Abstract
Different methods, including the use of nanoscale zero-valent iron (NZVI), have been used to treat arsenic (As)-contaminated environments, with much less data on the use of NZVI in arsenic-calcareous-polluted soils. Accordingly, two different experiments were conducted to investigate the effects of NZVI on the removal of As from three different calcareous-polluted soils. In the first experiment, the effects of soil type (differing in the rate of clay particles and organic carbon including S1 (8.0 and 0.05%), S2 (20 and 0.2%), and S3 (20.5 and 0.8%)) and NZVI concentration (0, 50, and 100 g kg−1 of dry soil) on the removal of As extractable with distilled water were evaluated using a factorial design with three replicates. In the second experiment, the NZVI concentrations were reduced to 0, 2.5, 5.0, and 25 g kg−1, and the NZVI contact time (0.5, 48, 96, 192, 384, and 768 h) was also tested. The analysis of variance in both experiments indicated the significant effects (P < 0.01) of the experimental treatments on the removal of As. The concentrations of available As in S3 (42.7 mg kg−1), S2 (20.22 mg kg−1), and S1 (24.22 mg kg−1) after using the 50 g kg−1 NZVI treatment decreased to 0, 0, and 0.05 mg kg−1, respectively, which was not significantly different from the 100 g kg−1 NZVI treatment. In the second experiment, using the 25 g kg−1 NZVI treatment, the concentration of available As significantly decreased in S1 from 16.48 to 0.1767 mg kg−1, in S2 from 13.34 to 0.31 mg kg−1, and in S3 from 33.67 to 0.84 mg kg−1. In the three soils, with increasing NZVI concentration and contact time, the concentration of available As in the solution phase significantly decreased (P = 0.01). S3, due to a higher rate of organic matter, was less responsive to the NZVI treatments than the other soils. The effectiveness of the nanoremediation method, tested in this research work, on the stabilization of As in calcareous soils, is verified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of soil is steadily increasing, worldwide. Industrial growth and the high use of chemicals result in the gradual pollution of soils with heavy metals. The heavy metals, which are of the highest concern, are lead, cadmium, mercury, and arsenic (As), which is actually a semi-metal with the mass of 74.92 g, from the fifth group of the periodic table of elements (Frost and Griffin 1977; Ernst 1996).
In nature, As is found in the form of organic and mineral; however, mineral As is more toxic and mobile than organic As. The most usual forms of As are the redox and the three- and the five-valent As. The mobility of As in the soil is determined by salinity; clay content; organic matter; surface release; and the presence of elements such as iron, manganese, calcium, and phosphate as well as by pH (Bissen and Frimmel 2003; Bauer and Blodau 2006). With respect to the chemical similarities of As and phosphorous (P), using P extractants is a suitable method for the extraction of As. For example, the Olsen’s method and the AB-DTPA methods are used for the extraction of exchangeable As, although the Olsen’s method is more common (Song et al. 2006).
Different methods have been used for the removal of As from the polluted environments including (1) oxidation techniques, (2) phytoremediation, (3) electro-chemical removal of As (ECAR), (4) coagulation-flocculation, (5) electrocoagulation, (6) adsorption, (7) electrokinetics, (8) ion exchange, (9) advanced hybrid and integrated technologies, (10) membrane technology, and (11) disposal of As sludges and wastes (Mohan and Pittman 2007; Singh et al. 2015). It has also been indicated that the use of iron nanoparticles may be a suitable method due to its high efficiency to reduce metal and semi-metal availability (Liu et al. 2008; Wang et al. 2014; Di Palma et al. 2015; Rahimi et al. 2015; Stefaniuk et al. 2016).
The remediation of polluted waters with arsenic, chromium, and lead has been effectively done using nanoscale zero-valent metals such as iron (Mueller et al. 2012; Yan et al. 2012). Using nanoscale zero-valent iron (NZVI) is a suitable method for the removal of As, which is due to its relatively high stability in the environment, ease of accessibility, high efficiency, quick and effective reaction, suitable expenses, and high efficiency in the removal of the usual forms of As, arsenite, and arsenate (Bezbaruah et al. 2014; Mu et al. 2017).
Research work has indicated that NZVI is a suitable method for the in situ remediation of ground waters polluted with inorganic pollutants (Kanel et al. 2005; Ali et al. 2011; Mondal et al. 2013; Datta et al. 2014). Bang et al. (2005) indicated that despite the complex nature of spectroscopic X-radiation, the final product of arsenate and arsenite reaction with zero-valent iron is the surface complex of arsenate and arsenite on the iron hydroxides. They found that the removal of As with zero-valent iron under anoxic conditions is related to the electrochemical reduction of arsenite with the less soluble zero-valent iron and the adsorption of arsenite and arsenate by ferric hydroxides (oxidation of Fe(0) by dissolved oxygen) on the surface of zero-valent iron. Accordingly, the quick removal of arsenite and arsenate under aerobic conditions is due to their surface adsorption on the formed ferric hydroxides by the oxidation of Fe(0) (Bang et al. 2005).
Kanel et al. (2005) indicated that the removal of arsenite in the presence of Fe(0) using NZVI is really quick (fast adsorption kinetic) and on a minute scale; however, the presence of free anions such as H2PO4 2−, HCO3 −, and H4SiO4 0 in the environment decreases the efficiency of removal. The use of Fe(0) filings is highly efficient in the removal of As from the polluted ground water, and at pHs less than 7, the removal of arsenite is more slow than arsenate (Bang et al. 2005). They also attributed the removal of As to the production of ferric hydroxides by the oxidation of NZVI under acidic conditions. Rahmani et al. (2011) found that NZVI is able to remove As from the saturated solution, quickly and in a wide range of pHs. There was also a positive correlation between the removal of As with contact time and NZVI amount.
Although there has been a few research work on the use of NZVI for treating contaminated soils (Zhang et al. 2010; Kim et al. 2012; Gil-Díaz et al. 2017), the one important aspect related to the novelty of this research work is the use of NZVI in calcareous soils with different rates of clay particles and organic matter. It is because, as mentioned earlier, the presence of calcium and carbonate as well as clay particles and organic matter can also affect the efficiency of NZVI method in contaminated soils. Accordingly, two different experiments were conducted. In the first experiment, the effects of NZVI concentration and soil type on the removal of As from the soil, and in the second experiment, in addition to such factors, the effects of NZVI contact time, were also investigated.
Materials and methods
Experiment I
The research work consisted of two different experiments in three replicates; in the first experiment, the effects of soil type and NZVI concentration on the removal of As, extractable with distilled water and Olsen’s extractant in contaminated calcareous soils were evaluated. Accordingly, a factorial experiment on the basis of a completely randomized block design in three replicates was conducted. The following experimental factors were tested: (1) soil type including soil 1 (S1) (clay and organic carbon at 8 and 0.05%, respectively), soil 2 (S2) (20 and 0.2%), and soil 3 (S3) (20.5 and 0.8%) and (2) the NZVI concentrations of 0 (NZVI0), 50 (NZVI50), and 100 g kg−1 dry soil (NZVI100). The properties of the soil types are presented in Table 1. The calcareous soils were treated according to the following. The NZVI treatments were mixed with 5 g of soil, and then mixed with 37.5 ml distilled water and shaken for 30 min. The samples were placed in plates and dried under room temperature. Two grams of each sample was taken and shaken with 40 ml sodium bicarbonate extractant for 2 h, and then centrifuged at 3000×g; the supernatant was filtrated using filter paper no. 42.
Experiment II
In the second experiment and according to the results of the first experiment, the concentrations of NZVI were reduced to 0 (NZVI0), 2.5 (NZVI2.5), 5 (NZVI5), and 25 g kg−1 dry soil (NZVI25). Accordingly, at the times of 0.5 (T1), 48 (T2), 96 (T3), 192 (T4), 384 (T5), and 768 h (T6) (as the third experimental treatment) after treating the samples, the removal of As was determined using the same methodology as indicated in the first experiment. During the experiment, the soils were regularly wetted and dried using distilled water.
NZVI synthesis
The NZVI was synthesized using the method of sodium borohydride according to the following details (Zhang 2003). First, 75 ml of methanol was poured into a 250-ml volumetric flask and was brought up to volume using distilled water. Five grams of iron sulfate was poured into the volumetric flask containing methanol and, using a stirrer, was well mixed. The acidity of the solution was adjusted, from 3.5 to 6–7, using a 2-N sodium hydroxide solution. The solution was mixed with sodium borohydride (2 g of sodium borohydride was poured into a 25-ml volumetric flask and the solution was brought up to volume), which was mixed with the solution gradually and stirred for 40 min. The solution was centrifuged for 15 min at 5000×g. The supernatant was poured away and the solid phase was filtrated using methanol and Whatman filter paper no. 42. The solid particles were finally dried under vacuum conditions using nitrogen gas. The SEM (scanning electron microscopy) method was used to indicate the physical properties of NZVI particles. Using freeze-dried particles and the Hitachi field emission scanning electron microscopy, the physical properties of the synthesized NZVI particles were determined (Kim et al. 2012).
Soil contamination with arsenic
The soil was contaminated with As according to the method of An and Zhao (2012) using sodium arsenate (NaAsO3) at the concentration of 150 mg L−1. For the contamination of the soil, 1 l of NaAsO3 solution was shaken with 800 g of the experimental soil, 2 h/day for 2 weeks. The supernatant was thrown away and the soil was dried at the temperature of the laboratory.
Measurement of extractable arsenic
The concentration of As extractable with Olsen’s extractant was determined using the following method with some modification (Rodriguez et al. 1999). The samples (2 g) were treated with 40 ml sodium bicarbonate 0.5 N, and shaken for 2 h using a reciprocal shaker at 150 rounds per minute. The suspension was then centrifuged for 15 m at 3000×g and the supernatant was filtrated using Whatman filter paper no. 42. The levels of As in the samples in the first and second experiments were determined using the hybrid part (model HS55) of atomic absorption instrument (Analytik Jena, Model Contra AA 300). Using the same method, the As extractable with distilled water was measured following the nano treatments. Accordingly, the samples (2 g) were treated with 15 ml of distilled water and shaken with the reciprocal shaker for 2 h at 150 rounds per minute. The suspension was centrifuged at 3000×g for 15 min, and the supernatant was collected and filtrated using Whatman filter paper no. 42.
Statistical analysis
The first experiment was a two-way factorial on the basis of a completely randomized block design including the experimental treatments of NZVI concentration and soil type. The second experiment was a three-way factorial on the basis of a completely randomized block design, which in addition to the treatments of the first experiment also tested the contact (shaking) time as the third experimental treatment. Data were subjected to analysis of variance using SPSS, and the significant differences among the treatments were determined. Using Duncan’s multiple range test, the means were compared at P = 0.05.
Results
Experiment 1
The SEM picture of the synthesized NZVI particles using the sodium borohydride method is shown in Fig. 1. The particles are aspheric with the average diameter of 45 nm. Analysis of variance indicated the significant effects of soil type and NZVI concentration as well as their interaction on the removal of As extractable with distilled water and Olsen’s extractant. This indicates that there were significant differences between soil types and NZVI concentrations affecting the removal of As from the soil (Tables 2 and 3).
Effects of NZVI concentration
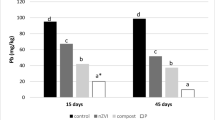
Regardless of the soil type, the NZVI50 and NZVI100 treatments were able to significantly immobilize or stabilize As from the calcareous polluted soils, compared with the control (NZVI0) treatment. However, as expected, water extracted less As than the Olsen extractant. The use of NZVI100 significantly decreased such levels (leachable As) to the amounts near zero (Figs. 2 and 3).
Effects of soil type
Regardless of the NZVI concentration, the mobility of As was significantly higher in S3 (highest rate of organic matter) compared with S2 (medium level of organic matter) and S1 (least rate of clay and organic matter) (Figs. 2 and 3).
The combined effects of NZVI concentration and soil type
The highest rate of leachable As was found in control treatments (NZVI0), related to the other NZVI treatments. Compared with NZVI0, the use of NZVI50 decreased the concentrations of As (using distilled water extractant) in S3 (42.7 mg kg−1), S2 (20.22 mg kg−1), and S1 (24.22 mg kg−1) to 0, 0, and 0.05 mg kg−1, respectively. The reduced concentrations of As (using the same extractant) with NZVI50 were not significantly different from those of the NZVI100 treatment (Fig. 2). The results also indicated that the concentration of As (using Olsen’s extractant) in S3 (85.1 mg kg−1) and S2 (24.3 mg kg−1), treated with NZVI50, decreased to 24.41 and 5.92 mg kg−1, respectively (Fig. 3).
Experiment II
According to the analysis of variance, there were significant differences between soil type, as well as the concentration and the contact time of NZVI on the removal of As extractable with distilled water (Table 4).
Effects of NZVI concentration
Similar to the first experiment and regardless of soil type, with increasing the concentration of NZVI, the leachability of As decreased and its immobilization and stabilization by NZVI increased. The most effective treatment was NZVI25, followed by NZVI5 and NZVI2.5. The immobilizing effects of NZVI25 treatment on As were significantly higher than of the other treatments (Figs. 4, 6, and 7).
Effects of soil type
The effects of the soil type was similar to the first experiment regardless of the NZVI concentrations, and soil 1 and soil 2 were the most responsive soils to the stabilization of As from the calcareous contaminated soils (Figs. 4, 5, and 7).
Effects of contact time
Regardless of NZVI concentrations and soil type, the contact times of 0.5, 48, 96, 192, and 384 h acted similarly on the immobilization of As. However, the NZVI dose was a determining factor on the effects of contact time affecting As removal, because higher doses allowed shorter contact time (Figs. 5, 6, and 7).
The combined effects of NZVI concentrations, soil type, and contact time
Figure 4 indicates how the removal of As (using water extractant) is affected by different NZVI concentrations in different soil types. There are no significant differences between 5 and 25 g kg−1 of NZVI in S2. In general, S1 and S2 showed similar results at the NZVI doses of 5 and 25 g kg−1 according to the As extracted with water. The least concentration of leachable As was related to S2 using the NZVI25 treatment, and the highest concentration was related to S3 using the NZVI0 treatment. The concentration of As, using S1 and the NZVI25 treatment, was equal to 0.1767 mg kg−1 indicating a significant decrease in As concentration, compared with the control treatment (16.48 mg kg−1). Such a reduction in S2 was equal to 13.34 and 0.31 mg kg−1, and in S3, it was equal to 33.67 and 0.84 mg kg−1, respectively (Fig. 4).
Figure 5 shows the interaction effects of NZVI contact time and soil type on the extraction of As (using water extractant). Accordingly, with increasing the contact time of NZVI, the removal efficiency of As significantly increased. The concentration of leachable As significantly decreased at the time of 768 h, compared with the time of 0.5 h, using different NZVI treatments, but not for soil 2.
The highest concentration of As (using water extractant) was related to S3 and the contact time of 0.5 h, and the least concentration was related to S2 and T6. According to the results, the concentration of As at T6 reduced to 24.66, 154.41, and 61.98% in S2 (As at T1 and T6 was equal to 3.69 and 2.97 mg kg−1, respectively), S3 (As at T1 and T6 was equal to 17.94 and 7.51 mg kg−1, respectively), and S1 (As at T1 and T6 was equal to 5.54 and 3.42 mg kg−1, respectively) compared with the control treatment (Fig. 5).
Figure 6 indicates the combined effects of NZVI contact time and concentration on the removal of As from the polluted soil. Accordingly, there were significant differences between the leachable As in the control treatment (NZVI0) with the other NZVI treatments (P = 0.01). After 48 h, the concentration of As extracted with water decreased from 23 mg kg−1 to 6.42, 2.29, and 0.44 mg kg−1 at the NZVI doses of 2.5, 5, and 25 g kg−1, respectively. There was also a similar trend for the concentration of leachable As in the other treatments.
Figure 7 indicates the interaction of soil type and contact time and the concentration of NZVI on the removal of As (using water extractant). According to the results, in all types of the soil, with increasing the concentration and the contact time of NZVI, the concentration of As in the solution phase significantly decreased (P = 0.01). However, the concentration of leachable As in all types of soil decreased to the concentrations less than 0.01 mg kg−1.
Discussion
The results indicated that the use of nanoscale zero-valent iron (NZVI) is an effective method for treating the calcareous soils polluted with As. It has been previously indicated that the use of zero-valent iron (ZVI) and nanoscale zero-valent iron are effective on the removal of inorganic As (arsenate and arsenite) from aqueous solutions (Bang et al. 2005; Kanel et al. 2005). However, only a few research works has addressed the effects of NZVI on the remediation of soils polluted with As (Zhang et al. 2010; Kim et al. 2012; Gil-Díaz et al. 2017).
In this research work, the effects of NZVI were examined, which have been indicated to be much more effective than the use of ZVI. For example, Kanel et al. (2005) found that NZVI is able to the decontamination of aqueous solutions with As with a speed of 1000 times faster than ZVI. This is due to the properties of the nanoparticles with a much higher rate of surface area, little size, and chemical activities. NZVI, as an excellent adsorbent and reductant, can be active for a period of 4–8 weeks and can be effectively used for the in situ remediation of a wide range of contaminants including As for a long time (Zhang 2003; Bae and Hanna 2015; Tiberg et al. 2016). The other important aspect related to this research work, which may also be considered the novelty of the research, is the use of calcareous soils differing in the rates of clay particles and organic matter.
A highly concentrated NZVI was used in the first experiment to find the most suitable concentrations, which could completely stabilize As and significantly decrease its leachability. The results indicated that the levels of NZVI, used in this research work, were totally effective on the removal of As from the soil. Due to the presence of clay particles and organic matter in the soil treatments, the effectiveness of NZVI could be affected. However, with respect to the results of the first experiment, the concentrations of NZVI were significantly decreased in the second experiment and some similar results were obtained.
Regardless of the NZVI concentration, the mobility of As was significantly higher in S3 (highest rate of organic matter) compared with S2 (medium level of organic matter) and S1 (least rate of clay and organic matter). This indicates that the higher rate of organic matter can significantly increase the leachability of As decreasing its mobilizations and stabilization. It also indicates that the effects of organic matter on the availability of As is higher than the clay particles tested in this research work (Figs. 2 and 3). The effect of the soil type was similar to the first experiment regardless of the NZVI concentrations, and soil 1 and soil 2 were the most responsive soils to the stabilization (removal) of As from the calcareous contaminated soils (Figs. 4, 5, and 7). Such results indicated how the properties of soil might affect the remediation processes of calcareous soils contaminated with As.
Different parameters including Ca2+ and HCO3 − affect the efficiency of As removal by NZVI. However, our results indicated that the NZVI concentrations tested in this research work were able to immobilize As from the soil solution and significantly decreased its rate of leaching in the tested calcareous soils. Zhang et al. (2010) indicated that the NZVI treatment at a Fe/As ratio of 100:1 significantly decreased the leaching rate of As in the soil. They also expressed that such a potential was a function of Fe concentration in the soil as in the soils with a less concentration of Fe (an orchard soil); the method of NZVI remediation could be more efficient. This can also be the case for this research work as in a calcareous soil the mobility of iron is much less than in an acidic soil. However, the effect of pH (alkaline in a calcareous soil) may not be an important factor affecting the efficiency of As removal (Kanel et al. 2005).
The other important factors, which were tested in this experiment, were organic matter and clay particles (S1, S2, and S3). The presence of organic matter may affect the speciation of As in the soil and its subsequent adsorption by NZVI because organic matter can (1) compete with As for adsorption by NZVI, (2) coagulate NZVI, and (3) decrease the corrosion rate of NZVI and hence the adsorbing sites by NZVI. The presence of clay particles, which can compete with NZVI, affects its potential for the adsorption of As. For example, kaolinite clay is a more positive clay and can adsorb more As than montmorillonite clay (Redman et al. 2002).
The presence of anions such as HCO3 − can significantly decrease the adsorption potential of NZVI due to their competition with NZVI and production of an inner complex in NZVI. However, cations such as Ca2+ and Mg2+ can significantly enhance the adsorbing potential of NZVI, which is due to the charge neutralization of adsorbate and adsorbent by such cations as well as due to their bridging potential (Murphy et al. 1990; Peng et al. 2005). Gil-Díaz et al. (2017) found that NZVI could effectively immobilize As in both acidic and calcareous soils; however, its efficiency was higher in the acidic soil.
Although there are some constrains in the use of NZVI for the treatments of environments polluted with the organic and inorganic compounds of As, the results are promising and such a technique can be used as a feasible method for the removal of As from the environment (Mueller et al. 2012; Gil-Díaz et al. 2014, 2017). The most important concerns related to the use of NZVI for the treatment of the environment polluted with As are (1) how the presence of NZVI may influence the environment in a long-term period, (2) the ecotoxicity of NZVI in the environment, and (3) lack of ample research work for the use of different NZVI products and development methods (Crane and Scott 2012).
According to the results of the two experiments, the removal of As from polluted soils is much more affected by soil organic matter than soil clay, which is due to a higher rate of cation exchange capacity in the soils with a higher rate of organic matter. According to the results, S3 (the soil with the highest rate of organic matter) was the least responsive to different concentrations of NZVI, at different contact times, resulting in the highest rate of leachable As. These results are somehow comparable to the results of Tiberg et al. (2016) using NZVI, as higher rate of organic matter results in the higher sorption of humic substances, due to their reaction with iron and aluminum hydroxide, and hence may similarly decrease the efficiency of NZVI treatment. Gil-Díaz et al. (2014) investigated the effects of commercial NZVI (1 and 10%) and the contact times of 3 days and 3 months on the immobilization of As in the soil. They found that As immobilization was more effective and stable at the highest dose of NZVI. The concentrations of NZVI, which were tested in this research, were effective on the removal of As from the calcareous-polluted soils and with time the efficiency of removal increased.
Conclusion
The method of nanoscale zero-valent iron (NZVI) for the treatments of calcareous soils contaminated with As was examined using two experiments with three different soils and different NZVI concentrations and contact times. These parameters significantly affected the removal of As from the polluted soils. Most of the experiments conducted so far using NZVI have been related to aqueous solutions, and there is just a few research work on the use of NZVI for remediation of As-polluted soils, especially calcareous soils. It is a promising method. The novelty of this research work is the use of calcareous soils differing in the rates of clay particles and organic matter. According to the results of this research work, the rate of organic matter is a more effective parameter affecting the efficiency of NZVI on the removal of As from the soil than the rate of soil clay. Such parameters must be considered when treating the soils contaminated with As. Studies including soils with higher content in organic matter are necessary. The results of this research work indicated that the NZVI concentration at the range of 2.5–25 g kg−1 dry soil could efficiently immobilize As in a calcareous soil with different rates of clay particles and organic matter, and with increasing time, the efficiency of method increased. With respect to the advantages of using NZVI, its use for the remediation of the calcareous soils polluted with As is promising although more research work is essential.
References
Ali I, Khan T, Hussain I (2011) Treatment and remediation methods for arsenic removal from the ground water. Int J Environ Eng 3:48–71
An B, Zhao D (2012) Immobilization of As (III) in soil and groundwater using a new class of polysaccharide stabilized Fe–Mn oxide nanoparticles. J Hazard Mater 211:332–341
Bae S, Hanna K (2015) Reactivity of nanoscale zero-valent iron in unbuffered systems: effect of pH and Fe (II) dissolution. Environ Sci Technol 49:10536–10543
Bang S, Johnson MD, Korfiatis GP, Meng X (2005) Chemical reaction between arsenic and zero-valant iron in water. Water Res 39:763–770
Bauer M, Blodau C (2006) Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci Total Environ 354:179–190
Bezbaruah AN, Kalita H, Almeelbi T, Capecchi CL, Jacob DL, Ugrinov AG, Payne SA (2014) Ca–alginate-entrapped nanoscale iron: arsenic treatability and mechanism studies. J Nanopart Res 16:1–10
Bissen M, Frimmel FH (2003) Arsenic—a review. Part I: occurrence, toxicity, speciation, mobility. Acta Hydrochim Hydrobiol 31:9–18
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211:112–125
Datta K, Petala E, Datta K, Perman J, Tucek J, Bartak P, Otyepka M, Zoppellaro G, Zboril R (2014) NZVI modified magnetic filter paper with high redox and catalytic activities for advanced water treatment technologies. Chem Commun 50:15673–15676
Di Palma L, Gueye MT, Petrucci E (2015) Hexavalent chromium reduction in contaminated soil: a comparison between ferrous sulphate and nanoscale zero-valent iron. J Hazard Mater 281:70–76
Ernst W (1996) Bioavailability of heavy metals and decontamination of soils by plants. Appl Geochem 11:163–167
Frost RR, Griffin RA (1977) Effect of pH on adsorption of arsenic and selenium from landfill leachate by clay minerals. Soil Sci Soc Am J 41:53–57
Gil-Díaz M, Alonso J, Rodríguez-Valdés E, Pinilla P, Lobo MC (2014) Reducing the mobility of arsenic in brownfield soil using stabilised zero-valent iron nanoparticles. J Environ Sci Health A 49:1361–1369
Gil-Díaz M, Pinilla P, Alonso J, Lobo MC (2017) Viability of a nanoremediation process in single or multi-metal (loid) contaminated soils. J Hazard Mater 321:812–819
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic (III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39:1291–1298
Kim K, Lee BT, Kim KW (2012) Arsenic stabilization in mine tailings using nano-sized magnetite and zero valent iron with the enhancement of mobility by surface coating. J Geochem Explor 113:124–129
Liu J, Zhao Z, Jiang G (2008) Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ Sci Technol 42:6949–6954
Mohan D, Pittman C (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53
Mondal P, Bhowmick S, Chatterjee D, Figoli A, Van der Bruggen B (2013) Remediation of inorganic arsenic in groundwater for safe water supply: a critical assessment of technological solutions. Chemosphere 92:157–170
Mu Y, Jia F, Ai Z, Zhang L (2017) Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ Sci: Nano 4:27–45
Mueller NC, Braun J, Bruns J, Černík M, Rissing P, Rickerby D, Nowack B (2012) Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res 19:550–558
Murphy E, Zachara J, Smith S (1990) Influence of mineral-bound humic substances on the sorption of hydrophobic organic compounds. Environ Sci Technol 24:1507–1516
Peng X, Luan Z, Chen F, Tian B, Jia Z (2005) Adsorption of humic acid onto pillared bentonite. Desalination 174:135–143
Rahimi S, Moattari RM, Rajabi L, Derakhshan AA, Keyhani M (2015) Iron oxide/hydroxide (α, γ-FeOOH) nanoparticles as high potential adsorbents for lead removal from polluted aquatic media. J Ind Eng Chem 23:33–43
Rahmani A, Ghaffari HR, Samadi MT (2011) Removal of arsenic (III) from contaminated water by synthetic nano size zerovalent iron. World Acad Sci Eng Technol 8:175–180
Redman AD, Macalady DL, Ahmann D (2002) Natural organic matter affects arsenic speciation and sorption onto hematite. Environ Sci Technol 36:2889–2896
Rodriguez RR, Basta NT, Casteel SW, Pace LW (1999) An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media. Environ Sci Technol 33:642–649
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Song J, Zhao F, McGrath S, Luo Y (2006) Influence of soil properties and aging on arsenic phytotoxicity. Environ Toxicol Chem 25:1663–1670
Stefaniuk M, Oleszczuk P, Ok Y (2016) Review on nano zerovalent iron (nZVI): from synthesis to environmental applications. Chem Eng J 287:618–632
Tiberg C, Kumpiene J, Gustafsson JP, Marsz A, Persson I, Mench M, Kleja DB (2016) Immobilization of Cu and As in two contaminated soils with zero-valent iron–long-term performance and mechanisms. Appl Geochem 67:144–152
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As (III) and As (V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Yan W, Vasic R, Frenkel AI, Koel BE (2012) Intraparticle reduction of arsenite (As (III)) by nanoscale zerovalent iron (nZVI) investigated with in situ X-ray absorption spectroscopy. Environ Sci Technol 46:7018–7026
Zhang WX (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Zhang M, Wang Y, Zhao D, Pan G (2010) Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles. Chin Sci Bull 55:365–372
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Azari, P., Bostani, A.A. Reducing As availability in calcareous soils using nanoscale zero valent iron. Environ Sci Pollut Res 24, 20438–20445 (2017). https://doi.org/10.1007/s11356-017-9447-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9447-x