Abstract
We carried out field studies and laboratory experiments to assess the impact of fluoride (F−) and turbidity on the freshwater snail Physella acuta in a polluted river receiving an industrial effluent (the middle Duraton River, Central Spain). Fluoride concentrations and turbidity levels significantly increased downstream from the industrial effluent (with the highest values being 0.6 mg F−/L and 55.2 nephelometric turbidity unit). In addition, higher deposition of fine inorganic matter was evident at polluted sampling sites. Conversely, the abundance of P. acuta significantly declined (until its virtual disappearance) downstream from the industrial effluent. Toxicity bioassays showed that P. acuta is a relatively tolerant invertebrate species to fluoride toxicity, with estimated safe concentrations (expressed as LC0.10 values for infinite hours of exposure) for juvenile and adult snails being 2.4 and 3.7 mg F−/L, respectively. Furthermore, juvenile snails (more sensitive than adult snails) did not show significant alterations in their behavior through 15 days of exposure to 2.6 mg F−/L: mean values of the proportion of test snails located on the water surface habitat, as well as mean values of the sliding movement rate (velocity) of test snails, never showed significant differences when comparing control and treatment glass vessels. It is concluded that instream habitat degradation, derived from increased turbidity levels, might be a major cause for significant reductions in the abundance of P. acuta downstream from the industrial effluent. The presence of the competing gastropod Ancylus fluviatilis could also affect negatively the recovery of P. acuta abundance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride (F−) must be regarded as a serious pollutant since its concentration in many aquatic ecosystems is significantly increasing because of man’s activities (World Health Organization 2002; Camargo 2003; Weinstein and Davison 2004; Jha et al. 2011; Chai et al. 2016). Aluminum and zinc smelters, phosphate fertilizer plants, plants manufacturing brick, ceramics and glass, the production and use of fluoride chemicals, and discharges of fluoridate municipal waters can cause significant increases in the fluoride concentration of surface waters (in some cases more than 100 times the natural background fluoride levels), resulting in considerable toxicity to sensitive aquatic animals (World Health Organization 2002; Camargo 2003; Weinstein and Davison 2004; Jha et al. 2011; Chai et al. 2016). Furthermore, field and laboratory studies indicate that, even at low pollution levels (0.5–1.0 mg F−/L), fluoride ions can adversely affect the normal behavior and competitive abilities of sensitive fish and freshwater invertebrates (Damkaer and Dey 1989; Camargo et al. 1992; Camargo 2003).
In spite of the current worldwide environmental concern about increased fluoride levels in surface waters, comparatively few studies have been conducted to assess the impact of those increased fluoride concentrations on aquatic animals (World Health Organization 2002; Camargo 2003; Weinstein and Davison 2004; Aguirre-Sierra et al. 2011; Alonso and Camargo 2012; Chai et al. 2016). Actually, the toxic effects of fluoride on freshwater gastropods have seldom been investigated, despite aquatic snails are common in freshwater ecosystems around the world, playing an important role within the food webs of these ecological systems (Dillon 2000; Tachet et al. 2003; Strong et al. 2008; Thorp and Covich 2010). As far as we know, fluoride toxicity has been only examined in the aquatic mud snail Potamopyrgus antipodarum (Aguirre-Sierra et al. 2011; Alonso and Camargo 2012).
On the other hand, fine inorganic suspended particulates and the ensuing water turbidity represent an anthropogenic environmental problem of increasing concern for freshwater ecosystems around the world (Quinn et al. 1992; Wood and Armitage 1997; Henley et al. 2000; Rowe et al. 2002; Suren et al. 2005; Bilotta and Brazier 2008; Bryce et al. 2010; Jones et al. 2012; Chapman et al. 2014; Naden et al. 2016). Although certain laboratory experiments (Rowe et al. 2002; Suren et al. 2005) have showed that supposedly sensitive benthic invertebrates, such as insects and crayfish, may be tolerant to the direct toxicity of high turbidity levels from fine suspended clay (up to 20,000 nephelometric turbidity unit (NTU) in short-term exposures and up to 1000 NTU in long-term exposures), numerous field studies have however showed that increases in water turbidity of rivers and streams can reduce light transmission and intensify siltation of suspended solids, these altered processes causing reductions in the abundance of sensitive benthic invertebrates due to decreases in food supply and quality, and also owing to alterations in available and suitable benthic substratum (Quinn et al. 1992; Wood and Armitage 1997; Henley et al. 2000; Bilotta and Brazier 2008; Bryce et al. 2010; Jones et al. 2012; Chapman et al. 2014; Naden et al. 2016).
Given the scarce information on fluoride toxicity to freshwater gastropods, and also the limited information on the adverse effects of turbidity on these mollusks, we carried out field studies and laboratory experiments to assess the impact of fluoride (F−) and turbidity on the bladder snail Physella acuta (Draparnaud, 1805) in a polluted river receiving an industrial effluent. Secondary aims were to estimate safe concentrations of fluoride for juveniles and adults of P. acuta, to evaluate the suitability of these estimated safe concentrations through behavioral toxicity testing, and to compare the tolerance of P. acuta to fluoride toxicity with previously published data regarding other freshwater benthic invertebrates.
P. acuta belongs to the monophyletic taxonomic family Physidae and, as other aquatic pulmonate gastropods, breathes via a lung-like pulmonary cavity located within the mantle, taking oxygen from the water or even from the air (Dillon 2000; Tachet et al. 2003; Thorp and Covich 2010). This physiological ability lets physids and other lunged aquatic snails use the surface environment of water, and even leave the water, to mitigate the effects of environmental stressors (e.g., aquatic predators) (Turner et al. 2000; Dalesman et al. 2007; Bernot and Brandenburg 2013). Other accepted physid species, such as Physa acuta, Physa (Physella) integra, and Physa (Physella) heterostropha, are currently recognized to be synonyms of P. acuta (Dillon et al. 2002; Anderson 2003; Soler et al. 2006; Thorp and Covich 2010). At present, P. acuta may be found in both lotic and lentic freshwater ecosystems of Europe, America, Africa, Asia, Australia, and New Zealand, being regarded as an invasive species in many geographical regions (Burch 1989; Dillon et al. 2002; Anderson 2003; Appleton 2003; Tachet et al. 2003; Cope and Winterbourn 2004; Soler et al. 2006; Albrecht et al. 2009; Zukowski and Walker 2009; Thorp and Covich 2010). Because of its widespread distribution around the world, P. acuta is usually considered to be tolerant to harsh environmental conditions, including water pollution. Nevertheless, certain laboratory studies have showed that P. acuta may be relatively sensitive to the toxicity of ionic compounds (Bernot et al. 2005; Zalizniak et al. 2009) and metal nanoparticles (Musee et al. 2010; Bernot and Brandenburg 2013).
Materials and methods
Field studies in the middle Duraton River
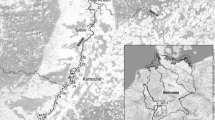
We carried out field studies in the middle Duraton River (Central Spain, Duero River Basin), in the vicinity of Burgomillodo Reservoir (Fig. 1). About 300 m downstream from Burgomillodo Dam, an industrial effluent enters Duraton River. During the industrial process, hydrofluoric acid (HF) is used to induce charge separation of silica and feldspar from sandy materials, which are subsequently used as raw materials for manufacturing ceramics and glass at other industrial plants. In spite of the industry’s wastewater treatment system, fluoride (F−) and suspended inorganic matter are discharged by the industrial effluent into the recipient river, causing fluoride pollution and increased turbidity downstream.
For the purpose of this investigation, three sampling sites (S-1, S-2, and S-3) were selected along the study area (Fig. 1). S-1 was placed about 0.2 km downstream from Burgomillodo Dam, subsequent to a small man-made waterfall (about 1 m in height) built to reinforce the re-oxygenation of hypolimnial waters released by the dam. This sampling site was used as a reference station. S-2 and S-3 were placed about 0.1 and 2.2 km downstream from the industrial effluent, respectively. The river bottom was mainly stony with cobbles and pebbles, but with an apparent deposition of fine inorganic matter at polluted sampling sites, particularly at S-2 where it was covered by a relatively thick layer of sandy sediment.
Sampling surveys to analyze river water properties were carried out through the summer of 2014. Water velocity, temperature, pH, and dissolved oxygen were analyzed in situ using specific meters according to standard guidelines (American Public Health Association 1998). In addition, water samples for analyzing turbidity and fluoride were collected at each sampling site using clean polyethylene containers. In the laboratory, turbidity was directly analyzed with a standard turbidimeter (American Public Health Association 1998). To analyze fluoride, water samples were first filtered through pre-rinsed 0.45-μm cellulose acetate filters, and then, water filtrates were used to determine fluoride concentrations by the standard SPADNS colorimetric method (American Public Health Association 1998). Throughout the whole sampling period, 12 measurements were taken for water velocity, temperature, pH, and dissolved oxygen and 6 measurements for turbidity and fluoride, at each sampling site.
A sampling survey to examine the abundance of aquatic gastropods at sampling sites was carried out in September 2014. A quantitative cylindrical bottom sampler (Hauer and Lamberti 1996; Wetzel and Likens 2000), which enclosed a sampling area of about 0.1 m2 and had a capturing net with a mesh size of 250 μm, was used to take five riffle bottom samples at each sampling site. Collected animals were preserved in 4% formalin until laboratory analyses. In the laboratory, aquatic snails were identified and counted using a light binocular stereomicroscope.
Laboratory experiments on fluoride toxicity
In order to examine the tolerance of P. acuta to the toxicity of fluoride ions, we conducted laboratory experiments on fluoride toxicity. P. acuta snails were obtained from the unpolluted Buendía Reservoir (Central Spain, Tajo River Basin). Animals were transported to our research laboratory using clean polyethylene containers. No mortality occurred during transportation. They were stocked in glass aquaria (filled with 30 L dechlorinated tap water) for acclimatization during 2 weeks. Cladophora and Chara algae from Buendia Reservoir were introduced into glass aquaria to provide natural food to aquatic snails.
After acclimatization, a static (with water renovation) short-term (4 days) toxicity bioassay was conducted in triplicate using 18 small glass vessels (each one with 150 mL of dechlorinated tap water) and 180 animals. Test snails were exposed to five nominal fluoride concentrations (50, 100, 150, 200, and 300 mg F−/L), plus a control (0.1 mg F−/L). These fluoride treatments were selected on the basis of previous short-term toxicity studies with juveniles and adults of the aquatic mud snail Potamopyrgus antipodarum (Aguirre-Sierra et al. 2011). To examine the effect of body size on the tolerance of P. acuta to fluoride toxicity, two groups of test snails with a clear difference in body size were established: in the group of smaller snails, mean shell height (±SD) was 3.3 ± 0.5 mm; in the group of larger snails, mean shell height (±SD) was 6.8 ± 0.7 mm. Because previous studies indicate that P. acuta can reach the adult stage at about 6 mm of shell height (Dillon 2000; Dillon et al. 2002; Cope and Winterbourn 2004; Bernot et al. 2005; Maqboul et al. 2014), we will refer to the group of smaller snails as juveniles and to the group of larger snails as adults.
The short-term toxicity bioassay was carried out using 10 snails (5 juveniles + 5 adults) per vessel/replicate and under a natural photoperiod. The main reason to test two body size groups of P. acuta snails at the same experimental units (glass vessels) was to ensure that both body size groups were exactly exposed to the same test fluoride concentrations and under the same water environmental conditions (temperature, pH, dissolved oxygen, and total hardness). Apparently, the only factor causing interference between both body size groups could be competition for space and food. However, at each experimental unit (glass vessel), there was enough space for the 10 snails, and also, all snails were fed, ad libitum, with Cladophora and Chara algae, placing pieces of these algae on the bottom of the glass vessels. In consequence, responses of juvenile and adult snails to fluoride toxicity may be considered independent of any interference derived from testing both body size groups at the same glass vessels.
Fluoride treatments were made from sodium fluoride (NaF ≥ 99%, Sigma, Germany). Mean values (±SD) of measured fluoride concentrations at each treatment were 44.7 ± 3.6, 90.8 ± 7.4, 136 ± 9.2, 174 ± 12.5, and 268 ± 20.3 mg F−/L. These fluoride concentrations were determined by the standard SPADNS colorimetric method (American Public Health Association 1998). Because some fluoride was expected to precipitate as calcium fluoride (CaF2), measured fluoride concentrations resulted to be lower than their respective nominal concentrations. Average values of water environmental conditions were 21.5 °C for temperature, 8.1 for pH, 6.2 mg O2/L for dissolved oxygen, and 147 mg CaCO3/L for total hardness. Water temperature, pH, and dissolved oxygen were measured using specific meters (American Public Health Association 1998). Total hardness was determined by the standard calmagite colorimetric method (American Public Health Association 1998). No aeration was provided during the toxicity bioassay since it is not needed for pulmonate gastropods (Bernot and Brandenburg 2013). In order to reduce evaporation and also prevent the escape of snails, glass vessels were covered with perforated plastic surfaces. Water renewal in glass vessels (including controls) was carried out after 48 h of starting the toxicity bioassay.
Dead snails were daily checked and removed. Snails were designated as dead when they remained immobile on (and no longer holding on to) the bottom of glass vessels, exhibiting no reaction of their body parts (foot, shell, tentacles) during 2 min after gentle prodding with a glass rod. Observations of test snails were facilitated by the use of a ×3 magnifying hand glass. In the case of snails being observed to stand on the water surface habitat (the water line or even out of the water), they were placed again on the bottom of their respective glass vessels using forceps. Recent laboratory studies (Bernot and Brandenburg 2013) have showed that physid snails can concentrate in the surface environment of water not only to avoid the action of aquatic predators but also to mitigate the toxicity of chemical substances.
A second toxicity bioassay was conducted to assess the tolerance of P. acuta to a lower nonlethal concentration of fluoride, but for a longer exposure time (15 days), using six glass vessels (each one with 150 mL of dechlorinated tap water) and 30 juvenile snails (more sensitive to fluoride toxicity than adult snails; see results in Table 2). Test animals were obtained from the unpolluted Buendia Reservoir and acclimatized to water laboratory conditions in stock glass aquaria for 2 weeks. After acclimatization, snails were exposed to a nominal fluoride treatment of 3 mg F−/L (measured fluoride concentration of 2.6 ± 0.2 mg F−/L), plus a control (0.1 mg F−/L), for 15 days. This fluoride treatment was selected on the basis of the estimated safe concentration for juvenile snails (see results in Table 2). The toxicity bioassay was conducted in triplicate using five snails per vessel/replicate. Mean values (±SD) of shell height for test snails at control and treatment vessels were 3.6 ± 0.4 and 3.4 ± 0.5 mm, respectively, with no significant difference (P > 0.05) between body sizes. Average values of water environmental conditions were 20.7 °C for temperature, 8.0 for pH, 6.6 mg O2/L for dissolved oxygen, and 135 mg CaCO3/L for total hardness. Test snails were fed, ad libitum, with Cladophora and Chara algae, placing pieces of these algae on the bottom of the glass vessels. In order to reduce evaporation and also prevent the escape of snails, glass vessels were covered with perforated plastic surfaces. Water renewal in glass vessels (including controls) was carried out every 3 days. As in the case of the first toxicity bioassay, test snails were placed on the bottom of their respective glass vessels just before the start of this second toxicity bioassay and also after each water renewal.
In addition to unlikely mortality, we checked every 3 days (before each water renewal) the proportion of test snails located on the water surface habitat (the water line or even out of the water) and the sliding movement rate of test snails. This sliding movement rate (expressed as mm/s) was determined using 1-mm square grid papers, which were placed under the bottom of glass vessels. With forceps, each snail was placed with the shell aperture down on the midpoint of the bottom of its respective glass vessel, and then, just after starting to slide, the distance covered by the snail during 10 s was recorded. The sliding movement rate (velocity) is a sensitive behavioral endpoint that may be used for assessing the impact of chemical pollutants on aquatic snails (Alonso and Camargo 2012; Bernot and Brandenburg 2013; López-Doval et al. 2014). Furthermore, in this case study, the choice of the sliding movement rate as a behavioral endpoint seems to be justified since fluoride ions can interrupt essential metabolic processes, such as glycolysis and synthesis of proteins, and also affect the normal function of nervous systems (Reddy et al. 1989; Reddy and Venu Gopal 1990; World Health Organization 2002; Camargo 2003).
Statistical analyses
Because parametric methods usually have more statistical power than nonparametric methods for detecting that the null hypothesis is false (Sokal and Rohlf 1995), we have preferred to use a parametric method in order to reduce the type II error (i.e., failing to detect pollution in the field study and failing to detect toxic effects in the second toxicity bioassay). In consequence, t tests were performed to check significant (P < 0.05) differences between pair of independent means: regarding the field study, we compared mean values at the reference station (S-1) with mean values at each polluted sampling site (S-2 or S-3); regarding the second toxicity bioassay, we compared mean values at the control (0.1 mg F−/L) treatment with mean values at the fluoride (2.6 mg F−/L) treatment. Normality and homoscedasticity of data were assumed for physicochemical and biological parameters (Sokal and Rohlf 1995).
On the other hand, 24, 48, 72, and 96-h LC50 values, along with their respective 95% confidence limits, for juvenile and adult snails of P. acuta were calculated using the multifactor probit analysis (MPA) software (US Environmental Protection Agency 1991). The MPA methodology solves the concentration-time-response equation simultaneously via the iterative reweighed least squares technique (multiple linear regression). The dependent variable was the probit of the proportion of snails responding to each fluoride treatment, and the independent variables were exposure times (24, 48, 72, and 96 h) and mean values of measured fluoride concentrations (44.7, 90.8, 136, 174, and 268 mg F−/L). In addition, safe concentrations for preventing mortality in juvenile and adult snails of P. acuta were also estimated (as LC0.10 values for infinite hours of exposure) using the MPA software and according to the US Environmental Protection Agency (1991). Significant (P < 0.05) differences between LC values were accepted when their 95% confidence limits did not overlap, since 85% confidence intervals seem to be enough to achieve a significance level of 0.05 for the P value (Payton et al. 2003). This criterion has already been used in other toxicity studies (Aguirre-Sierra et al. 2011; Pauget et al. 2013; Wagner et al. 2015).
Results
Mean values (±SD) of physicochemical and biological parameters along the study area are presented in Table 1. No significant (P > 0.05) differences between the reference station (S-1) and polluted sampling sites (S-2 and S-3) were found for water velocity, temperature, pH, and dissolved oxygen. As expected, fluoride concentrations and turbidity levels significantly (P < 0.05) increased downstream from the industrial effluent, with S-2 exhibiting the highest mean values (0.6 mg F−/L and 55.2 NTU). In contrast, mean abundances of P. acuta and Ancylus fluviatilis, the two competing species of lunged aquatic snails found in the study area, significantly decreased at S-2. Nevertheless, while it was evident that A. fluviatilis recovered its pre-effluent (S-1) abundance at S-3, P. acuta snails were absent at this polluted sampling site (Table 1).
In the short-term (4 days) toxicity bioassay, mortality of test snails increased with increasing both fluoride concentration and exposure time. In contrast, no dead snail occurred at control vessels. Twenty-four, 48, 72, and 96-h LC50 values, along with their respective 95% confidence limits, are presented in Table 2. Estimated LC50 values for juvenile snails were lower than estimated LC50 values for adult snails, this difference being statistically significant (P < 0.05) at 48, 72, and 96 h of exposure since 95% confidence limits of LC50 values did not overlap. Accordingly, the estimated safe concentration for juvenile snails (2.4 mg F−/L) was lower than the estimated safe concentration for adult snails (3.7 mg F−/L) (Table 2).
Behavioral responses of juvenile snails through 15 days of exposure to a lower nonlethal fluoride concentration are presented in Fig. 2. On the one hand, the proportion of test snails located on the water surface habitat was in general higher at treatment (2.6 mg F−/L) vessels than at control (0.1 mg F−/L) vessels. However, these differences were never significant (P > 0.05), even when comparing whole data sets. On the other hand, the sliding movement rate of test snails at fluoride treatment (2.6 mg F−/L) vessels exhibited both higher and lower mean values than the sliding movement rate of test snails at control (0.1 mg F−/L) vessels, but with differences between control and treatment vessels never being significant (P > 0.05).
Mean values (+SD) of behavioral responses of P. acuta juvenile snails at control (0.1 mg F−/L) and treatment (2.6 mg F−/L) glass vessels after 3, 6, 9, 12, and 15 days of exposure. No significant difference (P > 0.05) was found between control and treatment vessels throughout the toxicity bioassay, including for the whole data sets
Discussion
The presence of P. acuta and A. fluviatilis downstream from Burgomillodo Dam is not surprising since increased amounts of periphyton can occur downstream from dams as a consequence of nutrient enrichment and/or increased insolation, this resulting in greater abundances of invertebrate grazers/scrapers such as Physella and Ancylus snails (Camargo et al. 2005; Vaikasas et al. 2013; Benítez-Mora and Camargo 2014; Mbaka and Mwaniki 2015). Similarly, we expected to find increased fluoride concentrations and turbidity levels downstream from the industrial effluent, with higher deposition of fine inorganic matter at polluted sampling sites (S-2 and S-3), particularly at S-2 where the river bottom was covered by a relatively thick layer of sandy sediment. Because values of water velocity, temperature, pH, and dissolved oxygen were within normal ranges for supporting freshwater invertebrates (Wetzel and Likens 2000; Wetzel 2001; Tachet et al. 2003; Thorp and Covich 2010), with no significant differences between the reference station (S-1) and polluted sampling sites for these physicochemical parameters, it is reasonable to think that water pollution (i.e., increased fluoride concentrations and turbidity levels) and the resulting habitat degradation would be responsible, to a large extent, for significant reductions in the abundance of P. acuta and A. fluviatilis downstream from the industrial effluent.
Actually, it was obvious a decrease in water pollution and habitat degradation and, at the same time, a recovery of A. fluviatilis abundance, with increasing downstream distance from the industrial effluent (Table 1). However, this apparent reduction of water pollution and habitat degradation did not affect positively the abundance of P. acuta since it was absent at S-3. Because competition for available food and suitable benthic substratum between freshwater gastropods may be intense, particularly between pulmonate snails in which intraguild egg predation has been observed (Lombardo and Cooke 2004; Turner et al. 2007; Riley et al. 2008; Zukowski and Walker 2009; Riley and Dybdahl 2015), it is plausible that the recovery of A. fluviatilis abundance caused a serious constraint to the recovery of P. acuta abundance at the farthest sampling site (S-3).
On the whole, and according to the obtained results from laboratory experiments (Table 2 and Fig. 2), it seems that turbidity pollution might be much more responsible, than fluoride pollution, for the impact of the industrial effluent on P. acuta. Although increased fluoride concentrations at S-2 represent about six times the background fluoride concentrations at the reference station (S-1), these increased fluoride levels were only little higher than the proposed safe level of fluoride (<0.5 mg F−/L) for protecting sensitive freshwater animals (Camargo 2003). Besides, the estimated safe concentrations of fluoride for P. acuta juveniles and adults (Table 2) resulted to be much higher than the fluoride concentrations measured at S-2 (Table 1). And because juvenile snails (more sensitive to fluoride toxicity than adult snails) did not show significant alterations in their behavior during the second toxicity bioassay (Fig. 2), the estimated safe concentrations should be considered as suitable values for preventing mortality in P. acuta snails during long-term exposures to fluoride ions.
Assessing alterations in the behavior of animals exposed to chemical pollutants is gaining more recognition within the field of ecotoxicology as an important supplementary technique to conventional toxicity studies traditionally focusing on survival, growth, and reproduction, and with sensitive behavioral responses being used as early warning biomarkers for ecotoxicological risk assessment of environmental pollution (Zala 2004; Robinson 2009; Hellou 2011; Alonso and Camargo 2012; Benítez-Mora et al. 2014; Fernandes et al. 2016; Hansen and Roslev 2016). An overview of laboratory experiments with aquatic animals exposed to the toxic effects of chemical pollutants ultimately indicates that significant alterations in the movement behavior of test animals can depend not only on the kind of pollutant and the toxicant concentration but also on the sensitivity/tolerance of test animals. Regarding freshwater snails, Alonso and Camargo (2012) found that adult individuals of the mud snail Potamopyrgus antipodarum did not show significant changes in their velocity through a long-term exposure of 63 days to a mean fluoride concentration of 4.7 mg F−/L, but higher mean fluoride concentrations of 18.6 and 37.1 mg F−/L caused significant decreases in the velocity of P. antipodarum adults. This slower locomotion would be a direct consequence of the toxic action of fluoride ions since they can interrupt essential metabolic processes, such as glycolysis and synthesis of proteins, and also affect the normal function of nervous systems (Reddy et al. 1989; Reddy and Venu Gopal 1990; World Health Organization 2002; Camargo 2003). In contrast, other studies have reported that nonlethal concentrations of ionic liquids (Bernot et al. 2005) and silver nanoparticules (Bernot and Brandenburg 2013) caused significant increases in the sliding movement rate of adult individuals of P. acuta, this faster locomotion due to chemical stress being explained as a possible attempt of snails to migrate away from the toxic action of pollutants. Bernot and Brandenburg (2013) also found that silver nanoparticules, at concentrations as low as 1 μg/L and through exposures as short as 24 h, could cause significant increases in the proportion of P. acuta adult snails using the water surface habitat, this fact being similarly interpreted as an attempt of snails to escape from the toxic aquatic environment. More recently, López-Doval et al. (2014) reported that the herbicide diuron, at concentrations as high as 1172 μg/L and through exposures as long as 16 days, did not cause significant changes in the velocity of adult individuals of P. acuta. Moreover, the use of the surface environment of water by P. acuta adult snails during their exposure to the herbicide diuron was not notified in this laboratory study (López-Doval et al. 2014).
P. acuta hence seems to be a tolerant species to the toxicity of fluoride ions (Table 2 and Fig. 2). Actually, comparisons of 96-h LC50 values calculated in this study with previously published 96-h LC50 values for other freshwater benthic invertebrates indicate that P. acuta is more tolerant to fluoride toxicity than other invertebrate species (Table 3), with adult snails being even more tolerant than juvenile snails. According to our preceding laboratory investigations with different developmental stages of other aquatic invertebrate species (Camargo 2004; Aguirre-Sierra et al. 2011), significant differences in the tolerance to fluoride toxicity between juveniles and adults of P. acuta might be due mainly to lower metabolic rates, better osmoregulatory abilities, and/or greater detoxification mechanisms in adult snails than in juvenile snails.
On the other hand, the increased turbidity levels at S-2 represent about 17 times the background turbidity levels at the reference station (S-1). Although these increased turbidity levels are much lower than the highest turbidity levels without causing direct toxicity to freshwater invertebrates (Rowe et al. 2002; Suren et al. 2005), they might have caused considerable adverse effects on P. acuta and A. fluviatilis as a result of instream habitat degradation. Numerous field studies have clearly showed that increases in water turbidity of rivers and streams can reduce light transmission and intensify siltation of suspended solids, these altered processes causing reductions in the abundance of sensitive benthic invertebrates due to decreases in food supply and quality, and also owing to alterations in available and suitable benthic substratum (Quinn et al. 1992; Wood and Armitage 1997; Henley et al. 2000; Bilotta and Brazier 2008; Bryce et al. 2010; Jones et al. 2012; Chapman et al. 2014; Naden et al. 2016). In this regard, it is worth noting that Physella and Ancylus snails usually inhabit stony benthic substrata of freshwater ecosystems, mainly feeding on attached periphyton and vegetal detritus (Dillon 2000; Tachet et al. 2003; Soler et al. 2006; Thorp and Covich 2010).
Therefore, it seems evident that instream habitat degradation, derived from increased turbidity levels, might be a major cause for significant reductions in the abundance of P. acuta and A. fluviatilis downstream from the industrial effluent, particularly at the most polluted sampling site (S-2). However, because A. fluviatilis recovered its pre-effluent (S-1) abundance at S-3, whereas P. acuta snails were absent at this polluted sampling site (Table 1), it is plausible that P. acuta was more sensitive than A. fluviatilis to alterations in fluvial habitat, and with this greater sensitivity diminishing its competitive abilities. Recent studies (Extence et al. 2013; Murphy et al. 2015) indeed indicate that A. fluviatilis can exhibit a certain degree of tolerance to the effects of deposited fine sediments. In the case of S-2, the instream habitat degradation was so intense that competitive abilities of both A. fluviatilis and P. acuta were negatively affected, with the abundance of both gastropod species being dramatically reduced (Table 1). In the case of the reference station (S-1), both gastropod species were relatively abundant because of the absence of water pollution and habitat degradation, although the abundance of A. fluviatilis was higher than the abundance of P. acuta (Table 1), this fact suggesting superior competitive abilities of A. fluviatilis.
Conclusions
In the light of the obtained results from field studies and laboratory experiments, and their subsequent discussion, we can conclude that the industrial effluent caused significant increases in fluoride concentrations and turbidity levels of the recipient Duraton River, also generating deposition of fine inorganic matter on the river bottom. However, increased fluoride concentrations would not be responsible for significant reductions in the abundance of P. acuta downstream from the industrial effluent. Laboratory experiments showed that P. acuta is a relatively tolerant invertebrate species to fluoride toxicity, with estimated safe concentrations of fluoride for juveniles and adults of this gastropod species being much higher than fluoride concentrations measured at polluted sampling sites. Besides, long-term (15 days) toxicity bioassays have showed that behavioral toxicity testing may be satisfactorily used to evaluate the suitability of estimated safe concentrations of chemical pollutants (like fluoride ions) for aquatic animals. Overall, we can conclude that adverse changes in instream habitat conditions, derived from increased turbidity levels, might be a major cause for significant reductions in the abundance of P. acuta downstream from the industrial effluent. Additionally, the presence of the competing gastropod A. fluviatilis could have caused a constraint to the recovery of P. acuta abundance at the farthest polluted sampling site.
References
Aguirre-Sierra A, Alonso A, Camargo JA (2011) Contrasting sensitivities to fluoride toxicity between juveniles and adults of the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bull Environ Contam Toxicol 86:476–479
Albrecht C, Kroll O, Terrazas EM, Wilke T (2009) Invasion of ancient Lake Titicaca by the globally invasive Physa acuta (Gastropoda: Pulmonata: Hygrophila). Biol Inv 11:1821–1826
Alonso A, Camargo JA (2012) A video-based trackling analysis to assess the chronic toxic effects of fluoride ion on the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Ecotoxicol Environ Safe 81:70–75
American Public Health Association (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA-AWWA-WPCF, Washington, DC
Anderson R (2003) Physella (Costatella) acuta Draparnaud in Britain and Ireland. Its taxonomy, origins and relationships to other introduced Physidae. J Conchol 38:7–21
Appleton CC (2003) Alien and invasive freshwater Gastropoda in South Africa. Afr J Aquat Sci 28:69–81
Benítez-Mora A, Camargo JA (2014) Ecological responses of aquatic macrophytes and benthic macroinvertebrates to dams in the Henares River Basin (Central Spain). Hydrobiologia 728:167–178
Benítez-Mora A, Aguirre-Sierra A, Alonso A, Camargo JA (2014) Ecotoxicological assessment of the impact of nitrate (NO3 −) on the European endangered white-clawed crayfish Austropotamobius italicus (Faxon). Ecotoxicol Environ Safe 101:220–225
Bernot RJ, Brandenburg M (2013) Freshwater snail vital rates affected by non-lethal concentrations of silver nanoparticles. Hydrobiologia 714:25–34
Bernot RJ, Kennedy EE, Lamberti GA (2005) Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail, Physa acuta. Environ Toxicol Chem 24:1759–1765
Bilotta GS, Brazier RE (2008) Understanding the influence of suspended solids on water quality and aquatic biota. Water Res 42:2849–2861
Bryce SA, Lomnicky GA, Kaufmann PR (2010) Protecting sediment-sensitive aquatic species in mountain streams through the application of biologically based streambed sediment criteria. J N Am Benthol Soc 29:657–672
Burch JB (1989) North American freshwater snails. Hamburg, Michigan, The University of Michigan, Malacological Publications
Camargo JA (2003) Fluoride toxicity to aquatic organisms: a review. Chemosphere 50:251–264
Camargo JA (2004) Effects of body size and sodium chloride on the tolerance of net-spinning caddisfly larvae to fluoride toxicity. Bull Environ Contam Toxicol 72:579–585
Camargo JA, Tarazona JV (1990) Acute toxicity to freshwater macroinvertebrates of fluoride ion (F−) in soft water. Bull Environ Contam Toxicol 45:883–887
Camargo JA, Ward JV, Martin KL (1992) The relative sensitivity of competing hydropsychid species to fluoride toxicity in the Cache la Poudre River (Colorado). Arch Environ Contam Toxicol 22:107–113
Camargo JA, Alonso A, de la Puente M (2005) Eutrophication downstream from small reservoirs in mountain rivers of Central Spain. Water Res 39:3376–3384
Chai L, Dong S, Zhao H, Deng H, Wang H (2016) Effects of fluoride on development and growth of Rana chensinensis embryos and larvae. Ecotoxicol Environ Safe 126:129–137
Chapman JM, Proulx CI, Veilleux MAN, Levert C, Bliss S, André ME, Lapointe NWR, Cooke SJ (2014) Clear as mud: a meta-analysis on the effects of sedimentation on freshwater fish and the effectiveness of control measures. Water Res 56:190–202
Cope NJ, Winterbourn MJ (2004) Competitive interactions between two successful molluscan invaders of freshwaters: an experimental study. Aquat Ecol 38:83–91
Dalesman S, Rundle SD, Cotton PA (2007) Predator regime influences innate anti-predator behaviour in the freshwater gastropod Lymnaea stagnalis. Freshw Biol 52:2134–2140
Damkaer DM, Dey DB (1989) Evidence for fluoride effects on salmon passage at John Day dam, Columbia river, 1982-1986. N Am J Fish Manage 9:154–162
Dillon RT Jr (2000) The ecology of freshwater mollusks. Cambridge University Press, New York
Dillon RT Jr, Wethington AR, Rhett JM, Smith TP (2002) Populations of the European freshwater pulmonate Physa acuta are not reproductively isolated from American Physa heterostropha or Physa integra. Invert Biol 121:226–234
Extence CA, Chadd RP, England J, Dunbar MJ, Wood PJ, Taylor ED (2013) The assessment of fine sediment accumulation in rivers using macro-invertebrate community response. River Res Appl 29:17–55
Fernandes M, Amorim J, Vasconcelos V, Teles LO (2016) Resilience assessment of a biological early warning system based on the locomotor behavior of zebrafish (Danio rerio). Environ Sci Pollut Res 23:18858–18868
Gonzalo C, Camargo JA (2013) The impact of an industrial effluent on the water quality, submersed macrophytes and benthic macroinvertebrates in a dammed river of Central Spain. Chemosphere 93:1117–1124
Gonzalo C, Camargo JA, Masiero L, Casellato S (2010) Fluoride toxicity and bioaccumulation in the invasive amphipod Dikerogammarus villosus (Sowinsky, 1894): a laboratory study. Bull Environ Contam Toxicol 85:472–475
Hansen LR, Roslev P (2016) Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat Toxicol 179:36–43
Hauer FR, Lamberti GA (eds) (1996) Methods in stream ecology. Academic, San Diego
Hellou J (2011) Behavioural ecotoxicology, an “early warning” signal to assess environmental quality. Environ Sci Pollut Res 18:1–11
Henley WF, Patterson MA, Neves RJ, Lemly AD (2000) Effects of sedimentation and turbidity on lotic food webs: a concise review for natural resource managers. Rev Fish Sci 8:125–139
Jha SK, Mishra VK, Sharma DK, Damodaran T (2011) Fluoride in the environment and its metabolism in humans. Rev Environ Contam Toxicol 211:121–142
Jones JI, Murphy JF, Collins AI, Sear DA, Naden PS, Armitage PD (2012) The impact of fine sediment on macro-invertebrates. River Res Appl 28:1055–1071
Lombardo P, Cooke GD (2004) Resource use and partitioning by two co-occurring freshwater gastropod species. Arch Hydrobiol 159:229–251
López-Doval JC, Poquet M, Muñoz I (2014) Sublethal effects of the herbicide Diuron on the freshwater snail Physella acuta. Limnetica 33:205–216
Maqboul A, Aoujdad R, Fadli M, Fekhaoui M (2014) Population dynamics of Physa acuta (Mollusca: Pulmonata) in the lakes of Rif mountains (northern Morocco, Ouergha watershed). J Entomol Zool Stud 2:240–245
Mbaka JG, Mwaniki MW (2015) A global review of the downstream effects of small impoundments on stream habitat conditions and macroinvertebrates. Environ Rev 23:257–262
Metcalfe-Smith JL, Holtze KE, Sirota GR, Reid JJ, De Solla SR (2003) Toxicity of aqueous and sediment associated fluoride to freshwater organisms. Environ Toxicol Chem 22:161–166
Murphy JF, Jones JI, Pretty JL, Duerdoth CP, Hawczak A, Arnold A, Blackburn JH, Naden PS, Old G, Sear DA, Hornby D, Clarke RT, Collins AL (2015) Development of a biotic index using stream macroinvertebrates to assess stress from deposited fine sediment. Freshw Biol 60:2019–2036
Musee N, Oberholster PJ, Sikhwivhilu L, Botha AM (2010) The effects of engineered nanoparticles on survival, reproduction, and behavior of freshwater snail, Physa acuta (Draparnaud, 1805). Chemosphere 81:1196–1203
Naden PS, Murphy JF, Old GH, Newman J, Scarlett P, Harman M, Duerdoth CP, Hawczak A, Pretty JL, Arnold A, Laizé C, Hornby DD, Collins AL, Sear DA, Jones JI (2016) Understanding the controls on deposited fine sediment in the streams of agricultural catchments. Sci Total Environ 547:366–381
Pauget B, Gimbert F, Coeurdassier M, Crini N, Pérès G, Faure O, Douay F, Richard A, Grand C, de Vaufleury A (2013) Assessing the in situ bioavailability of trace elements to snails using accumulation kinetics. Ecol Indic 34:126–135
Payton ME, Greenstone MH, Schenker N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci 3:34
Quinn JM, Davies-Colley RJ, Hickey CW, Vickers ML, Ryan PA (1992) Effects of clay discharges on streams 2. Benthic invertebrates. Hydrobiologia 248:235–247
Reddy SLN, Venu Gopal NBRK (1990) Effect of fluoride on acetylcholinesterase activity and oxygen consumption in a freshwater field crab, Barytelphusa guerini. Bull Environ Contam Toxicol 45:760–766
Reddy SLN, Venu Gopal NBRK, Nagender Reddy A, Ramana Rao JV (1989) Fluoride-induced changes in carbohydrate metabolism in the tissue of freshwater crab Barytelphusa guerini. Ecotoxicol Environ Safe 18:59–67
Riley LA, Dybdahl MF (2015) The roles of resource availability and competition in mediating growth rates of invasive and native freshwater snails. Freshw Biol 60:1308–1315
Riley LA, Dybdahl MF, Hall RO (2008) Invasive species impact: asymmetric interactions between invasive and endemic freshwater snails. J N Am Benthol Soc 27:509–520
Robinson PD (2009) Behavioral toxicity of organic chemical contaminants in fish: application to ecological risk assessments (ERAs). Can J Fish Aquat Sci 66:1179–1188
Rowe DK, Suren AM, Martin ML, Smith JP, Smith BJ, Williams E (2002) Lethal turbidity levels for common freshwater fish and invertebrates in Auckland streams. Auckland Regional Council Technical Publication Number 337, New Zealand
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman, New York
Soler J, Moreno D, Araujo R, Ramos MA (2006) Diversidad y distribución de los moluscos de agua dulce en la Comunidad de Madrid (España). Graellsia 62:201–252
Strong EE, Gargominy O, Ponder WF, Bouchet P (2008) Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 595:149–166
Suren AM, Martin ML, Smith BJ (2005) Short-term effects of high suspended sediments on six common New Zealand steam invertebrates. Hydrobiologia 548:67–74
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2003) Invertebrés d’Eau Douce (Systematique, Biologie, Écologie). CNRS Editions, Paris
Thorp JH, Covich AP (eds) (2010) Ecology and classification of north American freshwater invertebrates, 3rd edn. San Diego, Academic
Turner AM, Bernot RJ, Boes CM (2000) Chemical cues modify species interactions: the ecological consequences of predator avoidance by freshwater snails. Oikos 88:148–158
Turner AM, Turner RR, Ray SR (2007) Competition and intraguild egg predation among freshwater snails: re-examining the mechanism of interspecific interactions. Oikos 116:1895–1903
US Environmental Protection Agency (1991) Multifactor probit analysis. US EPA 600/X-91-101, Washington DC
Vaikasas S, Palaima K, Pliuraite V (2013) Influence of hydropower dams on the state of macroinvertebrates assemblages in the Virvyte River, Lithuania. J Environ Eng Landsc Manage 21:305–315
Wagner N, Lötters S, Veith M, Viertel B (2015) Acute toxic effects of the herbicide formulation Focus® Ultra on embryos and larvae of the Moroccan painted frog, Discoglossus scovazzi. Arch Environ Contam Toxicol 69:535–544
Weinstein LH, Davison AW (2004) Fluorides in the environment: effects on plants and animals. CABI Publishing, Cambridge
Wetzel RG (2001) Limnology: Lake and rivers ecosystems, 3rd edn. Academic, San Diego
Wetzel RG, Likens GE (2000) Limnological analyses, 3rd edn. Springer, New York
Wood PJ, Armitage PD (1997) Biological effects of fine sediment in the lotic environment. Environ Manag 21:203–217
World Health Organization (2002) Environmental health criteria 227. Fluorides. WHO, Geneva
Zala SM (2004) Abnormal behaviors induced by chemical pollution: a review of the evidence and new challenges. Anim Behav 68:649–664
Zalizniak L, Kefford BJ, Nugegoda D (2009) Effects of different ionic compositions on survival and growth of Physa acuta. Aquat Ecol 43:145–156
Zukowski S, Walker KF (2009) Freshwater snails in competition: alien Physa acuta (Physidae) and native Glyptophysa gibbosa (Planorbidae) in the River Murray, South Australia. Mar Freshw Res 60:999–1005
Acknowledgments
Funds for this research came from the Spanish Ministry of Science and Innovation (research project CGL2011-28585). The University of Alcala provided logistical support for carrying out field studies and laboratory experiments. We also thank two anonymous reviewers for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Highlights
- The bladder snail Physella acuta appears to be a tolerant invertebrate species to fluoride toxicity.
- Estimated safe concentrations for juvenile and adult snails are 2.4 and 3.7 mg F−/L, respectively.
- Behavioral toxicity testing is used to evaluate the suitability of estimated safe concentrations.
- Instream habitat degradation, derived from increased turbidity levels, might be a major cause for the impact of the industrial effluent.
Rights and permissions
About this article
Cite this article
Camargo, J.A., Alonso, Á. Ecotoxicological assessment of the impact of fluoride (F−) and turbidity on the freshwater snail Physella acuta in a polluted river receiving an industrial effluent. Environ Sci Pollut Res 24, 15667–15677 (2017). https://doi.org/10.1007/s11356-017-9208-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9208-x