Abstract
The radiological and chemical pollution of a cluster of four lakes in a lignite mining area of North-Western Greece was investigated using a variety of analytical techniques. Alpha spectrometry was applied to measure the activity concentrations of the uranium radioisotopes (U-234, U-235, and U-238) in waters. The mass activities of U-238, Th-232, and K-40 in sediments were measured by high-resolution gamma spectrometry. Furthermore, the determination of the minor and trace elements was carried out by instrumental neutron activation analysis (INAA) in both water and sediments samples, respectively. Pollution levels were also evaluated by calculating enrichment factors (EFs), contamination factors (CFs) and pollution load index (PLI). The data were discussed taking into account several parameters such as the distance from the pollution source, temperature, and location and showed that the environmental impact in this region could not be considered as negligible. The deviation of the isotopic ratio of U-234/U-238 from the equilibrium value indicated waters with intensive dissolution of uranium. The activity values in both waters and sediments found to be low in cool periods and increased in warm periods. Moreover, the concentrations of the elements U, Zn, and Fe were raised in water samples indicating possible pollution as well as the CFs and PLI denoted accumulation in the sediments and moderate to severe contamination for Zn and Cr in some cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal thermal power plants are the main source of electricity in Greece, producing more than 73% of the country’s power requirements. It is worth to mention that coal exploitation has increased drastically in this country from 900,000 t/year in 1955 to over 75 million t/year in the last decade (Public Power Corporation of Greece 2003).
There are two main coal mining regions in Greece with coal-fired power plants: Megalopolis Lignite Center (MLC) in the Peloponnese, Southern Greece, and Ptolemaida Lignite Center (P-ALC) in Northern Greece consuming more than 60 million t of lignite per year and producing about 13 million t of total ash (Georgakopoulos et al. 2002; Karangelos et al. 2004; Papaefthymiou et al. 2007). The coal center of Northern Greece is considered the biggest, producing the 70% of the electricity with six Power Stations which are operating in Ptolemaida–Amynteon basin (total power 4440 MW).
It is well known that coal and the combustion residues such as fly ash produce significant amounts of trace elements and the naturally occurring radionuclides affecting air, soil, and water (Asokan et al. 2005; Mljač and Križman 1996). On the other hand, huge amounts of fly ash are disposed in specific sites in the surrounding area of the mines and therefore constitute a potential health hazard especially due to the leaching of radionuclides or toxic elements. Moreover, the radiation hazard from primordial radionuclides and toxic elements in the vicinity of a polluted environment such as lignite mine and thermal power plant is very significant (Papastefanou et al. 1999).
As it is known, lakes and their sediments are often used as indicators of environmental pollution especially for the deposition of heavy metals reflecting the effect of human activity on the aquifer (Arnason and Fletcher 2003; Joshi 1991). Heavy metals tend to accumulate and remain for long period with impact to the human health through several ways (water consumption by humans or animals, fishing farming, pollution of agricultural crops through irrigation). In our case, a potential pollution from the naturally occurring radionuclides of the decay of the U-235, U-238, and Th-232 series and the single decay radionuclide K-40 is of great concern due to lignite mining activities and sustainable disposal of associated by-products such as fly ash (Karamanis et al. 2009; Mljač and Križman 1996).
Several studies have been reported in the literature based on the presence of trace elements in the fly ashes from the Greek power stations near abandoned mines in the area of Ptolemais–Amynteon, but an essential assessment of the impact of the main radioisotopes and heavy metals in the surrounding region has not been appeared up to day (Adamidou et al. 2007; Karamanis et al. 2009; Pentari et al. 2006).
The present study was performed in four lakes in a lignite region of the North-Western Greece and focused on the investigation of natural radionuclides and heavy elements as well as their environmental impacts. The aim was to investigate the seasonal variations of the activity concentrations of the radioisotopes U-238, U-234, U-235, Th-232, and K-40 as well as of heavy metals in water and sediments samples of lakes in the largest lignite mining area of Greece. The results obtained will offer valuable information regarding the transfer and behavior of several trace elements connected with the utilization of lignite for the production of electricity. The activity concentrations of the natural radionuclides in water and sediment samples as well as the minor and trace elements were determined using radiochemical and nuclear techniques.
The study area
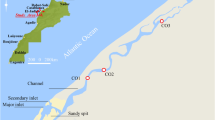
The study area with the four lakes (Cheimaditis, Zazari, Petron, and Vegoritis) which are protected by the European Ecological Network Natura 2000 is presented in Fig. 1a. The area belongs to the Florina–Ptolemais–Kozani basin in Northwestern Greece where the towns Amyntaion and Ptolemais are located as well as 14 villages with an average population of 50,000. The basement of the basin consists mainly of Palaeozoic and Mesozoic metamorphic and plutonic rocks, underlying Cretaceous limestone and flysch. The basement rocks are divided into four distinctive tectonic units (Pavlides and Mountrakis 1987; Megalovasilis et al. 2013). The cluster of the four lakes Cheimaditis, Zazari, Petron, and Vegoritis can be seen around the field mine of Amyntaion–Ptolemais (Fig. 1b). In the center of the region is the “Amyntaion” (600 MW) lignite power plant whereas four more plants (3500 MW) are located around, in distance of 30 km, and their effluents after a primary treatment are discharged through a system of channels in the lakes, especially in Vegoritis (Karamanis et al. 2009; Pentari et al. 2006). The Lake Vegoritis is the largest in Greece (54.31 km2) and receives water through canals and tunnel system from the other lakes, Petron (12.6 km2), Zazari (2.0 km2), and Cheimaditis (10.8 km2).
a Map of Greece with the study area. b Map showing the Ptolemais–Amynteon basin (study area), the cluster of the four lakes (1 Cheimaditis, 2 Zazari, 3 Petron, and 4 Vegoritis), the sampling locations (orange spots), the corresponding lignite mines A, B, and C (gray color), and the five power plants
The lithological composition of the Lake Vegoritis is represented by limestones while the Lake Petron is composed of sandy clays and limestones. At the Lake Zazari, recrystalline carbonate and metamorphic (marbles and schists) rocks are presented as well as at Cheimaditis, crystalline rocks (metamorphic marbles and schists) can be mainly found.
Materials and methods
Sampling and sample preparation
Water and sediment samples were collected at positions around the lakes and close to the villages. The sampling locations are shown in the Fig. 1b (orange spots). At each location, the samples were taken inside the lakes 1 to 2 m from the coastline. Three samples were collected along a line approximately 30 m. The sampling was performed during 1 year from December 2012 to September 2013. In order to estimate the seasonal variations of the investigated parameters, four different sampling periods were selected (first week of December, March, June, and September with temperatures 2–5, 9–11, 19–25, and 18–22 °C, respectively). Samples of sediments were collected using a sediment trap (thin surface layer up to 10 cm) in polyethylene containers after removing stones and algae. The sediments were dried first in air and then in an oven at a temperature of 65 °C until a constant weight was reached. The dried samples were mixed together in order to have one representative sample and defined as clay (more than 80% fine-grained material). Then, they were pulverized into a fine powder and passed through a standard 1-mm mesh size (ASTM No. 18). The homogenized samples were filled into cylindrical plastic containers (80 mL), which were then sealed with the aid of PVC tape to prevent the escape of Rn-222 and Rn-220 from the samples. All samples were weighed and stored for at least 1 month prior the measurement in order to attain radioactive secular equilibrium between Ra-226 and Rn-222 and their short-lived progenies (Tsabaris et al. 2007, Noli et al. 2017).
In the case of the water samples, the same sampling procedure was followed. A volume of approximately 5 L (3 × 1.5 L from the three points at each location) was collected at the same locations (average depth 1 m) and the pH and conductivity were measured immediately. Then, the samples were transported to the laboratory in polyethylene bottles, filtered through 0.45-μm filters, mixed, and acidified to pH 2 with 5 M HNO3 to avoid adsorption of radionuclides on the walls of the container. The pH and the electrical conductivity measurements were carried out using a Metrohm 941 pH meter and a Crison Basic 20 conductivity meter, respectively.
A relatively simple and effective method for the pre-concentration and separation of uranium by cation exchange resin and its alpha radiometric determination after electrodeposition on stainless steel discs was used. One hundred milliliters of each water sample after addition of U-232 tracer was pre-treated by Chelex 100-resin as described elsewhere (Kiliari and Pashalidis 2010; Noli et al. 2016). This pre-analytical procedure is selective for uranium and was carried out in parallel for every sample.
Furthermore, 1 L of water was evaporated at 65 °C leading to dry residue from 0.3 up to 1.2 g approximately. About 50–70 mg of each dry sample (water or sediment) was placed in special vials in order to analyze them by INAA.
Analytical methods
The activities of the radionuclides U-238 and Th-232 were determined assuming a state of secular equilibrium associated with decay of Th-234 (63 and 93 keV) as well as of Ac-228 (911.2 and 968.9 keV), Pb-212 (238.6 and 300.0 keV), and Tl-208 (583.1 keV), respectively. Additionally, the activity of K-40 at 1460.8 keV was also determined in all samples. The system used was a high-resolution gamma spectrometry with an HPGe detector (CANBERRA, REGe detector, efficiency 20%, energy resolution 2.1 keV for the 1332 keV 60Co γ-radiation). The detector was shielded using a 10-cm-thick low-background lead shield (model Canberra 747E). A standard computer-based gamma spectrometry setup (Ortec-Maestro-32) was used for collection of the detector signals. The gamma background spectra were measured, and the measuring time ranged from 15 to 25 h. The energy and efficiency calibrations were performed with standard reference sources of IAEA though the IAEA-CU-2007-03 worldwide open proficiency test and standards of a sediment sample spiked with Ba-133, Cs-137, and Eu-152 reference NIST solutions as described elsewhere (Noli et al. 2017).
The elemental composition of the samples was determined by instrumental neutron activation analysis (INAA), at the 2 MW pool-type research reactor (TU-Delft, The Netherlands) combined with high-resolution gamma spectrometry (neutron dose 4.5–5.3 × 1016 n/cm2 × s, activation time 4–5 h) as described elsewhere (Bode and Blaauw 2012). The system was calibrated using NIST-CRM mono-element solutions. The samples and certified standards (IAEA soil-5 and soil-7), ranging in mass from 60 to 80 mg, were sealed in nitric acid-cleaned polyethylene vials. A network of three gamma spectrometers with well-type detectors and two gamma spectrometers with coaxial detectors, as well as two spectrometers with coaxial detectors at the two fast rabbit systems. was used for the measurements.
The determination of the concentrations of uranium isotopes (U-234, U-235, and U-238) in water samples was performed by alpha spectrometry using an Ortec Dual 576A alpha spectrometer and PIPS detectors (450 mm2). Prior to sample measurement, the background was carefully measured under identical conditions and was found to be about 50 counts per day within the energy range of 3–8 MeV. The efficiency after the separation using the cation exchange Chelex 100-resin was of 85% as well as the electrodeposition of uranium on stainless steel discs was resulted in excellent yields, generally over 99%. The uranium activities were measured by alpha spectrometry with a detection counting efficiency of 8% while the minimum detectable activity of the method (95% confidence level) is 1.2 mBq/L U-238 (or 97 ng/L U-238). The determinations with gamma and alpha spectrometry were performed in duplicate.
EF, CF, and pollution load index (PLI)
To estimate the anthropogenic impact on sediments, calculation of a normalized enrichment factor (EF) was performed according to the Eq. (1). This factor is referred to metal concentrations above uncontaminated background levels. The background concentrations were based on the elemental abundances of continental crust, and iron (Fe) was used as the reference element for geochemical normalization (Rudnick and Gao 2014; Tamim et al. 2016) because it exhibits a geochemistry similar to that of many trace metals and its natural mass fraction is almost uniform.
Metal/Fe represents the mass fraction of metal to the mass fraction of Fe in the sample and in the background (reference values), respectively. When EF >1, the element is of anthropogenic origin. Values in the range 1.5–3, 3.01–5, 5.01–10, and >10 indicate minor, moderate, severe, and very severe modifications.
Contamination factors (CFs) of the specific heavy metals for a specific sampling site were determined, which can be described as follows (Hakanson 1980):
Values of CF <1, 1 ≤ CF < 3, 3 ≤ CF < 6, and CF ≥6 are of evidence of low contamination, moderate contamination, considerable contamination, and very high contamination (Hakanson 1980).
Another empirical index which characterizes the level of heavy metal pollution is the PLI calculated from the following equation (Tomlinson et al. 1980):
where CF1, CF2,CF3, and CF n represent the contamination factors. Values of PLI higher than 1 indicate progressive deterioration.
Results and discussion
Uranium activity concentrations in water
The activity concentrations of the uranium isotopes (U-238 and U-234) in water samples are listed in Table 1 with the associated standard deviations and the minimum and maximum values whereas U-235 was not detected. In the same table, parameters such as pH and conductivity as well as the U-234/U-238 activity ratio and the total uranium concentration are also presented.
As can be seen in Table 1, the pH values of the waters were slightly basic and varied from 7.2 to 9.1. Uranium can be found in aqueous environment due to the water-rock/sediment interaction. As it is known, the speciation of uranium depends on pH, concentration, redox potential, presence of other species, carbonate, and organic ligands. In alkaline aqueous solutions, uranium exists in the form of negatively charged species, uranyl carbonato (e.g., UO2(CO3)2 2−, UO2(CO3)3 4−), and uranyl hydroxy-carbonato (e.g., (UO2)2(CO3)(OH)3 −). These species are also frequently present in natural waters (Gavrilescu et al. 2009; Skwarzec et al. 2012; Wu et al. 2014). In our case, the pH and the carbonate content present in bedrock material of the investigated area favor dissolved uranium to exist in soluble di- and tri-carbonate complexes. The conductivity was also measured and varied from 109 to 926 μS/cm2 with higher values in warm periods. As it is known, high conductivity values indicate presence of ionic species due to dilution/dissolution processes. The presence of these species increases positively the solubility of uranium (Vidic et al. 2013, Wu et al. 2014). This could be an explanation of the elevated total uranium concentrations determined in the lakes of the investigated region in the present study compared to lower concentrations measured in other lakes in Greece (Kehagia et al. 2007). The activity concentration values varied in the range 7.01–79.9 and 6.22–119 mBq/L for U-238 and U-234, respectively, corresponding to a total uranium concentration of 0.57–6.10 μg/L. Although these values are much lower than the guideline value of the World Health Organization for drinking water (30 μg/L), the risk of uranium should be taken into account (WHO 2011). USEPA has classified uranium as a confirmed human carcinogen (group A), not only due to its radioactivity, but also due to its chemical toxicity and suggested zero tolerance as the only safe acceptable limit for the carcinogenic risk from that (UNSCEAR 2000).
Natural radioactive equilibrium (isotopic ratio of U-234/U-238 close to 1) was found only in two cases whereas deviations from the equilibrium value were observed in many cases (0.66 up to 2.77) as can be seen in Table 1. Deviations of the isotopic ratio of U-234/U-238 from the typical activity ratio are reported often in the literature. Higher values have been detected in groundwaters, and several theories to explain this phenomenon have been referred: (1) the U-234 atom displacement from the crystal lattice due to Th-234 ejection after the decay of U-238 and (2) the preferential leaching of U-234 which is more mobile and is taking place when the water–rock interaction is long and the dissolution rate is low (Al-Masri et al. 2004; Boryło 2013; Chkir et al. 2009; Skwarzec et al. 2009, 2012; Tripathi et al. 2013; Vidic et al. 2013).
The low values of the isotopic ratio of U-234/U-238 in our case indicate presence of “young” waters (no long water–rock interaction) and high dissolution rates of uranium because of the characteristics of the region (lignite field, waste of power plants, presence of carbonates, high conductivity values) (Abdul-Hadi et al. 2001; Grabowski and Bem 2012).
A significant variation of the uranium concentration in the water was observed for the four lakes between warm and cool periods as it is shown in Table 1. The concentration values were low in winter and then gradually increased in spring and summer. Similar observations have been referred by Skwarzec et al. (2009), Al-Masri et al. (2004), and Abdul-Hadi et al. (2001) for studies in Poland and Syria, respectively. This could be attributed to the enrichment of the lake water with the measured isotopes due to leaching after the snow melting and rainfalls. Other possible reasons are the evaporation and the increased operation of the power plants during this period due to higher power demand in the summer and the dry deposition of lignite and ash from the dust generated from mining operations, such as excavation, transport, and disposal of wastes close to the mines (Noli and Tsamos 2016; Triantafyllou et al. 2006).
Mass activities of radionuclides in sediments
The mass activities of U-238, Th-232, and K-40 in the sediments were also measured (uncertainty varied from 6 to 12%), and the results are graphically presented in Figs. 2, 3, and 4 (mean values of two measurements for each sample). The values ranged from 3.53 to 29.9, 0.41 to 38.7, and 1.29 to 585 Bq/kg for U-238, Th-232, and K-40, respectively, whereas the minimum detectable activity was 1.68, 0.24, and 1.03 Bq/kg.
The results were in good agreement with relevant results reported in the literature (Matveyeva et al. 2014; Santawamaitre et al. 2014). For example, Aytas et al. (2012) reported similar range for radionuclides in sediments in Danube and other rivers in several countries (Turkey, India, Nigeria). Comparable results were found by Otansev et al. (2016) in sediments in Marmara sea and Tsabaris et al. (2007) in sediments in Ionian sea whereas higher values were reported by Erenturk et al. (2014) for the Lake Van in Turkey (174–225, 63–70, 263–486 Bq/kg for U-238, Th-232, and K-40, respectively). Slightly higher mass activities were also reported for U-238 (25–87 Bq/kg) in a lake of Slovenia but lower for K-40 (210–380 Bq/kg) compared to our results (Mljač and Križman 1996). During a line of research in the same basin, Karamanis et al. (2009) also found comparable values with the results of this study, for soils irrigated with discharged water, but rather higher for the sediments at the bottom of the channels of the discharged water.
Seasonal variations of the mass activities of U-238, Th-232, and K-40 in sediments were observed with low values in winter due to accumulation effects, findings which were in good agreement with the results of Al-Masri et al. (2004), Skwarzec et al. (2009), and Noli et al. (2017). During the winter, rainfalls and snow lead to leaching and subsequently deposition of heavy and trace metals to the sediments and then higher values were detected in spring, summer, and autumn.
The lakes Zazari and Cheimaditis presented enhanced mass activity values for the nuclides U-238, Th-232, and K-40 (19.2–28.7, 11.0–38.7, and 183.0–585 Bq/kg) probably due to the higher degree of dry and wet deposition of fly ash because these lakes are close to the big Amyntaio lignite mine (point B) and the lignite power plants as it is shown in Fig. 1b (distance from the mine B: Cheimaditis 2.3 km, Zazari 5.1 km, Petron 8.4 km, and Vegoritis 10.6 km). The low mass activity values measured in sediments of the lakes Vegoritis and especially Petron for the same radionuclides (9.28–24.2, 0.43–12.7, and 1.11–145 Bq/kg) could be attributed to the geological background. A possible explanation could be that the presence of carbonate formations and ionic species, as it was indicated by the high conductivity values found in the Lake Petron, favors the dissolution of uranium from the sediments and transport into the water (Gavrilescu et al. 2009; Wu et al. 2014). This can be seen in Fig. 2 where a comparison of the concentration of uranium in both sediments and water samples at different seasons is presented. In the case of waters, a significant difference can be observed during warm and cool periods with low activity concentrations in winter, while in the case of sediments, the difference was moderate.
Minor and trace elements in water and sediments
In the case of the water samples, 17 minor metals and trace elements were determined. The range of the concentrations detected for the four periods as well as the mean values is reported in Table 2. The calculated values showed a decreasing order of Na > K > Fe > Sr > Zn > Ba > Rb > U > Cr > Mo > As > Ce > Co > La > Th > Sc > Cs. In the case of the sediments, the determined elements were 18. The corresponding results of the mass fraction are reported in Table 3 and followed a decreasing order of Fe > Na > K > Sr > Cr > Ba > Zn > Rb > Ce > Nd > La > Ga > Co > Sc > Th > As > U > Cs.
Concerning the waters, the concentrations of the majority of the elements for the four lakes found in this work were comparable with results that appeared in the literature and regarding studies in waters and mainly in rivers and lakes. High values were detected only for Zn (maximum literature values 34.0, 51.3, and 157 μg/L reported by Alvarado Corcho et al. (2014), Boskidis et al. (2011), and Simeonov et al. (2003), respectively) and Fe (10.581 by Alvarado Corcho et al. (2014) and 833 μg/L by Simeonov et al. (2003)). In the case of uranium, the concentrations measured with INAA were in agreement with the results of alpha spectrometry and higher than those found in a lake in Slovenia (0.2–0.66 μg/L, Mljač and Križman (1996)). Thorium was detected only in two water samples because this element exhibits limited solubility and normally is distributed in sediments.
Concerning the sediments, the detected mass fractions were comparable to relevant literature data reported for sediment samples except for Fe, Zn, and particularly Cr (Arnason and Fletcher 2003; Cheng et al. 2015; Erenturk et al. 2014; Mamat et al. 2016; Tamim et al. 2016). For example, values from 18 to 222 and 30 to 80 mg/kg were reported for Cr for the Lake Van in Turkey and lakes in Kazakhstan, respectively, whereas in our case, the values were extremely higher and varied from 30 up to 428 mg/kg. The lakes Zazari and Cheimaditis seem to be more affected by the lignite and, in this case, accumulate the elements which are the main constituents of lignite and fly ash. The most abundant elements in lignite in this region were found to be Fe, K, Na, Ba, Cr, Sr, Zn, Rb, As, Co, U, and Th as has been reported by Georgakopoulos et al. (2002) and Megalovasilis et al. (2013) while the composition of the fly ash, determined in this study by INAA, was Fe 4.88% w/w, K 0.89% w/w, Na 0.51% w/w, Ba 510.1 mg/kg, Sr 423.2 mg/kg, Rb 88.9 mg/kg, Ce 90.1 mg/kg, Zn 110.2 mg/kg, As 25.3 mg/kg, Co 20.9 mg/kg, Cr 10.7 mg/kg, U 29.3 mg/kg, and Th 26.7 mg/kg, respectively. It is interesting to mention that in these lakes, the elements Zn and Cr (as well as Ba and Sr) displayed variation in sediments in different seasons with high values in autumn as it is shown in Figs. 5 and 6. The same behavior was observed and in the case of the radionuclides U-238, Th-232, and K-40 as it was referred above. In Fig. 7, the seasonal variation of potassium is presented in both sediments and water samples for the four lakes where low values can be observed in winter and elevated in spring and summer following the trend for uranium. Potassium is a major element in lignite and fly ash and contributes to the radiological background (1 g of potassium contains K-40 corresponding to 30.3 Bq). A significant factor also for the high concentrations observed in spring and summer especially for the potassium could be the fertilizers used in agriculture during these periods (Noli and Tsamos 2016; Skwarzec et al. 2012).
In the Lake Petron, the concentrations of all elements in both water and sediments were considerably lower in comparison to the other lakes probably because in the area of this lake, the human activities are limited. It also seems that there is a trend of transfer of metals from the sediments in the water, indicated by the conductivity values especially in warm periods, most likely due to diffusion into the sediment pores and solubility in the water. Other possible reasons could be attributed to the geological background, but further investigation is required for that, which is beyond the aim of the present study.
Heavy metal contamination level
Figure 8 presents the enrichment factor (EF) for selected elements in the sediments of the four lakes in order to investigate whether these elements are of anthropogenic origin. As it is known, lignite produces incombustible elements with high content of the natural radionuclides and heavy metals contributing to the activity and chemical contamination of the surrounding area (Karangelos et al. 2004; Papaefthymiou et al. 2007).
The elements presented in the plot of Fig. 8 are the main elements of fly ash, and as it is shown, most of them exhibited values of EF >1, evidence of anthropogenic origin. Cr and Zn presented the highest EF values for the majority of the sediments: Cr from 3.1 up to 23.7 and Zn from 1.15 up to 6.32. In Table 4, the values of the contamination factors (CFs) and the PLI for the sediments are given, whereas low contamination is indicated in most of the cases. Only Cr in lakes Zazari and Vegoritis presented values indicating moderate to severe contamination and Zn in Zazari borderline contamination. These values are comparable with results reported by other researchers for sediments in sea and rivers (Cheng et al. 2015; Otansev et al. 2016; Tamim et al. 2016). The PLI of the Lake Zazari was >1 and suggests increasing deterioration.
In general, the results of this study showed a possible pollution of the lakes (radiological and chemical) reflecting the impact of the lignite production and utilization. The environmental impact is associated with the location depending on the distance from the lignite mines and power plants. Although the determined concentrations of the studied metals were not in excess to pose any threat for drinking purpose, agriculture, and fish activities, the findings were interesting because they can be served as a baseline for future comparison. The obtained data also provide information about the pollution in the vicinity of a lignite mining area regarding the accumulation of heavy metals and radionuclides and contribute to further research in these regions.
Summary
The radiological status and chemical pollution in a cluster of four lakes in a lignite mining area of North-Western Greece were estimated. Sediments and water samples were studied over a period of 1 year in different seasons in order to investigate seasonal variations of natural radionuclides (U-238, Th-232, and K-40) and minor and trace elements.
Τhe uranium concentrations determined for the waters were lower than the WHO guideline value of 30 μg/L but higher compared to values measured in other lakes in Greece, and the isotopic ratio of U-234/U-238 varied between 0.66 and 2.77 indicating presence of young waters with intensive dissolution of uranium.
In the case of the sediments, the measured mass activities were comparable to the literature data referring to regions with relevant activities.
For the most radionuclides, a significant variation was established between cool and warm periods. For the waters, the activity values were low in winter and then gradually increased in spring and summer whereas for the sediments, elevated values were detected in warm periods and mainly in autumn due to accumulation effects. The accumulation seems to depend on the distance from the pollution source whereas the sites close to mines and power plants exhibited higher activity values.
The concentrations of the majority of the elements determined by INAA in waters as well as the mass fractions in sediments were in agreement with results that appeared in the literature. Elevated concentrations were detected for U, Th, Zn, Fe, and mainly Cr indicating possible water pollution.
In sediments, Zn, Fe, and Cr were increased, probably due to migration of radionuclides and minor and trace elements through the aquatic pathway and seasonal variation was exhibited, with higher concentrations in autumn, especially in the lakes close to the lignite mines. The calculated CFs and PLI showed accumulation in the sediments and moderate to severe contamination for Zn and Cr only in some cases.
The obtained data indicated minor pollution of the lakes (radiological and chemical), slightly higher in warm periods because of leaching effects after the snow melting and rainfalls as well as the evaporation and increased industrialization activity especially in summer. These data can be served as a baseline for future comparison in lignite regions with mining and energy production activities.
References
Abdul-Hadi A, Alhassanieh O, Ghafar M (2001) Disequilibrium of uranium isotopes in some Syrian groundwater. Appl Radiat Isot 55:109–113
Adamidou K, Kassoli-Fournaraki A, Filippidis A, Christanis K, Amanatidou E, Tsikritzis L, Patrikaki O (2007) Chemical investigation of lignite samples and their ashing products from Kardia lignite field of Ptolemais, Northern Greece. Fuel 86:2502–2508
Al-Masri MS, Byrakdar ME, Mamish S, Al-Haleem MA (2004) Determination of natural radioactivity in Euphrates river. J Radioanal Nucl Chem 216:349–355
Alvarado Corcho JA, Balsiger B, Reollin C, Jakob A, Burger M (2014) Radioactive and chemical contamination of the water resources in the former uranium mining and milling sites of Mailuu Suu (Kyrgyzstan). J Environ Radioact 138:1–10
Arnason JG, Fletcher BA (2003) A 40+ year record of Cd, Hg, Pb, and U deposition in sediments of Patroon Reservoir, Albany County, NY, USA. Environ Pollut 123:383–391
Asokan P, Saxena M, Asolekar SR (2005) Coal combustion residues—environmental implications and recycling potentials. Res Conserv Recycl 43:239–262
Aytas S, Yusan S, Aslani MAA, Karali T, Turkozu DA, Gok C, Erenturk S, Gokcee M, Oguz KF (2012) Natural radioactivity of riverbank sediments of the Maritza and Tundja Rivers in Turkey. J Environ Sci Health A 47:2163–2172
Bode P, Blaauw M (2012) Performance and robustness of a multi-user, multi-spectrometer system for INAA. J Radioanal Nucl Chem 291:299–305
Boryło A (2013) Determination of uranium isotopes in environmental samples. J Radioanal Nucl Chem 295:621–631
Boskidis I, Gikas GD, Sylaios G, Tsihrintzis VA (2011) Water quantity and quality assessment of lower Nestos river, Greece. J Environ Sci Health A 46:1050–1067
Cheng Q, Wang R, Huang W, Wang W, Li X (2015) Assessment of heavy metal contamination in the sediments from the Yellow River Wetland National Nature Reserve (the Sanmenxia section), China. Environ Sci Pollut Res 22:8586–8593
Chkir A, Guendouz K, Zouari F, Ammarc H, Moulla AS (2009) Uranium isotopes in groundwater from the continental intercalaire aquifer in Algerian Tunisian Sahara (Northern Africa). J Environ Radioact 100:649–656
Erenturk S, Yusan S, Turkozu DA, Camtakan Z, Olgen MK, Aslani MA, Aytas S, Isik MA (2014) Spatial distribution and risk assessment of radioactivity and heavy metal levels of sediment, surface water and fish samples from Lake Van, Turkey. J Radioanal Nucl Chem 300:919–931
Gavrilescu M, Pavel LV, Cretescu I (2009) Characterization and remediation of soils contaminated with uranium. J Hazard Mater 163:475–510
Georgakopoulos A, Filippidis A, Kassoli-Fournaraki A, Iordanidis A, Fernández-Turiel JL, Ilorens JF, Gimeno D (2002) Environmentally important elements in fly ashes and their leachates of the power stations of Greece. Energy Sources 24:83–91
Grabowski P, Bem H (2012) Uranium isotopes as a tracer of groundwater transport studies. J Radioanal Nucl Chem 292:1043–1048
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Joshi SR (1991) Radioactivity in the Great Lakes. Sci Total Environ 100:61–104
Karamanis D, Ioannides K, Stamoulis K (2009) Environmental assessment of natural radionuclides and heavy metals in waters discharged from a lignite-fired power plant. Fuel 88:2046–2052
Karangelos DJ, Petropoulos NP, Anagnostakis MJ, Hinis EP, Simopoulos SE (2004) Radiological characteristics and investigation of the radioactive equilibrium in the ashes produced in lignite-fired power plants. J Environ Radioact 77:233–246
Kehagia K, Koukouliou V, Bratakos S, Seferlis S, Tzoumerkas F, Potiriadis C (2007) Radioactivity monitoring in drinking water of Atttika, Greece. Desalination 213:98–103
Kiliari T, Pashalidis I (2010) Simplified alpha-spectroscopic analysis of uranium in natural waters its separation by cation-exchange. J Radioanal Nucl Chem 284:547–551
Mamat Z, Haximu S, Zhang Z, Aji R (2016) An ecological risk assessment of heavy metal contamination in the surface sediments of Bosten Lake, northwest China. Environ Sci Pollut Res 23:7255–7265
Matveyeva I, Jacimovic R, Planinsek P, Stegnar P, Smodis B, Burkitbayev M (2014) Assessment of the main natural radionuclides, minor and trace elements in soils and sediments of the Shu valley (near the border of Kazakhstan and Kyrgyzstan). J Radioanal Nucl Chem 299:1399–1409
Megalovasilis P, Papastergios G, Filippidis A (2013) Behavior study of trace elements in pulverized lignite, bottom ash, and fly ash of Amyntaio power station, Greece. Environ Monit Assess 185:6071–6076
Mljač L, Križman M (1996) Radioactive contamination of surface waters from a fly-ash depository at Velenje (Slovenia). Environ Int 22:339–345
Noli F, Tsamos P (2016) Concentrations of heavy metals and trace elements in soils, waters and vegetables and health risk in the vicinity of a lignite-fired power plant. Sci Total Environ 563–564:377–385
Noli F, Kazakis N, Vargemezis G, Ioannidou A (2016) The uranium isotopes in the characterization of groundwater in the Thermi-Vasilika region, northern Greece. Isot Environ Health Stud. doi:10.1080/10256016.2015.1119134
Noli F, Tsamos P, Stoulos S (2017) Spatial and seasonal variation of radionuclides in soils and waters near a coal-fired power plant of Northern Greece: environmental dose assessment. J Radioanal Nucl Chem 311(1):331–338
Otansev P, Taskın H, Bassarı A, Varinlioglu A (2016) Distribution and environmental impacts of heavy metals and radioactivity in sediment and seawater samples of the Marmara Sea. Chemosphere 154:266–275
Papaefthymiou H, Symeopoulos BD, Soupioni M (2007) Neutron activation analysis and natural radioactivity measurements of lignite and ashes from Megalopolis basin, Greece. J Radioanal Nucl Chem 274:123–130
Papastefanou C, Manolopoulou M, Stoulos S, Ioannidou A, Gerasopoulos E (1999) Soil-to-plant transfer of 137Cs, 40K and 7Be. J Environ Radioact 45:59–67
Pavlides SB, Mountrakis DM (1987) Extensional tectonics of northwestern Macedonia, Greece, since the late Miocene. J Struct Geol 9:385–392
Pentari D, Typou J, Goodarzi F, Foscolos AE (2006) Comparison of elements of environmental concern in regular and reclaimed soils, near abandoned coal mines Ptolemais–Amynteon, northern Greece: impact on wheat crops. Intern J Coal Geol 65:51–58
Public Power Corporation of Greece (2003) https://en.wikipedia.org/wiki/Public_Power_Corporation. Accessed 21 Jan 2017
Rudnick RL, Gao S (2014) Composition of the continental crust. Treatise on Geochemistry 3:1–51
Santawamaitre T, Malain D, Al-Sulaiti HA, Bradley DA, Matthews MC, Regan PH (2014) Determination of 238U, 232Th and 40K activity concentrations in river bank soil along the Chao Phraya river basin in Thailand. J Environ Radioact 138:80–86
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A, Sofoniou M, Kouimtzis T (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Skwarzec B, Kabat K, Astel A (2009) Seasonal and spatial variability of 210Po, 238U and 239+240Pu levels in the river catchment area assessed by application of neural-network based classification. J Environ Radioact 100:167–175
Skwarzec B, Struminska-Parulska DI, Borylo A, Kabat K (2012) Polonium, uranium and plutonium radionuclides in aquatic and land ecosystem of Poland. J Environ Sci Health A 47:479–496
Tamim U, Khan R, Jolly YN, Fatema K, Das S, Naher K, Islam MA, Azharul Islam SM, Hossain SM (2016) Elemental distribution of metals in urban river sediments near an industrial effluent source. Chemosphere 155:509–518
Tomlinson L, Wilson G, Harris R, Jeffrey DW (1980) Problems in the assessments of heavy-metal levels in estuaries and formation of a pollution index. Helgoländer Meeresuntersun 33:566–575
Triantafyllou AG, Zoras S, Evagelopoulos V (2006) Particulate matter over a seven year period in urban and rural areas within, proximal and far from mining and power station operations in Greece. Environ Monit Assess 122:41–60
Tripathi RM, Sahoo SK, Mohapatra S, Lenka P, Dubey JS, Puranik VD (2013) Study of uranium isotopic composition in groundwater and deviation from secular equilibrium condition. J Radioanal Nucl Chem 295:1195–1200
Tsabaris C, Eleftheriou G, Kapsimalis V, Anagnostou C, Vlastou R, Durmishi D, Kedhi M, Kalfas CA (2007) Radioactivity levels of recent sediments in the Butrint Lagoon and the adjacent coast of Albania. Appl Radiat Isot 65:445–453
UNSCEAR (2000) Sources and effects of ionizing radiation. United Nations, New York
Vidic A, Ilic Z, Benedik L (2013) Recent measurements of 234U/238U isotope ratio in spring waters from the Hadzici area. J Environ Radioact 120:6–13
World Health Organization (2011) Guidelines for drinking-water quality, 4th edn. WHO, Geneva, p 205–213. http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf. Accessed 23 Nov 2016
Wu Y, Wang Y, Xie X (2014) Occurrence, behavior and distribution of high levels of uranium in shallow groundwater at Datong basin, northern China. Sci Total Environ 472(15):809–817
Acknowledgements
This research project has been supported by the European Commission under the Seventh Framework Programme through the “Research Infrastructures” action of the “Capacities” Programme, NMI3-II Grant number 283883. The authors would like to thank the people at the Reactor Institute (TU-Delft, The Netherlands) for the INAA measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Noli, F., Tsamos, P. Seasonal variations of natural radionuclides, minor and trace elements in lake sediments and water in a lignite mining area of North-Western Greece. Environ Sci Pollut Res 25, 12222–12233 (2018). https://doi.org/10.1007/s11356-017-9801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9801-z