Abstract
Donax trunculus is the most consumed bivalve by the local population of the Northeast Algeria for its nutritional value. Therefore, the aim of the current study was to determine the effects of cadmium (Cd), a known toxic metal, on the alterations in main essential omega-3 fatty acids, i.e., eicosapentaenoic acid (EPA; C20:5n-3) and docosahexaenoic acid (DHA; C22:6n-3), in male and female gonads of D. trunculus during the reproduction period at spring (before spawning). Additionally, this work seeks to describe the relation between EPA and DHA with non-methylene-interrupted dienoic (NMID) fatty acids, and explores their possible contribution of to protect against Cd stress. The samples were collected at El Battah, a relatively clean sea shore, and reared in the laboratory. Physico-chemical parameters such as temperature, pH, salinity, and dissolved oxygen were measured. Cd was added to the rearing water at two sublethal concentrations (LC10 and LC25-96h, as determined previously). A two-way ANOVA analysis indicated significant effects of concentrations and genders for both fatty acids. Our results showed a significant reduction in EPA and DHA concentrations in the both genders, with a strong effect in females. There was also a negative correlation between NMID fatty acids and the two essential omega-3 fatty acids for each gender.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Previous studies have reported markedly increased levels of pollution in the city of Annaba (Northeast Algeria), particularly by metal contamination (Beldi et al. 2006; Larba and Soltani 2014). Heavy metals constitute a core group of aquatic pollutants due to their bioaccumulative and non-biodegradable properties (Velma and Tchounwou 2010). Furthermore, Cd has been detected in Donax trunculus (Bivalvia, Donacidae), an edible species from the gulf of Annaba, with a variation in capturing site and seasons (Beldi et al. 2006). Recent studies have demonstrated that bivalves are successfully used as bioindicator species to monitor pollution in coastal areas (Amira et al. 2011; Soltani et al. 2012; Sifi et al. 2013; Hamza-Chaffai 2014; Karray et al. 2015). These species are chosen due to their ability to accumulate contaminants usually from water and food, reflecting the bio-available fraction (Chandurvelan et al. 2015). In addition, their relative immobility, wide distribution among different aquatic habitats, abundance, persistence, and ease of collection make them good long-term indicators of environmental contamination (Hamza-Chaffai 2014). Moreover, exposure of bivalves to certain metals may lead to changes in biochemical processes that might be potential biomarkers of the exposure as well as early warning signals of adverse effects of the metal accumulation within the bivalves (Le et al. 2016). Recent reports clearly demonstrate that some of heavy metals exert adverse effects on edible bivalves (Ali Abdel-Salam 2013; Borković-Mitić et al. 2013; Fokina et al. 2013; Nardi et al. 2017), by altering the levels of various minerals, vitamins, essential and non essential amino acids, protein, carbohydrate, and lipids. Cd is involved in growth retardation (Geret et al. 2002), endocrine disruption (Ketata et al. 2007), and interferes with reproduction (Smaoui-Damak et al. 2006; Yeung et al. 2016). In fact, metallic pollution could affect lipid metabolism during reproductive period (Hamdani and Soltani-Mazouni 2011; Sifi et al. 2013; Bensouda-Talbi and Soltani-Mazouni 2014; Rocha et al. 2016). Fatty acids (FA) being essential for cell membrane permeability, constitute the main components of lipids to be used as fuel during metabolic processes. Therefore, these agents play an important role in the modulation of biochemical and physiological responses (Neves et al. 2015). In recent years, there has been an upsurge of research on the beneficial effects of omega-3 fatty acids in health and disease (Swanson et al. 2012; De Camargo Talon et al. 2015; Drudi et al. 2017). Hence, highly polyunsaturated fatty acids (PUFA), e.g., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), play a key role in the modulation of physiological system of a number of species of animal kingdom including zoo planktons, fish, and bivalve. Interestingly, it is noteworthy that humans cannot synthesize de novo these fatty acids (Saito and Aono 2014; Gonçalves et al. 2012). However, very few studies are available on the possible effects of pollutants on these important nutrients. Seafood represents the primary source of two biologically active dietary omega-3 fatty acids. Recent investigations have demonstrated that the bivalve D. trunculus is an excellent source of omega-3 fatty acids (EPA and DHA) (Boussoufa et al. 2011). Therefore, the present study focused on the impact of sublethal exposure of cadmium (96h-LC10 and 96h-LC25) on the composition of the most important PUFA omega-3 (EPA and DHA), given their role on human health, and maintenance of essential functions such reproduction, in an edible mollusk species D. trunculus (in two genders) following an acute exposure (96 h) during the reproductive period, and correlate our findings with particular FA know as NMID (C22:2 NMID1 and C22:2 NMID2), which are distinguished by the presence of more than one methyl group between each double bond (Ojea et al. 2004), which makes them more resistant to oxidative stress (Barnathan 2009).
Experimental
Collection and animal rearing conditions
The gulf of Annaba is located in the east of Algeria. It is limited by the Cap Rosa (8° 15′ E and 36° 38′ N) in the East, and by the Cap Garde (7° 16′ E and 36° 68′ N) in the West (Fig. 1). The experiments were carried out in March, 2015. D. trunculus adults (shell length 25 ± 2 mm) were sampled at El-Battah beach (36° 50′ N–7° 50′ E), a coastal site far from any source of anthropogenic activities and subjected to an important hydrodynamic exposure. Animals were transported in cold boxes to the laboratory, and the genders were identified by macroscopic examination according to the color of gonads, i.e., dark blue in females and yellow-white in males (Manca Zeichen et al. 2002). The rearing was conducted in aquaria, containing sandy bottom and sea water, taken from the sampling area, and equipped with air pumps (Nirox X5). D. trunculus is preferentially distributed between 0 and 2 m of depth in the Mediterranean Sea (Gaspar et al. 2002). This species is a filter-feeding species and according to Mouëza and Chessel (1976), it absorbs the finest suspended particles (suspensivore) or those deposited on the sediment via to its elongated siphon (depositivore). The animals were kept unfed in an aquarium for 48 h to acclimatize prior to the start of the experiment of exposure to Cd (Belabed and Soltani 2013). No mortality was observed during the acclimatization. During all the experiments, the water was constantly aerated (dissolved oxygen 7.6–8.3 mg/L; pH 8.1–8.3; salinity 33.1–33.5 g/L; temperature 16 °C) and a 12 h light/dark cycle was maintained. After acclimatization, bivalves were fed daily with a commercial food mixture (Marine Invertebrate Diet. Carolina Ltd., NC, USA), and were exposed to Cd for 4 days while another set of bivalves was kept under control conditions. The metal was added to the seawater as cadmium chloride (CdCl2), according to 96h-LC10 and 96h-LC25 (lethal concentration inducing 10 and 25% mortality) of Cd treatment in the mollusk D. trunculus (Table 1) (Merad and Soltani 2015).
Fatty acid extraction and gas chromatography analysis
Individual gonads of four bivalves from control and treated series were used. Lipids were extracted according to the method of Bligh and Dyer (1959), in the presence of 100 μg of tripentadecanoic acid triglyceride (TG (15:0/15:0/15:0)) as internal standards, then transmethylated by BF3/methanol after saponification. Fatty acid methyl esters were analyzed by gas liquid chromatography using a Clarus 500 gas chromatograph (PerkinElmer, Waltham, MA, USA), equipped with a flame ionization detector and an Agilent VF-23ms capillary column (30 m × 0.32 mm) (Agilent, Santa Clara, CA, USA). The analysis conditions were as follows: oven temperature was 85 °C/1 min, increased to 150 °C at 30 °C/min, then increased at 3 °C/min to 215 °C. Helium was used as carrier gas, with a flow rate of 3 ml/min. Identification of different fatty acids was performed by comparison of relative retention times with those of commercial standards.
Identification of different fatty acids was performed by comparison of relative retention times with those of commercial standards. Fatty acids were quantified in reference to internal standard (C15:0) and were normalized to the weight of each tissue and expressed as μg/mg of dry weight of tissue.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using Prism version 6.01 for Windows (GraphPad software, La Jolla, CA, USA, www Graphpad.com). The homogeneity of variances was checked by Bartlett’s and Brown-Forsythe tests. One-way analysis of variance (ANOVA) with a Tukey post-hoc analysis HSD test was used to evaluate statistically significant differences in omega-3 fatty acid concentrations in control tissues (0 h) for each gender, to assess which sex is the richest in omega-3. Statistical differences among the means of control and Cd-exposed series (96 h) were determined using Dunnett’s test. To identify concentrations/genders relationships, a two-way analysis of variance (ANOVA) was performed. Statistical significance was set at p < 0.05 level.
Results
EPA and DHA levels recorded in gonads on untreated individuals at the beginning of the experiment (day 0) were summarized in Table 2. Results show that male controls presented significant higher values of EPA and DHA compared to females (p = 0.000 for EPA and p = 0.029 for DHA).
Effect of sublethal concentrations of cadmium on eicosapentaenoic acid amounts in the gonad of D. trunculus
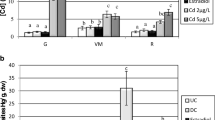
The impact of sublethal concentrations of Cd on the levels of eicosapentaenoic acid (EPA) varied as function treatment and gender (Table 3). After 96 h of exposure, a significant (p ≤ 0.01) decrease (37.28%) in EPA was observed in the treated series with the LC25 in males as compared with control males (Fig. 2). In females, a significant (p < 0.05) decrease in their EPA levels was observed with the two sublethal Cd concentrations (LC10: 31.58%; LC25: 37.60%) (Fig. 3). A two-way ANOVA indicated significant effects of concentrations (F 2, 23 = 9.79; p = 0.001) and genders (F 1, 23 = 6.79; p = 0.018). However, there was no significant effect of the interaction between concentrations and genders (F 2, 23 = 0.12; p = 0.884).
Effect of sublethal concentrations of cadmium on docosahexaenoic acid amounts in the gonad of D. trunculus
Table 3 shows the docosahexaenoic acid (DHA) levels in gonads after an acute exposure (96 h) to Cd. In males, a comparison between controls and treated series revealed a significant (p < 0.001) reduction (34. 86%) in this PUFA only with the highest concentration (LC25) of Cd (Fig. 4). As regards females, a significant reduction of in DHA levels was observed with the two tested concentrations (LC10: 27.72%, p < 0.05; LC25: 31.84%; p < 0.05) (Fig. 5). A two-way ANOVA indicated significant effects of concentrations (F 2, 23 = 12.07; p = 0.001) and genders (F 1, 23 = 25.54; p = 0.001), and no significant (F 2, 23 = 0.77; p = 0.476) effect of interaction (concentrations × genders).
Effect of sublethal concentrations of cadmium on non-methylene-interrupted dienoic amounts in the gonad of D. trunculus
The two C22 dienoic (C22:2 NMID1 and C22:2 NMID2) acids appeared successively between the C22:1n- 9 and C22:5n 6. They were present at low levels: between 0.45 and 1.13 (μg/mg of dry weight) in all samples. These FA increase in all treated individuals, except in male bivalves exposed to the lowest concentration of Cd (CL10). The increase in non-methylene-interrupted dienoic (NMID) levels coincided with a decrease in EPA and DHA in D. trunculus (Table 4).
Discussion
Cadmium have no physiological function for cells, and can cause toxic effects at sublethal concentrations in many bivalves (Fang et al. 2010; Company R et al. 2010; Yeung et al. 2016; Piló et al. 2017), due to the bioavailability of its free ionic form Cd2+ (Part et al. 1985; Wang and Rainbow 2006). Cd has the ability to break the balance between pro- and antioxidative systems, to cause oxidative stress in tissues, and the accumulation of lipid peroxidation products in bivalves (Dovzhenko et al. 2005; Franco et al. 2006; Regoli 2012). Indeed, the dysregulation of lipid metabolism is recognized among the most significant biochemical responses (Parrish 2013). Many studies have demonstrated that fatty acid composition can be altered in the aquatic environment pollution by anthropogenic activities (Rocchetta et al. 2006; Kainz et al. 2008; Penha-Lopes et al. 2009; Cheung et al. 2010; Perrat et al. 2013).
EPA and DHA are excellent energy sources, and involved in the maintenance of membrane structure and functions (Costa et al. 2015). The (n-3) PUFA have been reported to be essential for optimal growth for at least some species of juvenile bivalves (Bergé and Barnathan 2005). These omega-3 PUFA serve as precursors of eicosanoids (prostaglandins, thromboxanes, leukotrienes, etc.), which have a wide range of physiological actions in immune system, inflammatory response, neural function, reproduction, and enhancing the organisms adaptation to environmental stress (Vance and Vance 2002; Merzouk et al. 2008; Nani et al. 2015). Omega-3 fatty acids reduce cholesterol levels and the incidence of heart disease, stroke, and preterm delivery (Daviglus et al. 2002; Patterson 2002). According to the recent researches, DHA should be considered as a potential antiangiogenic candidate for future clinical trials (Siddiqui et al. 2011; Berger et al. 2013). For this reasons, the recommended intake of omega-3 fatty acids for healthy adults is 0.3–0.5 g/day of EPA + DHA and approximately 1 g/day for people with or at high risk for coronary heart disease (Kris-Etherton et al. 2002).
It has been reported that the EPA and DHA concentrations are generally decreased with increasing contamination levels and are often associated to peroxidation or decreasing membrane permeability (Filimonova et al. 2016; Signa et al. 2015). Our study showed that Cd can reduce levels of omega-3 PUFA significatively at sublethal concentrations following an acute exposure (Table 3) confirming previous reports. For instance, the bivalves Mytilus edulis exposed to 50 μg/L of copper after 24 and 72 h (Fokina et al. 2013), and Mizuhopecren yessoensis exposed to 0.25 ppm CdCl2 (Chelomin and Belcheva 1991) showed lower value of EPA and DHA, compared with controls. Mytilus galloprovincialis transplanted from a reference site to an impacted site, to assess the biochemical response of caged mussels to high trace element and polycyclic aromatic hydrocarbon (PAH) contamination, presented reduction in both EPA and DHA contents (Signa et al. 2015). Indeed, for the bivalve Scrobicularia plana, EPA percentages were particularly low in specimens from sites contaminated by PAH and pollutants with estrogenic activity (Perrat et al. 2013). Recently, Primextra ®Gold TZ, a herbicide, was found to cause a significant reduction in the amount of the omega-3 fatty acids in two marines bivalves, namely Cerastoderma edule and S. plana (Gonçalves et al. 2016). The same effect was observed in fish’s species like Lates calcarifer treated with nickel and mercury during 96 h (Senthamilselvan et al. 2016), and Oreochromis niloticus collected from contaminated sites by human activities, in particular with heavy metals (Muinde et al. 2013).
The reduction in n−3 PUFA observed in our study can be due to the activation of the lipid peroxidation mechanism: since PUFA are primary targets of ROS, when the process of lipid radical formation begins, higher lipid saturation and high oxygen concentrations cause the increase in the velocity of lipid radical chain reactions (Anacleto et al. 2014). NMID FA are seemingly ubiquitous lipid mollusk components (Ojea et al. 2004), and can be synthesized de novo by bivalves and used to replace the more sensitive FA (Barnathan 2009). Decrease observed for PUFA, especially for DHA coupled with increase in NMID FA, was a compensatory mechanism reported in bivalves to lower susceptibility to lipid peroxidation (Munro and Blier 2012; Signa et al. 2015) while maintaining proper membrane fluidity. NMID FA can be used to replace the more sensitive PUFA (EPA and DHA), because of an unusual structural property (i.e., isolated double bonds) which makes them more resistant to the oxidative stress (Barnathan 2009). The higher proportion of these PUFA reduces the susceptibility to lipid peroxidation. Indeed, NMID FA are distributed in higher quantities in the organs that are more exposed to the external environment, such as gills, mantle, and foot (Berge and Barnathan 2005).
Reproduction of intertidal bivalves includes gametogenesis, development and metamorphosis, all of which are energy-consuming processes (Martinez et al. 2000). The success of those processes depends on the physiological condition and especially the pre-spawning condition of the adult (Hendriks 2004). The DHA and EPA concentrations peaked in spring and winter coinciding with gametogenesis in D. trunculus (Boussoufa et al. 2011), and in other species of bivalve such as oyster (Dridi et al. 2007). Additionally, a direct and significant relationship was observed between EPA and DHA with the gonadosomatic index and ripe stage (Freites et al. 2010). The latter observation suggests the accumulation of the essential fatty acids in reproductive tissue during gametogenesis and oocyte maturation. EPA is used preferentially during embryonic development as an energy source (Freites et al. 2010; Martínez-Pita et al. 2012), while DHA plays an important role at the structural and functional levels of cell membranes involved in oogenesis and embryogenesis and it influences larval survival (Soudant et al. 1999; Pazos et al. 2003).
The fatty acid profile show differences between the genders in D. trunculus. Indeed, males present higher values in EPA and DHA. The content of 20:5n-3 and 22:6n-3 differs among bivalve’s species. In M. galloprovincialis, the percentage of the PUFA (20:5n-3 and 22:6n-3) were higher in males than in females (Martínez-Pita et al. 2012), and in the testes of Patella depressa (Morais et al. 2003) confirming our findings in D. trunculus. However, several investigators reported higher values of EPA in female gonads such as Argopecten purpuratus and Pecten maximus (Caers et al. 1999; Soudant et al. 1996). Similarly, the value of DHA is also more important in the females of P. maximus (Soudant et al. 1996). As previously reported, the differences in fatty acid composition between genders could be related to differences in the lipid composition since PUFA are mainly present in phospholipids, whereas neutral lipids such as TG and sterols ester accumulate 14:0 and MUFA (Caers et al. 1999; Ojea et al. 2004; Pazos et al. 2003; Saito 2004; Soudant et al. 1999). In addition, previous studies showed that Cd can strongly affect at low concentrations energy metabolism by suppressing the mitochondrial function and increasing basal energy demand of an organism to cover the energy costs of detoxification and damage repair (Cherkasov et al. 2006; Ivanina et al. 2009; Cannino et al. 2009). In addition, there is a difference in the energy demand of male and female gametes. Male bivalves produce small spermatozoa with few energy reserves by comparison with females which elaborate vitellin reserves for developing oocytes (Beninger and Le Pennec 1997) and have high needs of energy for oogenesis. Moreover, it has been demonstrated that the bioaccumulation of several heavy metals including cadmium depends on gender during the gametogenesis in M. galloprovincialis; this bioaccumulation was found higher in females (Richir and Gobert 2014). These findings can explain the difference in the responses observed between the genders in D. trunculus, especially the greater sensitivity of females, as they showed decreases in their omega-3 levels with the two test concentrations (CL10 and CL25) (Table 3).
Conclusions
Cadmium in both genders decreased omega-3 contents in gonads. The results of the experimental treatments point out the possibility to use EPA and DHA PUFA composition/parameters as biomarkers, reflecting the adverse effects of the heavy metals on bivalve mollusks, including the fact that cadmium can reduce significantly their nutritional value. The sensitivity of females could be correlated to different metabolic requirements during the reproductive period. Further more studies are wanting particularly on the enzymes, like elongases and desaturases which are involved in the synthesis of PUFA, for obtaining more information on their mode of action on reduction of FA.
References
Ali Abdel-Salam H (2013) Assessment of biochemical compositions and mineral contents of carapace of some important commercially crustaceans and mollusks organisms from Egyptian and Saudi Arabia coasts as a new animal feed. Am J of BioSci 1(2):35–43
Anacleto P, Maulvault AL, Bandarra NM, Repolho T, Nunes ML, Rosa R, Marques A (2014) Effect of warming on protein, glycogen and fatty acid content of native and invasive clams. Food Res Int 64:439–445
Amira A, Sifi K, Soltani N (2011) Measure of environmental stress biomarkers in Donax trunculus (Mollusca, Bivalvia) from the gulf of Annaba (Algeria). Eur J Exp Biol 1(2):7–16
Barnathan G (2009) Non-methylene-interrupted fatty acids from marine invertebrates: occurrence, characterization and biological properties. Biochimie 91:671–678
Belabed S, Soltani N (2013) Acute toxicity of cadmium on Donax trunculus: acetylcholinesterase, glutathione S-transferase activities and pattern of recovery. Eur J Exp Biol 3(2):54–61
Beldi H, Gimbert F, Maas S, Scheiffler R, Soltani N (2006) Seasonal variations of Cd, Cu, Pb and Zn in the edible mollusk Donax trunculus (Mollusca, Bivalvia) from gulf of Annaba, Algeria. Afr J Agr Res 1(4):85–90
Bergé JP, Barnathan G (2005) Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv in Biochem Eng Biot 96:49–125
Berger H, Végran F, Chikh M, Gilardi F, Ladoire S, Bugaut H, Mignot G, Chalmin F, Bruchard M, Derangére V, Chevriaux A, Rébé C, Ryffel B, Pot C, Hichami A, Desvergne B, Ghiringhelli F, Apetoh L (2013) SOCS3 Transactivation by PPARg Prevents IL-17–Driven Cancer Growth. Cancer Res 73(12)
Beninger PG, Le Pennec M (1997) Reproductive characteristics of a primitive bivalve from a deep-sea reducing environment: giant gametes and their significance in Acharaxaline (Cryptodonta: Solemyidae). Mar Ecol Prog Ser 157:195–206
Bensouda-Talbi L, Soltani-Mazouni N (2014) Measure of oxidative stress and neurotoxicity biomarkers in Donax trunculus from the gulf of Annaba (Algeria): case of the year 2012. Ann Rev Res Biol 4(12):1902–1914
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Borković-Mitić S, Pavlović S, Perendija B, Despotović S, Gavrić J, Gačić Z, Saičić Z (2013) Influence of some metal concentrations on the activity of antioxidant enzymes and concentrations of vitamin E and SH-groups in the digestive gland and gills of the freshwater bivalve Unio tumidus from the Serbian part of Sava River. Ecol Indic 32:212–221
Boussoufa D, Ghazali N, Viciano E, Navarro JC, El Cafsi M (2011) Seasonal variation in condition and fatty acid composition of coquina clam, Donax trunculus (Linnaeus 1758) (Mollusca: Bivalvia) from the Tunisian coast. Cah Biol Mar 52(1):47–56
Caers M, Coutteau P, Cure K, Morales V, Gajardo G, Sorgeloos P (1999) The Chilean scallop Argopecten purpuratus (Lamarck, 1819): I. Fatty acid composition and lipid content of six organs. Comp Biochem Physiol 123B:89–96
Cannino G, Ferruggia E, Luparello C, Rinaldi AM (2009) Cadmium and mitochondria. Mitochondrion 9:377–384
Chandurvelan R, Marsden ID, Glover CN, Gaw S (2015) Assessment of a mussel as a metal bioindicator of coastal contamination: relationships between metal bioaccumulation and multiple biomarker responses. Sci Total Environ 511:663–675
Chelomin VP, Belcheva NN (1991) Alterations of microsomal lipid-synthesis in gill cells of bivalve mollusk Mizuhopecten yessoensis in response to cadmium accumulation. Comp Biochem Physiol C 99:1–5
Cherkasov AS, Biswas PK, Ridings DM, Ringwood AH, Sokolova IM (2006) Effects of acclimation temperature and cadmium exposure on cellular energy budgets in a marine mollusk Crassostrea virginica: linking cellular and mitochondrial responses. J Exp Biol 209:1274–1284
Cheung SG, Wai HY, Shin PKS (2010) Fatty acid profiles of benthic environment associated with artificial reefs in subtropical Hong Kong. Mari Pollut Bull 60:303–308
Company R, Serafim A, Cosson RP, Fiala-Médioni A, Camus L, Serrão-Santos R, João Bebianno M (2010) Sub-lethal effects of cadmium on the antioxidant defence system of the hydrothermal vent mussel Bathymodiolus azoricus. Ecotox Environ Safe 73:788–795
Costa F, Robert R, Quéré C, Wikfors GH, Soudant F (2015) Essential fatty acid assimilation and synthesis in larvae of the bivalve Crassostrea gigas. Lipids 50:503–511
Daviglus M, Sheeshka J, Murkin E (2002) Health benefits from eating fish. Comment Toxicol 8:345–374
De Camargo Talon L, de Oliveira EP, Moreto F, Portero-McLellan KC, Burini RC (2015) Omega-3 fatty acids supplementation decreases metabolic syndrome prevalence after lifestyle modification program. J Funct Foods 19:922–928
Dovzhenko NV, Kurilenko AV, Nn B’c, Chelomin VP (2005) Cadmium-induced oxidative stress in the bivalve mollusk Modiolus modiolus. Russ J Mar Biol 3(5):309–313
Dridi S, Romdhane MS, El Cafsi M (2007) Seasonal variation in weight and biochemical composition of the Pacific oyster, Crassostrea gigas in relation to the gametogenic cycle and environmental conditions of the Bizerte lagoon, Tunisia. Aquaculture 263:238–248
Drudi LM, Schaller MS, Hiramoto J, Gasper W, Harris WS, Hills NK, Grenon SM (2017) Predictors of change in omega-3 index with fish oil supplementation in peripheral artery disease. J Surg Res 210:124–131
Fang Y, Yang H, Wang T, Liu B, Zhao H, Chen M (2010) Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp Biochem Physiol C 151: 325–333
Filimonova V, Gonçalves FG, Marques JC, Troch M, Gonçalves AMM (2016) Fatty acid profiling as bioindicator of chemical stress in marine organisms: a review. Ecol Indic 67:657–672
Fokina NN, Ruokolainen TR, Nemova NN, Bakhmet IN (2013) Changes of blue mussels Mytilus edulis L. lipid composition under cadmium and copper toxic effect. Biol Trace Elem Res 154:217–225
Franco JL, Trivella DBB, Trevisan R, Dinslaken DF, Marques MRF, Bainy ACD, Dafre AL (2006) Antioxidant status and stress proteins in the gills of the brown mussel Perna perna exposed to zinc. Chem Biol Interact 160:232–240
Freites L, García N, Troccoli L, Maeda-Martínez AN, Fernández-Reiriz MJ (2010) Influence of environmental variables and reproduction on the gonadal fatty acid profile of tropical scallop Nodipecten nodosus. Comp Biochem Physiol B Biochem Mol Biol 4:408–414
Gaspar MB, Chícharo LM, Vasconcelos P, Garcia A, Santos AR, Monteiro CC (2002) Depth segregation phenomenon in Donax trunculus (Bivalvia: Donacidae) populations of the Algarve coast (southern Portugal). Sci Mar 66(2):111–121
Geret F, Serafim A, Barreira L, Bebianno MJ (2002) Effect of cd on antioxidant enzymes in the gills of the clam Ruditapes decussatus. Biomarkers 7:242–256
Gonçalves AMM, Azeiteiro UM, Pardal MA, De Troch M (2012) Fatty acid profiling reveals seasonal and spatial shifts in zooplankton diet in a temperate estuary. Estuar Coast Shelf Sci 109:70–80
Gonçalves AMM, Mesquita AF, Verdelhos T, Coutinho JAP, Marques JC, Gonçalves F (2016) Fatty acids’ profiles as indicators of stress induced by of a common herbicide on two marine bivalves species: Cerastoderma edule (Linnaeus, 1758) and Scrobicularia plana (da Costa, 1778). Ecol Indic 63:209–218
Hamdani A, Soltani-Mazouni N (2011) Changes in biochemical composition of the gonads of Donax trunculus L. (Mollusca, Bivalvia) from the gulf of Annaba (Algeria) in relation to reproductive events and pollution. Jordan J Biol Sci 4(3):149–156
Hamza-Chaffai A (2014) Usefulness of bioindicators and biomarkers in pollution biomonitoring. Int J Biotech Well Indus 3:19–26
Hendriks IE (2004) Flow dependent processes in settlement of intertidal bivalve larvae s.n. Doctor of Philosophy, university of Groningen 160p
Ivanina AI, Taylor C, Sokolova IM (2009) Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat Toxicol 91:245–254
Kainz M, Arts MT, Mazumder A (2008) Essential versus potentially toxic dietary substances: a seasonal comparison of essential fatty acids and methyl mercury concentrations in the planktonic food web. Environ Pollut 155:262–270
Ketata I, Smaoui-Damak W, Guermazi F, Rebai T, Hamza-Chaffai A (2007) In situ endocrine disrupting effects of cadmium on the reproduction of Ruditapes decussatus. Comp Biochem Physiol C Toxicol Pharmacol 146:415–430
Karray S, Tastard E, Moreau B, Delahaut L, Geffard A, Guillon E, Denis F, Hamza-Chaffai A, Chénais B, Marchand J (2015) Transcriptional response of stress-regulated genes to industrial effluent exposure in the cockle Cerastoderma glaucum. Environ Sci Pollut R 22:17303–17316
Kris-Etherto PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757
Larba R, Soltani N (2014) Use of the land snail Helix aspersa for monitoring heavy metal soil contamination in Northeast Algeria. Environ Monit Assess 186(8):4987–4995
Le TT, Zimmermann S, Sures B (2016) How does the metallothionein induction in bivalves meet the criteria for biomarkers of metal exposure? Environ Pollut 212:257–268
Manca Zeichen M, Agnesi S, Maccaroni A, Ardizzone GD (2002) Biology and population dynamics of Donax trunculus L. (Bivalvia: Donacidae) in the South Adriatic Coast (Italy). Estuar Coast Shelf S 54:971–982
Martinez G, Brokordt K, Aguilera C, Soto V, Guderley H (2000) Effect of diet and temperature upon muscle metabolic capacities and biochemical composition of gonad and muscle in Argopecten purpuratus Lamarck 1819. J Exp Mar Biol Ecol 247:29–49
Martínez-Pita I, Sánchez-Lazo C, Ruíz-Jarabo I, Herrera M, Mancera JM (2012) Biochemical composition, lipid classes, fatty acids and sexual hormones in the mussel Mytilus galloprovincialis from cultivated populations in south Spain. Aquaculture 358–359:274–283
Merad I, Soltani N (2015) Environmental risks of cadmium on Donax trunculus (Mollusca, Bivalvia): sublethal effect on nucleic acid contents of gonads In: Proceeding of INOC- International Congress “Estuaries & Coastal Protected Areas, 04–06 November 2014, Izmir– TURKEY (pp. 260–267) Turkey: ECPA
Merzouk SA, Saker M, Briksi Reguig K, Soulimane N, Merzouk H, Guermouche B, Berrouiguet AY, Hichami A, Narce M, Khan NA (2008) N-3 polyunsaturated fatty acids modulate in-vitro T cell function in type I diabetic patients. Lipids 43:485–497
Morais S, Boaventura D, Narciso L, Re P, Hawkins SJ (2003) Gonad development and fatty acid composition of Patella depressa pennant (Gastropoda: Prosobranchia) populations with different patterns of spatial distribution, in exposed and sheltered sites. J Exp Mar Biol Ecol 294:61–80
Mouëza M, Chessel D (1976) Contribution à I’étude de la biologie de Donax trunculus L. (Mollusque Lamellibranche) dans l'Algérois: analyse statistique de la dispersion le long d'une plage en baie de Bou-lsmaïl. J Exp Mar Biol Ecol 2:211–221
Muinde VM, Nguu EK, Ogoyi DO, PShiundu PM (2013) Effects of heavy metal pollution on omega-3 polyunsaturated fatty acids levels in tilapia fish from Winam gulf of lake Victoria. Open Environ Eng J 6:22–31
Munro D, Blier PU (2012) The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging Cell 11:845–855
Nardi A, Mincarelli LF, Benedetti M, Fattorini D, d'Errico G, Regoli F (2017) Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere 25(169):493–502
Nani A, Belarbi M, Ksouri-Megdiche W, Abdoul-Azize S, Benammar C, Ghiringhelli F, Hichami A, Khan NA (2015) Effects of polyphenols and lipids from Pennisetum glaucum grains on T-cell activation: modulation of Ca2+ and ERK1/ ERK2 signaling. Complement Altern Med 15:426
Neves M, Castro BB, Vidal T, Vieira R, Marques JC, Coutinho JAP, Gonçalves F, Gonçalves AMM (2015) Biochemical and populational responses of an aquatic bioindicator species, Daphnia longispina, to a commercial formulation of a herbicide (Primextra®Gold TZ) and its active ingredient (S-metolachlor). Ecol Indic 53:220–230
Ojea J, Pazos AJ, Martínez D, Novoa S, Sánchez JL, Abad M (2004) Seasonal variation in weight and biochemical composition of the tissues of Ruditapes decussates in relation to the gametogenic cycle. Aquaculture 238:451–468
Parrish CC (2013) Lipids in marine ecosystems. ISRN Oceanography:1–16
Part P, Svanberg O, Kiessling A (1985) The availability of cadmium to perfused rainbow trout gills in different water qualities. Water Res 19:427–434
Pazos AJ, Sánchez JL, Román G, Pérez-Perellé ML, Abad M (2003) Seasonal changes in lipid classes and fatty acid composition in the digestive gland of Pecten maximus. Comp Biochem Physiol 134B:367–380
Patterson J (2002) Introduction-comparative dietary risk: balance the risks and benefits of fish consumption. Comment Toxicol 8:337–344
Penha-Lopes G, Torres P, Narciso L, Cannicci S, Paula J (2009) Comparison of fecundity, embryo loss and fatty acid composition of mangrove crab species in sewage contaminated and pristine mangrove habitats in Mozambique. J Exp Mar Biol Ecol 381:25–32
Perrat E, Couzinet-Mossion A, Fossi Tankoua O, Amiard-Triquet C, Wielgosz-Collin G (2013) Variation of content of lipid classes, sterols and fatty acids in gonads and digestive glands of Scrobicularia plana in relation to environment pollution levels. Ecotoxicol Environ Safe 90:112–120
Piló D, Carvalho S, Pereira P, Gaspar MB, Leitão A (2017) Is metal contamination responsible for increasing aneuploidy levels in the Manila clam Ruditapes philippinarum? Sci Total Environ 577:340–348
Regoli F (2012) Chemical pollutants and the mechanisms of reactive oxygen species generation in aquatic organisms. In: Abele D, Vázquez-Medina JP, Zenteno- Savín T (Eds). Oxidative stress in aquatic ecosystems. John Wiley & Sons Inc, Malden, pp 308–316
Rocha TL, Gomes T, Durigon EG, Bebianno MG (2016) Subcellular partitioning kinetics, metallothionein response and oxidative damage in the marine mussel Mytilus galloprovincialis exposed to cadmium-based quantum dots. Sci Total Environ 1(554–555):130–141
Richir J, Gobert S (2014) The effect of size, weight, body compartment, sex and reproductive status on the bioaccumulation of 19 trace elements in rope-grown Mytilus galloprovincialis. Ecol Indic 36:33–47
Rocchetta I, Mazzuca M, Conforti V, Ruiz L, Balzaretti V, de Molina MCR (2006) Effect of chromium on the fatty acid composition of two strains of Euglena gracilis. Environ Pollut 141:353–358
Saito H (2004) Lipid and fatty acid composition of the pearl oyster Pinctada fucata martensii: influence of season and maturation. Lipids 39:997–1005
Saito H, Aono H (2014) Characteristics of lipid and fatty acid of marine gastropod Turbo cornutus: high levels of arachidonic and n−3 docosapentaenoic acid. Food Cheme 145:135–144
Senthamilselvan D, Chezhian A, Suresh E (2016) Synergistic effect of nickel and mercury on fatty acid composition in the muscle of fish Lates calcarifer. J Fish Aquat Sci 11(1):77–84
Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD (2011) Docosahexaenoic acid, a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors 37:399–412
Sifi K, Amira A, Soltani N (2013) Oxidative stress and biochemical composition in Donax trunculus (Mollusca, Bivalvia) from the gulf of Annaba (Algeria). Adv Environ Biol 7(4):595–604
Signa G, Di Leonardo R, Vaccaro A, Tramati CD, Mazzola A, Vizzini S (2015) Lipid and fatty acid biomarkers as proxies for environmental contamination in caged mussels Mytilus galloprovincialis. Ecol Indic 57:384–394
Smaoui-Damak W, Rebai T, Berthet B, Hamza-Chaffai A (2006) Does cadmium pollution affect reproduction in the clam Ruditapes decussatus? A one-year case study. Comp Biochem Physiol C Toxicol Pharmacol 143:252–261
Soltani N, Amira A, Sifi K, Beldi H (2012) Environmental monitoring of the Annaba gulf (Algeria): measure of biomarkers in Donax trunculus and metallic pollution. Bull Soc Zool Fr 137(1–4):47–56
Soudant P, Marty Y, Moal J, Robert R, Quere C, Lecoz JR, Samain JF (1996) Effect of food fatty acid and sterol quality on Pecten maximus gonad composition and reproduction process. Aquaculture 143:361–378
Soudant P, Van Ryckeghem K, Marty Y, Moal J, Samain JF, Sorgeloos P (1999) Comparison of the lipid class and fatty acid compositions between a reproductive cycle in nature and a standard hatchery conditioning of the pacific oyster Crassostrea gigas. Comp Biochem Physiol B 123:209–222
Swanson D, Block R, Shaker AM (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. American Society for Nutrition Adv Nutr 3:1–7. doi:10.3945/an.111.000893
Vance DE, Vance JE (2002). Biochemistry of lipids, lipoproteins and membranes. In: 4th ed. Elsevier, 624
Velma V, Tchounwou PB (2010) Chromium induced biochemical,genotoxic and histopathologic effects in liver and kidney of goldfish Carassius auratus. Mutat Res 698(1–2):43–51
Wang W, Rainbow PS (2006) Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ Chem 3:395–399
Yeung JWY, Zhou GJ, Leung KMY (2016) Sub-lethal effects of cadmium and copper on RNA/DNA ratio and energy reserves in the green-lipped mussel Perna viridis. Ecotoxicol Environ Safe 132:59–67
Acknowledgements
We are grateful to the members of Physiologie de Nutrition & Toxicologie (NUTox) laboratory (Pr. N. Khan) for technical assistance with GC analyzes. This research was supported by the Algerian Fund for Scientific Research of Algeria (Laboratory Applied Animal Biology to Pr. N. Soltani) and by the Ministry of High Education and Scientific Research of Algeria (CNEPRU Project N° F01120140104 to Pr. N. Soltani).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Cinta Porte
Rights and permissions
About this article
Cite this article
Merad, I., Bellenger, S., Hichami, A. et al. Effect of cadmium exposure on essential omega-3 fatty acids in the edible bivalve Donax trunculus . Environ Sci Pollut Res 25, 18242–18250 (2018). https://doi.org/10.1007/s11356-017-9031-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9031-4