PAHs on urban atmosphere, they are good tracers of anthropogenic emissions (Vardar et al. 2008). PAHs are organic compounds formed from two or more fused aromatic carbon rings (Esen et al. 2008). They are mainly generated through pyrolytic processes and incomplete combustion of organic materials (Chrysikou and Samara 2009; Esen et al. 2008). Type and amount of PAHs are influenced by fuel type, the amount of oxygen, and combustion temperature (Lima et al. 2005). Low-temperature processes produce low-molecular-weight PAHs; in contrast to that, high temperature processes produce high-molecular-weight PAHs. The freshly generated PAHs are released in gaseous form and as ultrafine particles (Richter and Howard 2000). While PAHs with low molecular weight are mostly associated with gaseous phase, PAHs with high molecular weight are associated with particle phase (Hanedar et al. 2011). PAHs between two and four aromatic rings are partitioned between gaseous and particle phases, whereas PAHs with more aromatic rings accumulate in fine particle fraction (Akyüz and Çabuk 2010). PAH diagnostic ratios are used in many studies in order to identify their sources (Tobiszewski and Namieśnik 2012). However, these diagnostic ratios exhibit intrasource variability and intersource similarity (Galarneau 2008). For that reason, sources within the area of interest should be determined elaborately.
Numerous PAH compounds are released and over 100 different species are present in the environment (Karaca 2015). United States Environmental Protection Agency (US EPA) have listed 16 PAH compounds as priority pollutants due to their potentially harmful effects on human health (USEPA 2003). Seven compounds were classified as probable human carcinogen among the 16 priority PAHs (Shields et al. 2014). Carcinogenicity of PAH compounds increases with the increasing molecular weight of the compounds (Ravindra et al. 2001).
Due to increased anthropogenic activities, higher PAH levels are observed in winter. Approximately, an order of magnitude higher concentrations was observed in winter than in summer, in Bari (Amodio et al. 2009). Tsapakis and Stephanou (2005) expressed that there might be other reasons for the increased concentrations in winters such as lower planetary boundary layer and less atmospheric reactions. Regression between meteorological parameters and PAH concentrations suggest that there is a great dependence of PAHs to meteorological conditions (Amarillo and Carreras 2016).
Air pollution had been a serious problem in Istanbul before (Karaca et al. 2005). Especially, at the late 1980s and beginning of 1990s, particulate matter (PM) and SO2 episodes were observed in the city due to low-quality coal combustion (Unal et al. 2011). The worst of these episodes were observed on January 18, 1993. The measured total suspended particulate matter (TSP) and SO2 concentrations were 2662 and 4070 μg m−3, respectively (Batuk et al. 1997). In some specific days during the winter period, Istanbul is still exposed to severe air pollution. This situation occurs when high-pressure atmospheric conditions prevail. In this case, emissions, released from increased anthropogenic activities in winter, cannot disperse sufficiently. The aim of this study is to determine the variation of PAH at different atmospheric conditions in winter, identify potential air pollutant sources, and make toxicity assessment of the identified PAH species.
Materials and methods
Sampling campaign and location
A “Tecora ECHO HiVol” brand mark high-volume air sampler (HVAS) was used to collect ambient particle and gas phase pollutants. The HVAS was operated at the roof of the Yildiz Technical University Civil Engineering Faculty. Approximate height of the roof is 10 m above ground level. A Davis Vantage Pro-2 weather station is present next to HVAS. Meteorological data were recorded every half an hour. The sampling campaign was started on December 24, 2015, and finished on January 6, 2016. Daily samples were taken during this period. The device was calibrated with an external calibrator before each use. Each sampling was started at 9:00 AM and HVAS was operated for 24 h. Fourteen particle and gas samples were collected during this campaign. Particles were collected on a glass fiber filter (GFF) with a diameter of 90 mm. Gas phase pollutants were captured in two polyurethane foam (PUF) plugs, placed in series. Each PUF plug has 50-mm length and 65-mm diameter. The average operating flow rate of the HVAS was 0.3 m3 min−1.
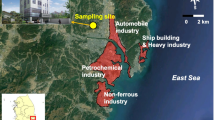
Conventional air pollutants are continuously monitored within a network in Turkey. There are 27 air quality monitoring stations in Istanbul. Instantaneous air pollutant concentrations can be tracked online from the website prepared by the Ministry of Environment and Urbanization. The closest air quality monitoring station is the Esenler station, which is being operated at the 1.6 km north of the sampling point. It is possible to get PM10 and CO level history from this station. NOx data archive was not available online in the Esenler station. NOx concentrations used in this study were gathered from another station which is located at 5 km southwest of the sampling station. The close vicinity of the sampling site is shown in Fig. 1 on a 3 km × 3 km map.
Geographical coordinates of the lower left corner of the map are 41° 01′ 04″ N and 28° 52′ 35″ E. Sampling station and air quality monitoring station are shown with bold black dots. Important areas were colored on the map and their description was given in the legend of Fig. 1. The remaining uncolored areas are residential sites.
Sample preparation
Collected samples were extracted, fractionated, and concentrated prior to gas chromatography (GC) analyses. Preparation of PAH samples was executed in accordance with (Kuzu 2016). PUFs were Soxhlet extracted and filters were ultrasonically extracted with 1:1 (v:v) acetone:hexane. The extraction duration of PUFs was 24 h, whereas filters were extracted for 1 hour. Filters were extracted with two equal aliquots in two sets of 30 min. In the first set, filters were extracted at a solvent mixture of 25 ml volume for 30 min. In the second set, 25 ml more solvent mixture was included and filters were extracted for another 30 min. After extraction of the PUFs and filters were finished, their solvent volumes were reduced to 2 ml by rotary evaporator. Samples were then cleaned up and fractionated through a column of 3 g silicic acid (3 % water), 2 g neutral alumina (6 % water), and 2 g Na2SO4. The column was pre-washed with 20 ml of petroleum ether and 20 ml of dichloromethane, respectively. Next, the sample in 2 ml of hexane was poured into the column, and PAHs were eluted with 20 ml of dichloromethane. Solvent exchange procedure was applied to samples. The volume of the samples was reduced to 2 ml under a gentle nitrogen blow-down system, and 15 ml of hexane was included. This step was repeated twice and the final extracts were taken into hexane. Samples were concentrated to 1 ml after the solvent exchange. All samples were taken to GC vials. For volume correction, the internal standard of pyrene-d10 was included to each vial before capping them.
PAHs were quantified by gas chromatography Perkin Elmer Clarus 500 equipped with a Clarus 560S Mass Spectrometer (MS) detector. HP-5MS (30 m × 0.25 mm × 0.25 μm) capillary column was used. Splitless injection was used for the samples. Injection volume was 1 μL. The inlet temperature of the GC oven was 250 °C. The mass inlet line temperature was kept at 180 °C and the source temperature was kept at 200 °C. The oven temperature program was held at 50 °C for 1 min, then raised to 200 °C at a rate of 25 °C min−1, finally raised to 300 °C at a rate of 8 °C min−1, and kept 5.5 min at 300 °C. The carrier gas was ultra pure helium at 1.0 ml min−1 flow. Seven different levels of standards were prepared to range between 0.05 and 10 ng μl−1 for calibration. The investigated PAHs were Naphthalene (Np), acenaphthylene (Acy), acenaphthene(Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), benz(a)anthracene (BaA), chrysene (Chr), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), indeno(1,2,3-c,d)pyrene (IcdP), dibenz(a,h)anthracene (DahA), and benzo(g,h,i)perylene (BghiP).
Quality assurance/quality control
Glassware products used in the laboratory were washed with pure water, methanol, and dichloromethane, then dried and covered with aluminum foil to protect from contact with air. Aluminum foils, used during the sample preparation and handling, were pre-baked at furnace and hexane rinsed. PUF plugs were cleaned with 1:1 (v:v) acetone:hexane for 24 h before sampling. After cleaning, PUFs were dried at 70 °C for 24 h. Filters were wrapped with aluminum foil and baked at 450 °C for 6 hours in a furnace to remove any organic residual. After the cleaning processes, filters and PUFs were cooled and kept in a desiccator.
All samples were spiked with a surrogate standard solution prior to extraction step. The surrogate standard solution included napthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12. The concentration of these compounds in the surrogate standard solution was 5 μg ml−1. After volume correction, recoveries were calculated. Recoveries for particle phase PAHs were between 51 and 102 %. The recoveries of gas phase were between 56 and 109 %.
After each 10 sample injections, stability was checked with the medium standard. The values of determination coefficient of the calibration curve for distinct species were above 0.995. Blank samples were taken with each data set. Limit of detection (LOD) was calculated according to Dennis (2005) in Eq. (1).
where, σ is the residual standard deviation of the calibration line and S is the standard deviation of the calibration curve. LODs were ranged from 0.1 to 1.8 ng μL−1 for BkF and BaP, respectively. All samples were recovery and blank corrected.
Results and discussion
Air pollution is an important issue in urban areas. Higher air pollution could be observed especially during the winter season due to increased anthropogenic emissions. Additionally, decreased mixing height worsens the state of atmospheric conditions. In this case, pollutants are emitted and dispersed in smaller air volume. Over 14 millions of people live in Istanbul. Province surface area is 5461 km2. All the produced pollutants are emitted within this area. PM10, CO, and NOx concentrations gathered from the air quality monitoring stations are given in Fig. 2.
The concentrations were from September 15, 2015, till January 7, 2016. This time span was selected in order to show the concentration trend from fall to winter. A similar pattern of CO and PM10 is observed in Fig. 2, especially in the winter. The statistical relation was determined for two different periods. The first one was between September 15 and October 15. These dates represent the transition from warm period to cold period. Domestic heating is not effective. The relation of CO and PM10 is not much significative (p < 0.05). After this period, from October 16 until January 7, a strong correlation was present (p < 0.001) between the two pollutants. Statistical analyses show that PM increases after October 15 can be attributed to an increase in anthropogenic activities. However, in both periods, there was a strong correlation between CO and NOx (p < 0.001).
CO pollution in the atmosphere is the result of incomplete combustion. However, it is not possible to differentiate sources behind CO occurrence. PAHs are also emitted as the result of incomplete combustion. These two pollutants indicate similar sources. Diagnostic ratios of PAHs help the researchers to identify the likely sources of the pollution. In this manner, it is essential to determine the levels of PAHs and then to make an assessment based on diagnostic ratios in order to identify the pollutant sources. An emission inventory was prepared for traffic, industry, and domestic heating in Istanbul. According to this inventory, traffic, industry, and domestic heating contributed to 89.4, 6.1, and 4.5 % of NOx emissions; 19.7, 28.8, and 51.5 % of PM10 emissions; 68.3, 0.4, and 31.3 % of CO emissions, respectively (Kara et al. 2014). The highest contributor was traffic for NOx and CO, while it was domestic heating for PM10 emissions.
Ambient PAH levels
Sequential samples were collected and analyzed in order to determine the PAH compounds and concentrations. Total concentrations of Ʃ16PAHs in gaseous and particle phases were given in Fig. 3.
Gas phase concentrations were ranged from 69.3 to 1026.6 ng m−3 with an average of 241 ng m−3. The particle phase concentrations varied between 5.8 and 253.6 ng m−3 with an average of 60.4 ng m−3. Total (gas + particle) PAH concentrations were between 76.4 and 1280.3 ng m−3, with an average of 301.4 ng m−3. 18.5 % of the PAHs resided on particles. Meteorological conditions during the sampling and TSP concentration are given in Table 1.
Summak (2015) monitored ambient PAHs between February 2013 and June 2014 in Yildiz Technical University Campus. The average PAH concentration of the 16-month long sampling period was 124 ng m−3. The seasonal distributions of total PAH concentrations were 82, 47, 66, and 280 ng m−3 for spring, summer, fall, and winter, respectively. In another study, which was conducted between September 2006 and December 2007, 100.7 and 84.6 ng m−3 total PAH concentrations were reported for two urban sites in Istanbul (Hanedar et al. 2011). The average PAH concentration of this study is considerably higher than the previously reported long-term average concentrations in Istanbul. In Nanjing, China, a study was conducted in winter, where highest PM2.5 bound-PAH levels were 114 ng m−3 during severe haze occurrence (Kong et al. 2015).
High-pressure prevailed from December 24, 2015, to January 2, 2016. The highest concentration was observed on December 24. Total (gas + particle) concentration was 1280.3 ng m−3. On that day, TSP concentration was 252 μg m−3. Inceci̇k (1996) stated that anticyclonic pressure conditions and low surface wind speeds lead to unfavorable conditions for air pollution episodes over the city atmosphere. Radiosonde measurements indicate that there is a strong inversion on 24th December. SkewT log(p) plot for the inversion is given in Fig. 4.
At the time of the radiosonde observation, the ground-level ambient temperature was 6.4 °C (base height is 18 m). The vertical temperature profile was 8.8, 7.8, 8.6, 9.6, and 10.2 °C at 26, 139, 250, 276, and 485 m elevations, respectively. The strong ground-level temperature inversion favored unpleasant conditions for air pollutants to accumulate near the ground level.
The lowest concentration was observed on December 29; the sum of gas and particle phase concentrations was 76.4 ng m−3. The reason for the concentration decrease could be the increased wind speed and wet precipitation. The increased wind speed has the ability to disperse the emitted pollutants from their sources. PAH and CO concentrations were of statistically high significance (p = 0.001), indicating similar sources. The variation of individual PAH compounds is given in Fig. 5.
The average individual compound concentrations ranged from n.d. to 99.2 ng m−3 in the gaseous phase, while the range was from n.d. to 11.5 ng m−3 in particle phase. The highest contributing compound was Phe, which was followed by Flt. The average (gas + particle) concentration of these two PAHs was 150.8 ng m−3. This is corresponding to the half of the total PAH concentration. These two compounds were also the highest contributors in Bursa atmosphere (Birgul and Tasdemir 2015). BaP is considered as a marker of total and carcinogenic PAHs (Di Vaio et al. 2016). PM10 fraction of BaP should not exceed 1 ng m−3 over a calendar year according to European Commission (Directive 2004). BaP was detected in 6 days out of 14. The concentrations were considerably higher than the threshold value. They ranged between 5.5 and 14.8 ng m−3. The average of the whole sampling duration, including the days when BaP was not detected, was 3.7 ng m−3.
Light-molecular-weight PAHs were dominant at gaseous phase, whereas heavy-molecular-weight PAHs were dominant at particle phase. Their distribution is shown in Fig. 6.
Five-ring and six-ring PAHs were only detected on particles. Four-ring PAHs were detected at both phases except BaA. Phe and Ant were detected at both phases among the three-ring PAHs. The remaining was only detected at gaseous phase. Nap, comprising the two-ring PAHs, was only detected at gaseous phase. Regression between TSP-particle phase PAHs and TSP-gaseous phase PAHs indicated that there is strong relation for both (p < 0.001). This significant relation suggests that gas/particle partitioning can be at equilibrium. The state of partitioning is determined by Eq. (2).
where, \( {P}_L^0 \) is the super-cooled vapor pressure of the compound, which is the vapor pressure of the compound at the verge of phase transition, and Kp is the gas/particle partition coefficient, given in Eq. (3).
where, Cp and Cg are particle phase and gas phase PAH concentrations, respectively. The partition coefficient is determined by normalizing the particle-bound PAHs with the TSP concentration. The unit of the partition coefficient is m3 μg−1.
Sub-cooled vapor pressures of PAHs were calculated according to Eq. 4. Experimental mL and bL values of Odabasi et al. (2006) were used in this study.
logKp and \( \mathit{\log}{P}_L^0 \) values are plotted to determine the state of the equilibrium. In ideal conditions, equilibrium can be reached when the slope of the regression line (mr) is equal to −1 (Pankow 1994). However, it is also stated that equilibrium can be achieved when the slope is different than −1 (Goss and Schwarzenbach 1998). Pankow and Bidleman (1992) indicated that the presence of non-exchangeable compounds on/in the particles, differences in the excess heat of desorption of the various compounds, slow adsorption/desorption kinetics, and sampling artifacts, could lead shallow slopes. mr values were ranged between −0.52 and −0.93, with an average of −0.71, having a high coefficient of determination (r2 = 0.96). Akyüz and Çabuk (2010) stated that slope values close to −1 indicate long-range transportation of the pollutants, whereas shallower slopes denote the contribution of local sources. In this study, relatively shallower slope was obtained, showing the contribution from local sources.
Source identification
Molecular diagnostic ratios of PAHs are commonly exploited in determining their actual sources. In urban areas, relatively away from industrial sites, combustion due to domestic heating and motor vehicle exhaust emissions are effective. The ratio of Ant/(Ant + Phe) determines whether the PAHs are from pyrolytic sources or petrogenic sources. If this ratio is <0.1, petrogenic sources are effective; in the transverse case (>0.1), pyrogenic sources are effective (Pies et al. 2008). In this study, Ant/(Ant + Phe) ratio was between 0.08 and 0.12 with an average of 0.11, which is slightly over 0.1. In 11 of the samples, it can be commented that pyrolytic sources are effective; one sample is on the boundary and in two of them, petrogenic sources seem to be effective. Flt/(Flt + Pyr) ratio determines the type of the fuel used. The ratios can be listed in three categories as <0.4, 0.4–0.5, and >0.5, which correspond to petrogenic sources, liquid fuels, grass, and wood or coal combustion, respectively (De La Torre-Roche et al. 2009). This ratio was between 0.54 and 0.87; the average was 0.71. Solid fuel combustion is effective on the atmospheric compounds. Another method of classifying petrogenic, petroleum, and solid fuel combustion is the ratios of IcdP/(IcdP + BghiP). The ratios of <0.2, 0.2–0.5, and >0.5, corresponds to petrogenic sources, petroleum combustion, and soil fuel combustion, respectively (Yunker et al. 2002). The average ratio was found to be 0.54, ranging between 0.49 and 0.6. This ratio also indicated that solid fuel combustion is effective. Pio et al. (2001) stated that IcdP/(IcdP + BghiP) ratios of 0.18–0.4, 0.56, and 0.62 correspond to vehicular emission, coal combustion, and wood combustion, respectively. The average result in this study that was 0.54, which is very close to 0.56, showed the dominance of coal combustion within the solid fuels. Petrol and diesel emissions among the vehicular emission can be differentiated according to Flu/(Flu + Pyr) ratio. The ratio <0.5 indicates petrol emissions, whereas >0.5 indicates diesel emissions (Ravindra et al. 2008). The average of this study was 0.51, slightly over the threshold, showing the contribution of diesel emissions. Alternatively, BghiP/BaP ratio differentiates the gasoline and diesel contribution. Ratios between 1.2–2.2 and 2.5–3.3 show diesel and gasoline sources, respectively (Manoli et al. 2004). The ratios were between 1.20 and 1.57. The average of this ratio was 1.52, suggesting diesel contribution from vehicular emissions.
Coal combustion and diesel emissions are major contributors to the atmosphere in Istanbul according to diagnostic ratios. Relatively less cost of the diesel fuel in Turkey has led an increase in the number of diesel vehicle sale. Moreover, the sampling station is in close proximity (approximately 2 km) to the Central Coach Station of Istanbul. The dominance of diesel contribution over gasoline combustion might be due to these two factors.
The contribution of coal combustion is more significant than diesel vehicle emissions. Natural gas piping system is integrated around Istanbul. Incecik and Im (2012) expressed that natural gas piping system covers 95 % of the city. In this case, it might be surprising that the use of coal is contributing to air pollution in Istanbul. On the other hand, Incecik and Im (2012) also included that coal consumption is still an important factor in domestic heating even though it has a limited consumption. They also stated that some parts of the city use lignite coal, although these houses are equipped with natural gas piping system, due to high natural gas prices. Their suggestion is consistent with the diagnostic results of this study.
Toxicity assessment
PAHs are toxic compounds. Toxicities of individual PAH compounds considerably differ from each other. Due to its high carcinogenic potential, BaP is considered as a tracer for risk assessment. The concentration of each PAH compound was converted to BaP equivalency in order to make the toxicity assessment. In this approach, the concentration of each compound was multiplied with their own toxic equivalency factor (TEF) to make the conversion. The relation is shown in Eq. (5).
According to the equation, each PAH compound is multiplied by its own toxic equivalency factor. This yields the BaP equivalency for each PAH component. Then, the sum of each individual BaP equivalencies gives the total BaP equivalency of each sampling campaign. TEF values were gathered from Nisbet and LaGoy (1992). By including the TEFs and concentration of each PAH compound, Eq. 5 can be written as Eq. (6).
In Eq. 6, the compounds having same TEF values were grouped under the same multiplier. The average BaP(eq) concentration of each PAH compound with their standard deviation are shown in Fig. 7.
In this study, the total BaP(eq) concentrations ranged between 0.4 and 30.0 ng m−3. The average BaP(eq) concentration was 7.2 ng m−3. As with the highest ambient PAH concentrations, the highest average BaP(eq) concentration was measured during the air pollution episode on 24th December. Five percent of the BaP(eq) concentration belonged to the gaseous phase on average. Particle phase contributed to the remaining greater portion in terms of BaP(eq) concentration. The highest contributor compound to BaP(eq) was the BaP with 3.7 ng m−3 on average. BbF and IcdP were the second and third prominent compounds, respectively. Average BaP(eq) concentration of BbF and IcdP was 1.1 and 0.7 ng m−3, respectively. BaP, BbF, and IcdP constituted the 76 % of the total BaP(eq) concentration.
Albuquerque et al. (2016) reported 1.2 ng m−3 average BaP(eq) concentration in Porto, Portugal. The average BaP(eq) concentrations at three different stations in Istanbul between September 2006 and December 2007 were 2.2, 2.6, and 1.3 ng m−3 (Hanedar et al. 2014). The result of this study is approximately three to six times of the previously reported values for Istanbul.
Conclusion
Istanbul is a highly populated megacity between Asia and Europe continents. Due to rapid population growth and use of low quality fossil fuels, many air pollution episodes had been observed in Istanbul. The worst recorded concentrations was on January 18, 1993. TSP and SO2 concentrations were 2662 and 4070 μg m−3, respectively. Stagnant high-pressure conditions favored these high pollutant levels. After the shift of coal with natural gas, the air pollution levels decreased gradually. However, sometimes, air pollution episodes are observed during winter-time in Istanbul. In a recent report of NASA, NOx concentrations were decreased around the globe. But there was an increasing trend in some specific cities such as in Istanbul. This showed that combustion sources affect the air pollution levels but sources were unclear.
In this study, combustion-originated air pollution was evaluated by PAH sampling and analysis. Ambient air samples were collected between December 24, 2015, and January 7, 2016. Anticyclonic pressure conditions prevailed from the start of the sampling campaign until January 2, 2016. There was a strong inversion on December 24, 2015 when the highest ambient concentrations were observed. Total PAH concentrations were 76.4 and 1280.3 ng m−3. The average concentration was 301.4 ng m−3, which is considerably higher than previously reported long-term levels in Istanbul. PAH concentrations were in good agreement with CO concentrations, suggesting similar sources. Also, the correlation between NOx and CO was strong. Subsequently, this study was focused on the identification of air pollution sources. Diagnostic ratios were utilized in order to determine possible sources contributing to ambient air. Diagnostic ratios suggested that coal combustion and diesel vehicle exhaust emissions contributed to urban air pollution. Average BaP concentration was 3.7 ng m−3, which is higher than the legislation threshold value of 1 ng m−3 in particles. Toxicity assessment was made based on the BaP equivalency of individual PAH compounds. The average BaP(eq) concentration was 7.2 ng m−3. BaP(eq) of particle phase had a higher share than the gaseous phase. Results of this study present the actual sources of the air pollution in Istanbul metropolitan city. Local authorities can take measures considering the results presented in this study.
References
Akyüz M, Çabuk H (2010) Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak, Turkey. Sci Total Environ 408:5550–5558. doi:10.1016/j.scitotenv.2010.07.063
Albuquerque M, Coutinho M, Borrego C (2016) Long-term monitoring and seasonal analysis of polycyclic aromatic hydrocarbons (PAHs) measured over a decade in the ambient air of Porto, Portugal. Sci Total Environ 543(Part A):439–448. doi:10.1016/j.scitotenv.2015.11.064
Amarillo AC, Carreras H (2016) Quantifying the influence of meteorological variables on particle-bound PAHs in urban environments. Atmos Pollut Res. doi:10.1016/j.apr.2016.02.006
Amodio M, Caselli M, de Gennaro G, Tutino M (2009) Particulate PAHs in two urban areas of Southern Italy: impact of the sources, meteorological and background conditions on air quality. Environ Res 109:812–820. doi:10.1016/j.envres.2009.07.011
Batuk DN, Gursoy E, Ertut H, Erdun H, Incecik S (1997) Analysis of SO2 and TSP under meso-scale weather conditions in Istanbul. Environ Res Forum 7–8:73–87
Birgul A, Tasdemir Y (2015) Concentrations, gas-particle partitioning, and seasonal variations of polycyclic aromatic hydrocarbons at four sites in Turkey. Arch Environ Contam Toxicol 68:46–63. doi:10.1007/s00244-014-0105-8
Chrysikou LP, Samara CA (2009) Seasonal variation of the size distribution of urban particulate matter and associated organic pollutants in the ambient air. Atmos Environ 43:4557–4569. doi:10.1016/j.atmosenv.2009.06.033
De La Torre-Roche RJ, Lee W-Y, Campos-Díaz SI (2009) Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: analysis of a potential problem in the United States/Mexico border region. J Hazard Mater 163:946–958. doi:10.1016/j.jhazmat.2008.07.089
Dennis RJ (2005) Response spectrum in chromatographic analysis. In: Encyclopedia of chromatography, second edition. CRC Press, pp 1439–1453. doi:10.1201/NOE0824727857.ch313
Di Vaio P et al (2016) Level, potential sources of polycyclic aromatic hydrocarbons (PAHs) in particulate matter (PM10) in Naples. Atmos Environ 129:186–196. doi:10.1016/j.atmosenv.2016.01.020
Directive (2004) Directive 2004/107/EC of the European parliament and of the council of 15 December, 2004. Relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air
Esen F, Tasdemir Y, Vardar N (2008) Atmospheric concentrations of PAHs, their possible sources and gas-to-particle partitioning at a residential site of Bursa, Turkey. Atmos Res 88:243–255. doi:10.1016/j.atmosres.2007.11.022
Galarneau E (2008) Source specificity and atmospheric processing of airborne PAHs: implications for source apportionment. Atmos Environ 42:8139–8149. doi:10.1016/j.atmosenv.2008.07.025
Goss K-U, Schwarzenbach RP (1998) Gas/solid and gas/liquid partitioning of organic compounds: critical evaluation of the interpretation of equilibrium constants. Environ Sci Technol 32:2025–2032. doi:10.1021/es9710518
Hanedar A, Alp K, Kaynak B, Baek J, Avsar E, Odman MT (2011) Concentrations and sources of PAHs at three stations in Istanbul, Turkey. Atmos Res 99:391–399. doi:10.1016/j.atmosres.2010.11.017
Hanedar A, Alp K, Kaynak B, Avşar E (2014) Toxicity evaluation and source apportionment of polycyclic aromatic hydrocarbons (PAHs) at three stations in Istanbul, Turkey. Sci Total Environ 488–489:437–446. doi:10.1016/j.scitotenv.2013.11.123
Inceci̇k S (1996) Investigation of atmospheric conditions in i̇stanbul leading to air pollution episodes. Atmos Environ 30:2739–2749. doi:10.1016/1352-2310(95)00366-5
Incecik S, Im U (2012) Air pollution in mega cities: a case study of Istanbul In: Khare DM (ed) Air pollution—monitoring, modelling and health. InTech. doi: 10.5772/32040
Kara M, Mangir N, Bayram A, Elbir T (2014) A spatially high resolution and activity based emissions inventory for the metropolitan area of Istanbul, Turkey. Aerosol Air Qual Res 14:10–20. doi:10.4209/aaqr.2013.04.0124
Karaca G (2015) Spatial distribution of polycyclic aromatic hydrocarbon (PAH) concentrations in soils from Bursa, Turkey. Arch Environ Contam Toxicol 70:406–417. doi:10.1007/s00244-015-0248-2
Karaca F, Alagha O, Ertürk F (2005) Statistical characterization of atmospheric PM10 and PM2.5 concentrations at a non-impacted suburban site of Istanbul, Turkey. Chemosphere 59:1183–1190. doi:10.1016/j.chemosphere.2004.11.062
Kong S et al (2015) Variation of polycyclic aromatic hydrocarbons in atmospheric PM2.5 during winter haze period around 2014 Chinese spring festival at Nanjing: insights of source changes, air mass direction and firework particle injection. Sci Total Environ 520:59–72. doi:10.1016/j.scitotenv.2015.03.001
Kuzu SL (2016) Compositional variation of PCBs, PAHs, and OCPs at gas phase and size segregated particle phase during dust incursion from the Saharan Desert in the Northwestern Anatolian peninsula. Adv Meteorol 2016:12. doi:10.1155/2016/7153286
Lima ALC, Farrington JW, Reddy CM (2005) Combustion-derived polycyclic aromatic hydrocarbons in the environment—a review. Environ Forensic 6:109–131. doi:10.1080/15275920590952739
Manoli E, Kouras A, Samara C (2004) Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 56:867–878. doi:10.1016/j.chemosphere.2004.03.013
Ravindra, Mittal AK, Van Grieken R (2001) Health risk assessment of urban suspended particulate matter with special reference to polycyclic aromatic hydrocarbons: a review. Rev Environ Health 16:169–189
NASA (2015) NASA images show human fingerprint on global air quality—release materials. http://svs.gsfc.nasa.gov/12094. Accessed 20.05.2016 20.05.2016
Nisbet ICT, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300. doi:10.1016/0273-2300(92)90009-X
Odabasi M, Cetin E, Sofuoglu A (2006) Determination of octanol–air partition coefficients and supercooled liquid vapor pressures of PAHs as a function of temperature: application to gas–particle partitioning in an urban atmosphere. Atmos Environ 40:6615–6625. doi:10.1016/j.atmosenv.2006.05.051
Pankow JF (1994) An absorption-model of gas-particle partitioning of organic-compounds in The atmosphere. Atmos Environ 28:185–188. doi:10.1016/1352-2310(94)90093-0
Pankow JF, Bidleman T. F (1992) Interdependence of the slopes and intercepts from log-log correlations of measured gas-particle partitioning and vapor pressure-I. The theory and analysis of available data. Atmos Environ:1071–1080. doi: 10.1016/0960-1686(92)90039-N
Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 72:1594–1601. doi:10.1016/j.chemosphere.2008.04.021
Pio CA, Alves CA, Duarte AC (2001) Identification, abundance and origin of atmospheric organic particulate matter in a Portuguese rural area. Atmos Environ 35:1365–1375. doi:10.1016/S1352-2310(00)00391-5
Ravindra K, Wauters E, Van Grieken R (2008) Variation in particulate PAHs levels and their relation with the transboundary movement of the air masses. Sci Total Environ 396:100–110. doi:10.1016/j.scitotenv.2008.02.018
Richter H, Howard JB (2000) Formation of polycyclic aromatic hydrocarbons and their growth to soot—a review of chemical reaction pathways. Prog Energy Combust Sci 26:565–608. doi:10.1016/S0360-1285(00)00009-5
Shields WJ, Ahn S, Pietari J, Robrock K, Royer L (2014) Chapter 6—atmospheric fate and behavior of POPs. In: Sandau GOS (ed) Environmental forensics for persistent organic pollutants. Elsevier, Amsterdam, pp. 199–289. doi:10.1016/B978-0-444-59424-2.00006-2
Summak G (2015) Analysing the polyaromatic hydrocarbons (PAHs) on size fractionated particle phase and gas phase in urban atmosphere. Dissertation, Yildiz Technical University
Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119. doi:10.1016/j.envpol.2011.10.025
Tsapakis M, Stephanou EG (2005) Occurrence of gaseous and particulate polycyclic aromatic hydrocarbons in the urban atmosphere: study of sources and ambient temperature effect on the gas/particle concentration and distribution. Environ Pollut 133:147–156. doi:10.1016/j.envpol.2004.05.012
Unal YS, Toros H, Deniz A, Incecik S (2011) Influence of meteorological factors and emission sources on spatial and temporal variations of PM10 concentrations in Istanbul metropolitan area. Atmos Environ 45:5504–5513. doi:10.1016/j.atmosenv.2011.06.039
USEPA (2003) Appendix A to 40 CFR, Part 423–126 Priority pollutants
Vardar N, Esen F, Tasdemir Y (2008) Seasonal concentrations and partitioning of PAHs in a suburban site of Bursa, Turkey. Environ Pollut 155:298–307. doi:10.1016/j.envpol.2007.11.026
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515. doi:10.1016/S0146-6380(02)00002-5
Acknowledgment
The author would like to thank the Istanbul Metropolitan Municipality, Marmara Clean Air Center, and the Ministry of Environment and Urbanization owing to operating and sharing the ambient concentration values of the air quality monitoring stations. The author would also like to acknowledge the Earth System Research Laboratory for producing the SkewT log (p) plot and permission to use in scientific studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Constantini Samara
Rights and permissions
About this article
Cite this article
Kuzu, S.L. Source identification of combustion-related air pollution during an episode and afterwards in winter-time in Istanbul. Environ Sci Pollut Res 26, 16815–16824 (2019). https://doi.org/10.1007/s11356-016-7831-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7831-6