Abstract
The present manuscript studies the effectiveness of commercial nano zero valent iron (nZVI) particles in decreasing the availability of Cd, Cu, Ni, and Pb in spiked soil samples and to remove the same heavy metals from aqueous solutions. The difference of nZVI efficiency between single and multi-metal contamination was evaluated. The application of nZVI in water samples showed higher effectiveness in the cases of single metal contamination. The effectiveness of single- and multi-metal (mixtures of Cu, Ni, Pb and Cd, Cu, Ni, Pb) immobilization in soil using different doses (0%, 0.85%, 1.7%, 2.55%, and 5.1%) of nZVI was determined. Immobilization efficiency was assessed using the leaching procedure and depended on a particular metal and the dose of nZVI. In all cases, it was determined that an increasing amount of nZVI resulted in decrease in the leaching of analysed metals. In cases, where higher nZVI doses were used, higher immobilization efficiency was observed for heavy metals in multi-metal contamination. The application of nZVI significantly reduced leaching of all heavy metals and this strategy can be successfully used for heavy metals stabilization in soils.
Article Highlights
-
The highest removal efficiencies from aqueous solutions were for Cu2+ and Pb2+.
-
The percentages of metal immobilized were higher in multi-metal polluted soils.
-
Using nZVI the exchangeable fraction of heavy metals can be significantly reduced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are constantly getting into the soil because of human activities (Zeng et al. 2018). In contaminated areas, heavy metals like lead (Pb), cadmium (Cd), copper (Cu), and nickel (Ni) are often detected (Wuana and Okieimen 2011). To protect human health and soil quality, treatment of the soil contaminated by heavy metals is relevant.
Nano zero valent iron (nZVI) particles can be used to immobilize heavy metals and for the remediation of contaminated sites. Iron nanoparticles have a higher sorption capacity than conventional sorbents and can adsorb many pollutants including metals and metalloids (Boparai et al. 2011). Because of their sorption features, these particles can be used for treating soil contaminated with heavy metals, and also for the remediation using the in situ method (Komárek et al. 2013).
Zero valent iron has a strong reducing power (Zhao et al. 2016). Most studies using Fe0 nanoparticles were performed in water samples (Kanel et al. 2005, 2006; Li and Zhang 2007; Klimkova et al. 2011; Boparai et al. 2013; Wang et al. 2016). The studies done by scientists provided knowledge about the possibilities of Fe0 nanoparticles in transforming metal cations in the water. nZVI can quickly eliminate and/or reduce the amount of these ions in water samples. Heavy metals are reduced on the surface of Fe0 nanoparticles (Boparai et al. 2011). Under environmental conditions, nZVI reacts well in water and can be a good electron donor (Li and Zhang 2006; Cook 2009):
The reaction mechanisms of Fe0 nanoparticles with heavy metals include (O’Carroll et al. 2013): adsorption (Pb, Ni, Cd), oxidation/reoxidation (Pb), reduction (Cu, Pb, Ni), precipitation (Cu, Pb, Cd) and co-precipitation (Ni). The controlling mechanism is the standard electrode potential (E0) of the heavy metal (Boparai et al. 2011). The immobilization mechanisms of nZVI mainly include adsorption/surface complexation for metal ions, whose electrode potential is very close to or more negative than Fe0 (− 0.44 V) (Liang et al. 2014). For example, the standard Cd2+ redox potential (− 040 V, 25 °C) is very close to zero valent iron, and therefore the removal of Cd2+ ions by nZVI is due to sorption (Li and Zhang 2007). On the surface, adsorbed Cd2+ ions become non-mobile. Lead (Pb2+) can be removed from aqueous solutions by reducing to Pb0 or by adsorbing it on an iron (hydr)oxide layer. After reaction with nanoparticles, Pb2+ precipitates as Pb(OH)2 or oxidizes as α-PbO2 (Ponder et al. 2000; Xi et al. 2010). For metal ions such as Cu2+, whose E0 is more positive compared to Fe0, the removal of metal ions mainly occur through the surface-mediated reduction reactions. Cu2+ can be removed from aqueous solutions through the chemical reduction to elemental form and sorption on the surface of oxidized nZVI (Karabelli et al. 2008; Üzüm et al. 2009; Yan et al. 2010). Both reduction and surface complexation (sorption) are observed when removing Ni2+ from the aqueous medium. The ability of nZVI to remove Ni2+ was experimentally determined to reach 0.13 g Ni2+/g Fe0. High-resolution X-ray photoelectron spectroscopy (HR-XPS) has shown that the amount of Ni0 on the surface of the nZVI increases with time. At equilibrium, about 50% of Ni2+ is reduced to Ni0 and 50% of Ni2+ remains adsorbed on the surface of iron nanoparticles. The surface complex is nickel hydroxide (Li and Zhang 2006).
Studies on the use of nZVI for the immobilization of heavy metals in soil have been initiated lately. Compared to the other heavy metals, most of the studies have focused on the immobilization of As (Gil-Díaz et al. 2014, 2017a; Vítková et al. 2017) and Cr in soil samples using nZVI (Xu and Zhao 2007). Franco et al. (2009), Di Palma et al. (2015) investigated the reduction of Cr6+ in soil in batch or column tests. Iron nanoparticles can also be used to remove heavy metals from the soil by washing (Mohamadiun et al. 2018). However, there is a lack of data that analyses the effectiveness of nZVI in immobilizing Cu and Ni in soil. Also, the interaction of nZVI with several heavy metals present in soil has been only slightly investigated.
In soil, Fe0 oxidizes and forms Fe hydroxides (Fe(OH)3). The used amount of iron should be up to 5% by weight. The application of more than 5% of Fe0 in soil can have negative effects on soil structure and vegetation (Mench et al. 2000).
The study primarily aimed at investigating the efficiency of immobilization of heavy metals cadmium (Cd), copper (Cu), lead (Pb), nickel (Ni) and their mixtures in soil using different doses of nZVI. To investigate the dependencies of immobilization efficiency on the amount of nZVI, the selected doses of Fe0 in soil samples were between 0 and 5.1% by weight. In this study, the available fraction of heavy metals was determined using CaCl2 solution. This is one of the methods used to determine the bioavailable fraction of heavy metals in the aqueous phase, which has similar ionic strength as many soil solutions. The removal of the same heavy metals from their aqueous solutions using nZVI was also investigated.
Materials and Methods
Chemicals and Iron Nanoparticles
The heavy metal salts Cd(NO3)2·4H2O, Cu(NO3)2·3H2O, Ni(NO3)2·6H2O, and Pb(NO3)2 were of analytical grade and were purchased from Merck, Darmstadt, Germany. All heavy metal solutions were prepared by dissolving the corresponding amounts of metal salts in deionized water. During the investigations, nZVI suspension (NANOFER 25S) purchased from Nano Iron s.r.o. (Czech Republic) was used. A detailed chemical composition of nZVI suspension is shown in Table 1.
According to the supplier information, the amount of Fe0 in the used suspension was 17%. Particles of nZVI are coated with polyacrylic acid, which stabilizes nanoparticles and prevents the nanoparticles from settling and agglomeration. The average particle size is 50 nm, the particle size distribution is 10–100 nm and the average surface area is 20–25 m2/g.
Investigation of Cd2+, Ni2+, Cu2+, Pb2+ Removal from Aqueous Solutions
To investigate how heavy metals are removed from aqueous solutions, individual aqueous solutions of heavy metals (Cd2+, Ni2+, Cu2+, Pb2+) and a solution with a mixture of all heavy metals were prepared. The pH of the prepared heavy metal solutions was not adjusted. The concentration of each heavy metal in the solutions was 250 mg/L. Experiments were carried out in 100 ml plastic bottles. 1 g of nZVI suspension (or 0.17 g of Fe0) was added to the solutions. The solutions were mixed for 3 h in rotoshaker (15 rpm) and then filtered through 0.45 μm cellulose acetate filters. Before and after the treatment, the pH of the solutions was determined. pH values were measured using Mettler Toledo SevenMulti pH metre. The same solutions of heavy metals without nanoparticles were also prepared. The uptake of heavy metals was calculated by dividing the difference between the amount of heavy metal ions added at the beginning and the residual amount after sorption from the amount of used nZVI. Experiments were performed in triplicate. The concentrations of heavy metals were determined using the atomic absorption spectroscopy analysis method using the Buck Scientific 2010 VGP. Depending on the concentration, heavy metals were analysed using a flame atomizer spectrophotometer or a electrothermal atomizer. Before each test, the solutions were acidified with a few drops of concentrated HNO3 acid.
Soil Sampling and Spiking
Sandy soil was collected from a forested place in Vilnius at a depth between 0 and 20 cm in the month of December. Inert materials were used for taking and storing samples. Before analysis, the samples were air dried, sieved (< 2 mm) and homogenized. Soil properties were analysed (Table 2).
Soil pH and electrical conductivity were determined according to ISO 10390. The ratio of soil to deionized water and soil to 1 M KCl suspension (w:v) was 1:5. The soil organic matter (OM) was calculated from the loss of ignition (LOI) and determined as a percentage loss of weight after ignition in a muffle furnace at a temperature of 550 °C for 2 h. Before the ignition, the soil sample was dried at 105 ± 2 °C to the constant mass. The texture of the soil was analysed following the hydrometer method (Bouyoucos 1962). Calcium carbonate equivalent (CCE) in soils was determined by the titration method. The cation exchange capacity (CEC) was determined using ammonium acetate.
Air-dried soil samples (200 g) were spiked with heavy metal solutions (soil/solution ratio was 2 g: 1 ml), which were prepared from the corresponding metal salts by dissolving them in deionized water. The target concentrations in the soil for heavy metals Cu, Pb, Ni were 1000 mg/kg, Cd—300 mg/kg. Soil samples were mixed with heavy metal nitrate solutions using a glass rod (about 5 min) so that the heavy metals could be evenly distributed in the soil. Spiked soil samples were incubated for 30 days (without humidity control) at room temperature (about 20 °C). After 30 days, the soil was air dried and sieved (< 2 mm).
The total concentration of heavy metals in soil samples was determined by acid digestion of 0.5 g of soil for 45 min using a mixture of 3 mL of nitric acid (69% purity) and 9 mL of hydrochloric acid (37% purity) in a microwave reaction system. After acid digestion, the solutions were filtered, poured into 50 mL flasks and diluted to 50 mL using deionized water.
The obtained concentrations of Cd, Cu, Ni, and Pb, in soil spiked with single metal, were 296 mg/kg, 1035 mg/kg, 836 mg/kg, 1214 mg/kg, respectively, and in soil spiked with three metals (Cu, Ni and Pb), the concentrations were 1056 mg/kg, 766 mg/kg, 1498 mg/kg, respectively, while in soil spiked with four metals (Cd, Cu, Ni, and Pb), the concentrations were 348 mg/kg, 1060 mg/kg, 828 mg/kg, 1654 mg/kg, respectively.
Experimental Setup
Batch leaching tests were performed to evaluate the efficiency of heavy metals immobilization using zero valent iron nanoparticles.
Soil samples (40 g) were placed in plastic vials of 100 ml and treated with nZVI suspension at the doses of 0, 5, 10, 15, and 30% by weight. This is the equivalent of applying 0, 0.85, 1.7, 2.55, and 5.1% of Fe0 or 0 mg, 8.5 mg, 17 mg, 25.5 mg, and 51 mg of Fe0 in 1 g of soil. Deionized water was added to reach a soil to water ratio of 2:1 (w:w). The soil was thoroughly mixed with nZVI suspension. Then, the mixture of soil and nZVI was air dried.
The effectiveness of immobilization was investigated after 1 month. Control tests were performed in parallel with the same amount of soil and deionized water without nZVI. Each test was performed in duplicate.
The leaching procedure according to Houba et al. (2000) was used to determine the proportion of bioavailable elements in the soil. 10 g of soil was transferred to 150 mL glass vials and a 100 mL of 0.01 M CaCl2 extraction solution was added to obtain a ratio of 1:10 (m:V). Samples were mixed in a rotoshaker for 2 h. The liquid was filtered and acidified with few drops of 65% HNO3.
Results and Discussion
Removal of Heavy Metals from Aqueous Solutions
Table 3 shows the removal of single heavy metal cations from aqueous solutions. The highest removal efficiencies were observed for Cu2+ and Pb2+ and exceeded 99%. Cu2+ and Pb2+ electrode potentials (E0) are much more positive than Fe. The electrode potentials of copper and lead electrodes are 0.338 V (Cu2+/Cu) and − 0.126 V (Pb2+/Pb), respectively. A higher potential difference generates a higher driving force of electrons’ transport from nZVI to mentioned metal ions. The efficiency of removal of cadmium, whose electrode potential (− 0.404 V) is close to iron, was the lowest.
Table 4 shows the removal of heavy metal cations from multi-metal solution. As in the single metal treatment, the highest removal efficiencies were observed for Cu2+ and Pb2+, however, the removal of Cd2+ and Ni2+ decreased significantly. The competition between different metal cations led to a relatively low Ni and Cd removal efficiency. Also, the lower efficiency of nZVI could have been due to the fact that the pH value of the mixture of heavy metals was small and reached ~ 5. At low pH, nanoparticles have a positive charge and attract anionic ligands. When the pH of the solution is above the isoelectric point (zero point of charge of nZVI ≈ 8), the surface of nZVI becomes negatively charged and the sorption of metal cations is more favourable (Li et al. 2006).
After measuring the pH of the heavy metal solutions before and after the use of nZVI, pH of all solutions (except for Pb solution) decreased. After the treatment, the pH of the solution containing Pb ions increased from 5.49 to 9.09. One of the reasons for the decrease in pH is that soluble iron (Fe2+) forms compounds with OH− ions and more free H+ ions can appear in the solutions. After analysing the amount of iron in solutions after the use of nZVI (analysis was done at pH < 2), it was observed that if the solution had a high pH, it contained less iron. After the use of nZVI, the lowest iron content (0.77 mg/L) was in a solution with a pH of 9.09, and the maximum iron concentration (222 mg/L) was in a solution with a pH of 4.46 (in a solution of all heavy metals). In addition, the consumption of OH− ions in the reactions with the remaining heavy metals reduced the pH of solutions.
The Immobilization of Heavy Metals in Soil
Soil pH
Soil pH is one of the parameters that determine the mobility of heavy metals. Soil pH (using 1 M KCl) was determined before and after the treatment of the soil with nZVI. The pH dependence of soil samples spiked with different heavy metals on the amount of nZVI suspension used is shown in Table 5.
The pH of used nZVI suspension was 11.5. Contrary to the removal of heavy metals from aqueous solutions in which the pH of the solutions (except for Pb solution) decreased after the application of nanoparticles, the use of nZVI in the soil increased its pH. As can be seen from Table 3, the soil pH increased in all cases by increasing the amount of nZVI in the soil. The highest pH increment was found in soil spiked with mixtures of heavy metals: from 4.91 to 6.62 when the soils were spiked with three heavy metals and from 4.98 to 6.65 when the soils were spiked with four heavy metals.
Immobilization of Heavy Metals
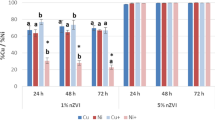
The leaching of heavy metals was tested using the CaCl2 solution. As can be seen from Fig. 2, in all cases, it was found that an increasing amount of nZVI in the soil resulted in a decrease of the leaching of all analysed heavy metals. The increasing pH of the soil, when the amount of nZVI increased, was one of the reasons for the decrease of heavy metal leaching. The increase of OH− anions in soil also causes the precipitation of studied metal cations. So, the increase of the immobilization efficiency is influenced by the increase of both soil pH and nZVI dose.
While performing tests in aqueous solutions, heavy metal removal efficiency was significantly reduced in a solution containing a mixture of heavy metals. However, the higher effectiveness of heavy metal immobilization was observed in soil samples spiked with several heavy metals (Fig. 2). In the control samples spiked with several heavy metals, the pH of the soil was below 5, and the leaching of metals was much higher than that of soil samples spiked with single heavy metals. Increasing the amount of nZVI suspension in soils spiked with several heavy metals from 0 to 30% increased its pH much more than in soils that were spiked with single heavy metals. This could have been one of the reasons why the effectiveness of immobilization was higher in soils spiked with several heavy metals. Also, one of the reasons for more efficient immobilization in the case of soil spiked with several heavy metals is that Ni and Cu act as catalysts and they are reduced by Fe0 forming bimetallic nanoparticles, i.e., y Fe0/Ni0 and Fe0/Cu0 (O’Carroll et al. 2013). According to other researchers (Hu et al. 2010; Schrick et al. 2002), the reactivity of bimetallic nanoparticles is higher than that of iron nanoparticles. Such catalysts can be used to improve the efficiency of heavy metal immobilization, taking into account that the soil may be spiked with several heavy metals (Lien et al. 2007).
Figure 1 shows the images of untreated and treated soil samples. Images of treated soil samples were taken after 1 month after immobilization. Using 30% nZVI in the soil, after immobilization it was observed that the soil spiked with Cu and Pb got a dark red colour. This indicates that excess amounts of nZVI have been used and there are a lot of unreacted iron nanoparticles in the soil that were oxidized to trivalent iron. Figure 1b and c show red Fe(III) oxide deposits on soil particles.
The image of soil samples (40 times magnification by Motic optical microscope): a soil spiked with Cd + Cu + Ni +Pb without nZVI; b soil spiked with Pb and treated with nZVI suspension (30% by weight); c soil spiked with Cu and treated with nZVI suspension (30% by weight); d soil spiked with Cd and treated with nZVI suspension (30% by weight); e soil spiked with Ni and treated with nZVI suspension (30% by weight); f soil spiked with Cd + Cu + Pb + Ni and treated with nZVI suspension (30% by weight)
In cases where the soil was spiked with heavy metal mixtures, the soil colour was almost unchanged (since the soil was spiked with several heavy metals, a major portion of nZVI was used for reactions with heavy metals).
Since Cu and Pb formed complexes with soil organic matter and were bound, in the soil samples which were spiked with Cu and Pb, a small amount of nZVI reacted with the mentioned metals.
The dependencies of leaching (mg/kg) of heavy metals and the effectiveness of immobilization on the used quantity of nZVI is presented in Fig. 2.
With increasing concentrations of nZVI suspension in the soil from 0 to 30%, cadmium leaching decreased when the soil was spiked with only Cd and when the soil was spiked with a mixture of heavy metals. Cadmium is a very mobile metal in the soil. When the soil was spiked only with Cd, its leaching was 44.7 mg/kg, i.e., 15.1% of Cd has been leached from total Cd content. After using the dose of 5%, 10%, 15%, 30% of nanoparticles in soil, Cd leaching decreased, respectively, to 5.9%, 4.0%, 3.7%, 2.9% (Fig. 2a). Compared to the other heavy metals (in soil samples spiked with single metal), the efficiency of cadmium immobilization was the lowest. Also, the removal of Cd from aqueous solutions using the same nZVI was the lowest. The lowest Cd immobilization efficiency in soil spiked with single metal was obtained by Gil-Díaz et al. (2017a, b). When soil was spiked only by Cd, the immobilization efficiency was 61%, 73%, 76%, 81%, respectively (Fig. 2b). One of the reasons why Cd immobilization in the soil was the lowest is that Cd2+ ions are sequestrated on the iron (hydr)oxide shell only by adsorption (Soto-Hidalgo and Cabrera 2018).
In untreated soil spiked with several heavy metals, the leaching of Cd reached 117 mg/kg, i.e., 33.6% of Cd had been leached from the total Cd content. After using 5%, 10%, 15%, 30% of nZVI suspension in soil, Cd leaching decreased, respectively, to 11%, 4.9%, 2.1%, 0.74% of total Cd content in soil. When the soil was spiked with four different metals, the immobilization efficiency was higher than when the soil was spiked only with Cd. With increasing concentration of Fe0 in the soil, the Cd immobilization efficiency was 68%, 85%, 94%, 98%. Using the same nanoparticles, Gil-Díaz et al. (2017a, b)studied heavy metal immobilization and found that in the calcareous soil spiked with several heavy metals, at nZVI dose of 5% and 10%, the percentage of exchangeable Cd in the soil decreased more than in the soil spiked only with Cd.
With increasing concentrations of nZVI suspension in the soil from 0 to 30%, the leaching of Cu decreased from both single and multi-metal spiked soil. Cu is a slightly mobile metal in the soil and its available fraction in the soil was very small. Cu has a high affinity for organic matter and insoluble organic matter can bind significant amounts of Cu in soil (Kumpiene et al. 2008). From the control samples, where the soil was spiked only with copper, its leaching amounted to 8.83 mg/kg, i.e., only 0.85% of the total Cu content had been leached. After using 5%, 10%, 15%, 30% of nZVI suspension in soil, Cu leaching decreased to 0.29%, 0.25%, 0.17%, 0.15%, respectively (Fig. 2c). The efficiency of copper immobilization reached 66%, 71%, 81%, 82%, respectively. Cu2+ ions adsorb to iron (hydr)oxides as inner-sphere complexes (Peacock and Sherman 2004). The increase of immobilization efficiency was also influenced not only by the increase of nZVI dose, but also by the increase of pH. Cu2+ adsorption to nZVI and soil organic matter increases with increasing pH (Tiberg et al. 2016). As only a small amount of Cu was available in soil, a large part of Fe0 nanoparticles was unused in the reaction with mentioned metal. This caused the change in soil colour from dark to reddish brown.
In the untreated soil spiked with several heavy metals, the leaching of Cu was higher and amounted to 2.0% of the total Cu content in the soil. As it can be seen from Fig. 2d, the efficiency of immobilization was higher when the soil was spiked with several heavy metals. When soil samples were spiked with three heavy metals, using 5%, 10%, 15%, 30% of nZVI suspension in soils, the Cu immobilization efficiency was 79.1%, 81.6%, 82.2%, and 87.6%, respectively. When soil samples were spiked with four heavy metals, using 5%, 10%, 15%, 30% of nZVI suspension in soils, the Cu immobilization efficiency was high and amounted to 85%, 87%, 89%, 92%, respectively.
Like cadmium, nickel is a very mobile metal in the soil. E0 of Ni is slightly more positive than E0 of Fe. Therefore, the mechanisms of immobilization of Ni2+ in soil are reduction and adsorption on nZVI. With increasing concentrations of nZVI suspension in the soil from 0 to 30%, the leaching of Ni decreased from both single and multi-metal spiked soil. When the soil was spiked only with Ni, its leaching from control sample was 12% of the total Ni contained in the soil. After using 5%, 10%, 15%, 30% of nZVI suspension in soil, Ni leaching, respectively, decreased to 2.34%, 0.25%, 0.17%, 0.15% of the total amount of it in soil. The efficiency of nickel immobilization was the highest compared to other metals (in cases where the soil was spiked with one metal) and, respectively, reached 80%, 81%, 86%, 90%.
When the soils were spiked with several heavy metals, the percentage of leached Ni from untreated soil reached 36% (Cu + Ni + Pb) and 45% (Cd + Cu + Ni + Pb) from the total amount of Ni in soil. Compared to the other heavy metals, the Ni immobilization efficiency was the highest in multi-metal spiked soils (except in the case of Pb, the immobilization efficiency of which was the greatest with 5% and 10% of nZVI suspension used in soil). When soil samples were spiked with three heavy metals, using 5%, 10%, 15%, 30% of nZVI suspension in soil, resulted in Ni immobilization efficiency of 83.5%, 92.4%, 96.8%, 98.6%, respectively. When soil samples were spiked with four heavy metals, using 5%, 10%, 15%, and 30% of nZVI suspension in soil, resulted in Ni immobilization efficiency of 94.8%, 95.7%, 98.1%, 99.3%, respectively. One of the reasons why Ni immobilization efficiency was very high is that its sorption in the soil was low and that a large portion of Ni was exposed to nanoparticles.
With increasing dose of nZVI in the soil, the leaching of Pb decreased both from the soil which was spiked only with Pb and from soil spiked with several heavy metals. Pb is a slightly mobile metal in soil. When the soil was spiked only with Pb, its leaching reached 0.4% of the total Pb contained in the soil. Pb leaching decreased to 0.11%, 0.09%, 0.06%, 0.05% of total Pb in soil after treating the soil with 5%, 10%, 15%, 30% of nZVI suspension (Fig. 2g). Pb immobilization efficiency was 74%, 79%, 85%, and 87%, respectively. As only a small amount of Pb was available in soil, a large part of nZVI remained unused. As it was mentioned before, this caused the change in soil colour from dark to reddish brown.
When the soils were spiked with several heavy metals, the percentage of leached Pb from the total amount was higher and reached 0.62% (Cu + Ni + Pb) and 0.93% (Cd + Cu + Ni + Pb). Compared to the control samples, the leaching of Pb was most decreased in soil spiked with several heavy metals. When soil samples were spiked with three heavy metals, using 5%, 10%, 15%, 30% nZVI suspension in soil, Pb immobilization efficiency was 91.8%, 92.5%, 94.7%, and 95.2%, respectively. When soil samples were spiked with four heavy metals, using 5%, 10%, 15%, 30% nZVI suspension in soil, immobilization efficiency of Pb was 96.3%, 97.0%, 97.2%, 97.9%, respectively. As E0 of Pb is slightly more positive than E0 of Fe, the mechanisms of immobilization of Pb2+ in soil were reduction and adsorption on nZVI surface.
Conclusions
The immobilization of Cd2+, Cu2+, Ni2+, and Pb2+ using nZVI reduced the available fraction of heavy metals in the soil. The immobilization of heavy metals, when the soil was spiked with single heavy metals and their mixtures was different: immobilization efficiency in all cases was higher in soils which were spiked with several heavy metals. In both cases, the best immobilization was found for Ni. While individually immobilizing heavy metals, the worst immobilization efficiency was for Cd, however, as the amount of nZVI in the soil increased, Cd immobilization efficiency has increased the most compared to other heavy metals. With the use of 15% and 30% of nZVI suspension in soil spiked with several heavy metals, immobilization efficiency of Cd reached over 90%. However, such an amount of nZVI could be used in industrial contaminated sites, where it is important to control heavy metal migration.
Although the removal of Cu and Pb from aqueous solutions was the highest, the immobilization efficiency of Cu and Pb in single metal polluted soil was only slightly higher than Cd. This can be explained by the fact that there was a considerable amount of organic matter in the soil in which these metals were sorbed. For Cu and Pb immobilization in soils with high insoluble organic matter content, it is recommended not to use higher than 5% of nZVI suspension according to the soil mass. Also, further experiments are needed to evaluate the effectiveness of nZVI in other types of soils.
References
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186:458–465. https://doi.org/10.1016/j.jhazmat.2010.11.029
Boparai HK, Joseph M, O’Carroll DM (2013) Cadmium (Cd2+) removal by nano zerovalent iron: surface analysis, effects of solution chemistry and surface complexation modeling. Environ Sci Pollut Res 20:6210–6221. https://doi.org/10.1007/s11356-013-1651-8
Bouyoucos GJ (1962) Hydrometer method improved for making particle size analyses of soils. Agron J 54:464–465. https://doi.org/10.2134/agronj1962.00021962005400050028x
Cook S (2009) Assessing the use and application of zero-valent iron nanoparticle technology for remediation at contaminated sites. Jackson State University, Jackson; U.S. EPA, Office of Solid Waste and Emergency Response Office of Superfund Remediation and Technology Innovation, Washington, DC
Di Palma L, Gueye MT, Petrucci E (2015) Hexavalent chromium reduction in contaminated soil: a comparison between ferrous sulphate and nanoscale zero-valent iron. J Hazard Mater 281:70–76. https://doi.org/10.1016/j.jhazmat.2014.07.058
Franco DV, Da Silva LM, Jardim WF (2009) Reduction of hexavalent chromium in soil and ground water using zero-valent iron under batch and semi-batch conditions. Water Air Soil Pollut 197:49–60. https://doi.org/10.1007/s11270-008-9790-0
Gil-Díaz M, Alonso J, Rodríguez-Valdés E et al (2014) Reducing the mobility of arsenic in brownfield soil using stabilised zero-valent iron nanoparticles. J Environ Sci Heal-Part A Toxic/Hazard Subst Environ Eng 49:1361–1369. https://doi.org/10.1080/10934529.2014.928248
Gil-Díaz M, Alonso J, Rodríguez-Valdés E et al (2017a) Comparing different commercial zero valent iron nanoparticles to immobilize As and Hg in brownfield soil. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.02.011
Gil-Díaz M, Pinilla P, Alonso J, Lobo MC (2017b) Viability of a nanoremediation process in single or multi-metal(loid) contaminated soils. J Hazard Mater 321:812–819. https://doi.org/10.1016/j.jhazmat.2016.09.071
Houba VJG, Temminghoff EJM, Gaikhorst GA, van Vark W (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal 31:1299–1396. https://doi.org/10.1080/00103620009370514
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39:1291–1298. https://doi.org/10.1021/es048991u
Kanel SR, Grenèche J-M, Choi H (2006) Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol 40:2045–2050. https://doi.org/10.1021/es0520924
Karabelli D, Çaǧri Ü, Shahwan T et al (2008) Batch removal of aqueous Cu2+ions using nanoparticles of zero-valent iron: a study of the capacity and mechanism of uptake. Ind Eng Chem Res 47:4758–4764. https://doi.org/10.1021/ie800081s
Klimkova S, Cernik M, Lacinova L et al (2011) Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere 82:1178–1184. https://doi.org/10.1016/j.chemosphere.2010.11.075
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag. https://doi.org/10.1016/j.wasman.2006.12.012
Li XQ, Zhang WX (2006) Iron nanoparticles: the core–shell structure and unique properties for Ni(II) sequestration. Langmuir 22:4638–4642. https://doi.org/10.1021/la060057k
Li X, Zhang W (2007) Sequestration of metal cations with zerovalent iron nanoparticles: a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946. https://doi.org/10.1021/jp0702189
Li XQ, Elliott DW, Zhang WX (2006) Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State Mater Sci 31:111–122. https://doi.org/10.1080/10408430601057611
Liang W, Dai C, Zhou X, Zhang Y (2014) Application of zero-valent iron nanoparticles for the removal of aqueous zinc ions under various experimental conditions. PLoS One. https://doi.org/10.1371/journal.pone.0085686
Lien H-L, Jhuo Y-S, Chen L-H (2007) Effect of heavy metals on dechlorination of carbon tetrachloride by iron nanoparticles. Environ Eng Sci 24:21–30. https://doi.org/10.1089/ees.2007.24.21
Mench M, Vangronsveld J, Clijsters H et al (2000) Phytoremediation of contaminated soil and water. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, Boca Raton, pp 327–362
O’Carroll D, Sleep B, Krol M et al (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122. https://doi.org/10.1016/j.advwatres.2012.02.005
Peacock CL, Sherman DM (2004) Copper(II) sorption onto goethite, hematite and lepidocrocite: a surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim Cosmochim Acta. https://doi.org/10.1016/j.gca.2003.11.030
Ponder SM, Darab JG, Mallouk TE (2000) Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol 34:2564–2569. https://doi.org/10.1021/es9911420
Soto-Hidalgo KT, Cabrera CR (2018) Nanoscale zero valent iron for environmental cadmium metal treatment. Green Chemistry. InTech, London
Tiberg C, Kumpiene J, Gustafsson JP et al (2016) Immobilization of Cu and As in two contaminated soils with zero-valent iron—long-term performance and mechanisms. Appl Geochem 67:144–152. https://doi.org/10.1016/j.apgeochem.2016.02.009
Üzüm Ç, Shahwan T, Eroǧlu AE et al (2009) Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl Clay Sci 43:172–181. https://doi.org/10.1016/j.clay.2008.07.030
Vítková M, Rákosová S, Michálková Z, Komárek M (2017) Metal(loid)s behaviour in soils amended with nano zero-valent iron as a function of pH and time. J Environ Manage 186:268–276. https://doi.org/10.1016/j.jenvman.2016.06.003
Wang W, Hua Y, Li S et al (2016) Removal of Pb(II) and Zn (II) using lime and nanoscale zero-valent iron (nZVI): a comparative study. Chem Eng J 304:79–88
Wuana RA, Okieimen FE (2011) Heavy Metals in Contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol 2011:1–20. https://doi.org/10.5402/2011/402647
Xi Y, Mallavarapu M, Naidu R (2010) Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron—a SEM, TEM and XPS study. Mater Res Bull 45:1361–1367. https://doi.org/10.1016/j.materresbull.2010.06.046
Xu Y, Zhao D (2007) Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res 41:2101–2108. https://doi.org/10.1016/j.watres.2007.02.037
Yan W, Herzing AA, Kiely CJ, Zhang WX (2010) Nanoscale zero-valent iron (nZVI): aspects of the core-shell structure and reactions with inorganic species in water. J Contam Hydrol 118:96–104. https://doi.org/10.1016/j.jconhyd.2010.09.003
Zeng J, Zhou S, Lv L et al (2018) Soil heavy metal contamination in rural land consolidation areas in the Yangtze River Delta, China. J Environ Eng Landsc Manag 26:28–37. https://doi.org/10.3846/16486897.2017.1346512
Zhao X, Liu W, Cai Z et al (2016) An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res 100:245–266. https://doi.org/10.1016/j.watres.2016.05.019
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasarevičius, S., Danila, V. & Paliulis, D. Application of Stabilized Nano Zero Valent Iron Particles for Immobilization of Available Cd2+, Cu2+, Ni2+, and Pb2+ Ions in Soil. Int J Environ Res 13, 465–474 (2019). https://doi.org/10.1007/s41742-019-00187-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-019-00187-8