Abstract
Nitrogen (N) deposition due to anthropogenic pollution is a major driver of the global biodiversity loss. We studied the effect of experimental N and phosphorus (P) fertilization (0, 10, 20, and 50 kg N ha−1 year−1 and 14 kg P ha−1 year−1 over the background deposition levels) on plant cover dynamics of a rosemary (Rosmarinus officinalis L.) shrubland after 8 years of nutrient addition in a semiarid Mediterranean ecosystem from Central Spain. We specifically aimed at testing whether N deposition has the potential to influence the observed expanding trend of woody vegetation into areas dominated by grassland, biological soil crusts, and bare soil. Our results show that N addition loads above 10 kg N ha−1 year−1 reverted the cover dynamics of shrubs. Under N addition conditions, N was no longer a limiting nutrient and other elements, especially P and calcium, determined the seasonal growth of young twigs. Interestingly, N fertilization did not inhibit the growth of young shoots; our estimates point to a reduced rosemary leaf lifespan that is driving individuals to death. This may be triggered by long-term accumulation of N compounds in leaves, suggesting the need to consider the old organs and tissues in long-lived perennial plants, where N toxicity effects could be more mediated by accumulation processes. Shrublands are a widely distributed ecosystem type in biodiverse Mediterranean landscapes, where shrubs play a key role as nurse plants. Therefore, the disappearance of shrublands may accelerate the biodiversity loss associated with other global change drivers, hamper the recruitment of seedlings of woody species, and, as a consequence, accelerate desertification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are generally subjected to several co-occurring environmental stresses that may be far from additive (Chapin et al. 1987; Austin et al. 2004; van der Waal et al. 2009). Mediterranean ecosystems are acknowledged as biodiversity hotspots and a prime target for conservation efforts (Myers et al. 2000). High temperatures, high light irradiance, and aridity are three of the main features of Mediterranean ecosystems determining the structure and dynamics of their vegetation (Nardini et al. 2014). To cope with these stressing factors, plants in the Mediterranean biome have developed different mechanisms of resistance (Pugnaire et al. 1996a; Qian et al. 1997; Werner et al. 1999; Balaguer et al. 2002). However, global environmental change is adding stress to natural extreme environmental factors by increasing temperatures, reducing water availability, and increasing air pollution, including N deposition (Bobbink et al. 2010; Ochoa-Hueso et al. 2011; Dias et al. 2014). Although plant adaptations to natural environmental stresses in Mediterranean ecosystems have been relatively well studied (Bussotti et al. 2014; Garbulsky et al. 2014; Matesanz & Valladares 2014), there is a need of more studies focusing on the effects of anthropogenic stressing factors, including N deposition, to understand, predict, and prevent future undesirable changes in these ecosystems that could lead to a loss of fundamental ecosystem services. Nitrogen deposition, associated with the continuous emission of anthropogenic reactive N into the atmosphere, is a direct driver of biodiversity loss (Sala et al. 2000; Galloway et al. 2004; Dentener et al. 2006; Phoenix et al. 2006; Basto et al. 2015), while it can also alter competitive interactions between plant species and modify plant community composition (Bobbink et al. 2010).
Shrublands are characteristic components of Mediterranean landscapes and are subjected to degradation in the Mediterranean basin where they may indeed cover more land surface of forests (Tomaselli 1977). Nitrogen deposition has the potential to trigger the loss of shrub cover in Mediterranean systems. This has been particularly well demonstrated in ecosystems from California (Padgett and Allen 1999), while in the Mediterranean basin, the evidence of the potential effects of N deposition on shrub cover is much scarcer. The few experimental studies carried out in Mediterranean basin shrublands consistently reveal a variable response to increased N availability that could depend on N form, plant species present, and local edapho-climatic conditions (e.g., soil chemistry and fertility, rainfall regime, water and other limiting nutrient availability, or plant interactions) (Ochoa-Hueso et al. 2011; Dias 2012). A N addition study carried out in a dense Portuguese maquis showed that higher NH4 + availability reduced scrub cover promoting soil erosion and N leaching, while higher NO3 − availability led to biomass accumulation which may increase fire risk (Dias et al. 2014). The role of P availability seems to be particularly relevant in the response of N-saturated Mediterranean-type ecosystems to increased N deposition. For example, in a study carried out in Central Spain, N addition only increased the abundance of nitrophilous annual plants where P availability was naturally high, particularly during wet years (Ochoa-Hueso and Manrique 2014). In dryland ecosystems, shrubs play a crucial role by ameliorating environmental conditions (water, fertility), providing shelter to other plant and animal species, and representing true islands of fertility. Furthermore, shrubs are fundamental to maintain soil structure and fertility after degradation processes (Sardans et al. 2005). Therefore, the loss of this kind of vegetation could compromise a wide array of ecological functions and thus the ability of these systems to provide key ecosystem services, including carbon sequestration.
In this ecological context, we aimed at studying the impacts of increasing N and P availability on the cover dynamics, the net primary production (NPP), the tissue N:P stoichiometry, and a proxy indicator of leaf lifespan (LL) of a rosemary (Rosmarinus officinalis L.) population in a limestone semiarid Mediterranean shrubland in Central Spain after 8 years of N application in the form of NH4NO3 (0, 10, 20, and 50 kg N ha−1 year−1 over the background level of ca. 6–7 kg N ha−1 year−1) and 1 year of P addition in the form of KH2PO4 (14 kg P ha−1 year−1, alone or in combination with 20 kg N ha−1 year−1). Given that the dominant mineral N form in soil according to our data was NO3-N during the 7-year experiment, we predict that rosemary cover may be increasing under current conditions but also that this trend might be reverted under the experimental N treatments due to the accumulation of reduced N (Dias et al. 2014). Secondly, we investigated the proximal causes of the harmful effect that long-term N addition may have caused in rosemary cover. We hypothesize that the rosemary cover decrease reported might be due to either a decay in NPP or a shorter LL, both driving to a reduction in photosynthetic material and triggering plant mortality. However, given that NPP responses to N may also be linked to nutritional imbalances (with P and possibly other nutrients) (Sardans et al. 2005), we hypothesize that responses to long-term N addition will promote growth in combination with P addition but only at low doses—i.e., when both nutrients are limiting. At high doses, N addition will result in a reduction of rosemary cover due to the overriding effects of ammonium toxicity.
Methods
Nitrogen fertilization experiment
We carried out a simulated N deposition experiment in the Nature Reserve El Regajal-Mar de Ontígola, 40° 00′ N, 3° 36′ W, near Madrid urban Area (Spain) (500–600 masl). The climate of the area is continental-Mediterranean with cold and wet winters and warm and dry summers. Plants are active all year round, but there are two characteristic plant activity peaks per year, one in spring and another in autumn (Navarro and Cabezudo 1998). Total rainfall amount is approximately 425 mm year−1, predominantly falling between October and May (Rivas-Martínez 1987). In Spain, kermes oak (Quercus coccifera L.) chaparral—a tall (around 2 m in our site) shrubland constituted by several individuals growing very close one each other—is frequent in thermic Mediterranean regions with marked semiarid climate (Alcaraz et al. 1987) but it is also present in Mediterranean-continental areas in Madrid province due to local particularities, sharing the space with short shrubs (20–60 cm) like rosemary, thyme, and other chamephytes (Izco 1972). The vegetation in our study site is a mosaic with two clearly different vegetation physiognomies: (1) kermes oak dense tall shrubland (hereafter chaparral patches) and (2) open interspaces where scattered small sclerophyllous rosemary shrubs are separated by open spaces where bare soil, a diverse and well-developed biological soil crust, and short-lived therophytes during the growing seasons are mixed (Crespo and Barreno 1975; Ochoa-Hueso et al. 2013a) (hereafter rosemary patches). Despite other small shrubs species different than rosemary are present in the rosemary patches at the study site, rosemary is the dominant short shrub and within our plots it is close to monospecific.
In autumn 2007, six randomized blocks were established in flat or slightly sloped areas exclusively covering rosemary patches (Fig. 1). Within each block, four 2.5- × 2.5-m plots were established, resulting in a total of 24 plots. Plot edges were not juxtaposed to avoid cross-contamination between treatments; a 1-m buffer band was disposed between contiguous plots (Fig. 2), a distance considered sufficient given the flatness of the territory. In each block, treatments were randomly assigned to each plot: 0, 10, 20, and 50 kg N ha−1 year−1. In one slightly sloped block, the plots were assigned a treatment from low N concentrations in the top of the hill to high N concentrations in lower parts to minimize the effect of unlikely cross-contamination events. A background deposition of ∼6 kg N ha−1 year−1 was estimated based on the CHIMERA model (Vivanco et al. 2009). Nitrogen was applied monthly with a hand sprayer or a watering can in 2-L doses of an aqueous solution of ammonium nitrate (NH4NO3). Due to the seasonality and unpredictability of rains in Mediterranean climate, it is common that the reactive dry N accumulate in soils and become suddenly available in high concentration peaks with the onset of rains (Fenn et al. 2003; Ochoa-Hueso et al. 2013b). The treatment simulates dry deposition because the water from the aqueous solution evaporates a few minutes after application, and only when natural precipitations occur the treatment has an effect. However, to avoid interference due to our experimental treatment, control plots are also watered with the same volume of water. The use of a 1:1 proportion of oxidized and reduced N is in agreement with rain chemistry measurements at the study site (Ochoa-Hueso et al. 2013c). Despite initially the solution was applied monthly from October to June, during summers (July–September), and since 2012 all year, fertilization was applied in a 3-month basis, adjusting the total N load.
L eft: Map of the study site and the location of the six experimental blocks (B1 to B6) in the open spaces between kermes oak tall shrublands, where the vegetation is a mixture of scattered rosemary small shrubs and bare soil, a well-developed biological crust, and annual plants during the growing seasons. Photo by courtesy of Google Earth 2016
Rosemary shrubs had been previously found to be unresponsive to N fertilization even though P addition promoted their growth (Sardans et al. 2005), suggesting that P could be the real limiting nutrient in sites similar to our study area where P is usually immobilized under insoluble forms (calcium phosphate). Therefore, to assess the effects of increased P availability and its interactions with N deposition, two new plots with the same vegetation characteristics were set up in early March 2015 in each previously defined block (Fig. 2), summing a total of 36 plots, six per block. The new plots were assigned to one of the following treatments: (1) 0 kg N ha−1 year−1 + 14 kg P ha−1 year−1 and (2) 20 kg N ha−1 year−1 + 14 kg P ha−1 year−1. This amount of added P is sufficient to increase P availability to enhance plant growth in P-depleted soils (Kanzler et al. 2015). The application of N in these new plots was done following the methodology described above. Phosphorus was added once per year in a 2-L aqueous solution in the form of KH2PO4. This form has been chosen because our results over 7 years showed no response of rosemary plants to soil K measured levels.
Soil analysis
We collected and pooled three soil cores (2.0 cm diameter × 0–4 cm depth) in each of the 24 plots as described in Ochoa-Hueso et al. (2013b) by the end of each of the following seasons: autumn 2008, winter 2008, spring 2009, summer 2009, spring 2010, spring 2011, autumn 2011, autumn 2013, autumn 2014, spring 2015, and autumn 2015 (Fig. 3). Soil extractable NO3-N and NH4-N were included in a N dataset (N24) containing six variables with 24 data each (N treatment plots only) representing soil extractable NH4 and NO3 data collected in autumn 2014, spring 2015, and autumn 2015. The variables are as follows: [NO3]autumn 2014, [NH4]autumn 2014, [NO3]spring 2015, [NH4]spring 2015, [NO3]autumn 2015, and [NH4]autumn 2015.
Extractable P for soil samples from autumn 2008 until autumn 2013 was extracted following the Burriel and Hernando (1950) method (see Ochoa-Hueso et al. 2013b); also, extractable P of samples from spring 2010 until spring 2016 was measured in the same extract made with 1 M KCl. A P dataset (P24) was put together with four variables with 24 data each (N treatment plots only) representing soil extractable P measures, grouped to obtain mean values for 2 to 3-year periods. The variables are as follows: [P2O5]2008–2009 (mean of Burriel-Herrando extractions for autumn 2008, winter 2008, spring 2009, and summer 2009), [P2O5]2010–2011 (mean of Burriel-Herrando extractions for spring 2010, spring 2011, and autumn 2011), [PO4]2010–2011 (mean of KCl extractions for spring 2010, spring 2011, and autumn 2011), and [PO4]2013–2014 (mean of KCl extractions for autumn 2013, autumn 2014, spring 2015, and autumn 2015).
In autumn 2015, a complete soil nutrient analysis was performed in samples collected for both the 24 eight-year-old plots and the 12 new P plots. We collected data for extractable NO3, NH4, PO4, total C, total N (organic N + inorganic N) and total P, pH, and extractable K, Mg, Ca, Fe, Mn, Zn, and Cu. A first soil nutrient (Sn36) dataset groups 15 variables with 36 data each (24 N treatment and 12 new P treatment plots). The variables are all the measures from autumn 2015 (see above). A second soil micronutrient (Sm24) dataset includes three variables with 24 data each (N treatment plots only) representing soil micronutrient data collected on autumn 2015. The micronutrients selected for Sm24 are those affecting rosemary NPP according to our data analysis presented below. Variables are Na, Ca, and Mn (extractable).
Rosemary measures
To analyze the dynamics of rosemary population, we accounted for four response variables. The main response variable studied was Δcover (difference in % rosemary at the beginning and the end of the 7-year period). In order to understand the mechanisms involved in the cover dynamics response, we measured several physiological response variables, including net primary production (NPP as dry weight biomass production per twig), N:P stoichiometry in new leaves, and the plant volume defoliated (VD) that serves as a proxy of LL.
Δcover was calculated in the 24 N-treated plots based on the assumption that the horizontal geometric projection of each individual shrub has a shape similar to an ellipse. We measured firstly the maximum horizontal diameter of each individual (D 1) and secondly its perpendicular horizontal diameter intersecting with the former one in the geometric center of the projection of the shrub (D 2). The ellipse area of each individual was estimated by means of the formula π · D 1 · D 2, and the plant cover in each plot was then expressed as the summed area of the ellipses in each plot on a percentage basis of the total plot area. This percentage cover was measured twice, first in spring 2008 and second in summer 2015. The evolution of rosemary cover (Δcover) is the increment in shrub cover, calculated as follows:
Both in 2008 and 2015 all plots located in rosemary patches were rather covered by rosemary short shrubs or deprived of woody vegetation cover, and kermes oak recruitment was not reported within our plots by the end of the period. Therefore, a positive Δcover value indicates an expansion of rosemary over bare soil, biological crust, and annual forbs space, and a negative value indicates the opposite dynamics, i.e., decay in short shrub encroachment and expansion of bare soil, biological crust, and annual forbs.
NPP was measured in October 2015, by the end of the autumn activity peak and after the summer drought which could interfere with the plant response to N in a dose-dependent manner. We randomly collected five twigs from five rosemary shrubs growing in each plot—including those treated with P. The twigs collected for the measurement fully grew during a plant activity peak posterior to the establishment of the P-treated plots, and therefore we assume that this biomass production was affected by the treatment in equal condition that the one exposed to the N treatment. Each twig was processed in the laboratory to separate the last growth from the older parts of the plant. While old parts of the stems present a gray chapped epithelium and scarcity of leaves, the new growth of the stems can be easily discerned by the russet color of the epithelium, the abundance of new leaves, and especially by the presence of ramifications in the base of the new annual growth. In apices where annual growth was scant and neither new twigs nor ramifications were discernible, we assumed that the last leaves, with a lighter green color, developed in the immediate previous growing season. New growths were cut from the twigs, counted, oven dried at 65 °C to constant weight, and weighted. The mean value per twig in each plot of the new growths’ dry weight in grams was used as an NPP estimate.
N:P stoichiometry in plant tissues is a reliable indicator of the limitation and co-limitation levels of both N and P in the soil (Tessier and Raynal, 2003) and is often studied as an indicator of the ecosystem response to N deposition (Sardans et al. 2012). Data on N:P stoichiometry of leaves from the last activity peak were collected by the end of June 2015, using the same collection process described above for NPP, in all the 36 plots including new P-treated plots. The tissue collected for the chemical analysis grew in an activity peak posterior to the establishment of the P-treated plots, and therefore we assume this tissue was affected by the treatment. Twigs were oven dried at 65 °C to constant weight and then milled. Due to its hardness and high resin content, leaf dried tissues were first frozen in liquid N, crushed in a mortar, and then powdered in a ball mill for chemical analysis. The plant powder was digested following the Kjeldahl protocol and analyzed for total P (ammonium molybdate method, Murphy and Riley 1962) and organic N.
The VD measures where estimated in the 24 N-treated plots by the end of June 2016. For each individual shrub, VD was visually estimated by two independent researchers previously trained as 3D volume included in the shrub geometric space that was lacking leaves, in a scale from 0 to 100% in intervals of 10%. The final value for each shrub was the mean value estimated by both researchers. The VD value for each plot was calculated as the mean value of the VD estimated for all the individual shrub within the plot. The volume of the plant that is lacking leaves is inversely proportional to the volume populated with leaves, and therefore VD is the result of an equilibrium between the leaf production (proportional to NPP) and the leaf fall (explained by LL). Controlling for NPP, VD is therefore a reliable proxy of LL: for a similar NPP, a higher VD will indicate shorter LL. A plot showing a higher VD but same NPP than other growing under optimal conditions would be indicating premature defoliation.
Statistical analysis
Δcover dataset including 24 observations for N-treated plots was firstly analyzed by means of a one-way ANOVA model considering only the N treatment and secondly by means of ANCOVA models considering the N treatment as factor and the soil phosphorus dataset P24 and soil micronutrient dataset Sm24 as covariates. The NPP dataset including 36 observations for both N- and P-treated plots was firstly analyzed by means of two-way ANOVA models considering the N and P treatments and their interaction. Secondly, we developed linear models where the soil nutrient dataset Sn36 was used as independent variables. Finally, we performed ANCOVA models combining the N and P treatments as factors and Sn36 as covariates excluding in this case extractable NO3, NO2, NH4, and PO4 measurements that would not be independent of the factors. The N:P ratio dataset including 36 observations for both N- and P-treated plots was also analyzed by means of a two-way ANOVA including both N and P treatments as factors. The VD dataset including 24 observations for N-treated plots was analyzed by means of ANCOVA models using the soil nitrogen dataset N24 as covariates. Despite N24 and the N treatment are not completely independent, we decided to use this dataset as covariate in the VD models because it helps to understand the intrinsic variability of the soils in each block and the differential effect of reduced and oxidized forms of N. All the above models were validated checking the homoscedasticity and normality and visualizing the Leverage and Cook’s distance values for all the observations. In the case of NPP, models including factors were fitted using the sum contrast matrix. All linear models where performed using a [3, −1, −1, −1] contrast matrix, as previous prospection of the data for all the variables considered in this study clearly revealed no differences between the three N addition levels but pointed to differences with the control.
For each response variable, best-fitting models were selected using the Akaike information criterion for small samples (AICC), a Bayesian likelihood-based measure of model fit. The method, based on the information theory, allows to select the models that minimize the Kullback–Leibler distance between the model and the truth (Burnham and Anderson 2002). Model selection was carried out using a stepwise regression procedure. To avoid relying on a single best model when alternative models are similarly well fitted (Whittingham et al. 2006), we calculated AICC weights (w i), which provides a relative weight of evidence for each model. Models were compared to the best-fitting model by means of the Kullback–Leibler evidence ratio (K-L) (w i/w best-fitting). Models that resulted to be less than four times worse than the best-fitting model (K-L ratio ≤ 4) are reported as “similar” to the best-fitting model (Wagenmakers and Farrell 2004). The parameter significance in the best-fitting model and the similar models was checked to find significant factors or covariates affecting the response variable. All analyses are performed using R version 3.2.2 (R Core Team 2016) in Rstudio (version 0.99.879).
Results
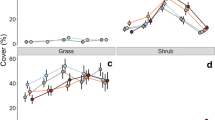
We found that 7 years of N addition reduced rosemary shrub cover. Changes in cover did not differ from zero or were negative in the N-treated plots, showing a trend to recession or stabilization of shrub encroachment, while it was positive under control conditions, indicating encroachment over the same period (Fig. 4a and Table 1). The Δcover best-fitting model also points to a significant role of soil extractable P measured in 2010–2011, Na, and Ca (Suppl. Tables 1A, B, C).
Plot of mean values and standard errors. a Cover change of rosemary shrub between 2008 and 2015 for the different N fertilization treatments. b NPP measured as twig annual growth. c VD. d N:P stoichiometry in leaves of new bourgeons; all variables presented for the different N fertilization treatments. 0 N, 10 N, 20 N, and 50 N refer to the treatments with 0, 10, 20, and 50 kg N ha−1 year−1, respectively. Significant different groups (p < 0.05) in all best-fitting models are marked with the asterisk symbol
Regarding the proximal causes of this decrease in cover over time in response to nutrient addition, neither N fertilization (Fig. 4b) nor P fertilization did affect NPP, which was better explained by linear models including only soil measures. All NPP best-fitting models showed significant effects of pH, Na, Ca, Mn, and P2O5 (Suppl. Tables 2A, B, C, D). In the NPP best-fitting model, P2O5 and Ca had markedly high p values and positive slopes (Suppl. Table 1A and Fig. 5), suggesting an effect mediated by P that was not detected with our P treatment. In contrast, N addition significantly increased VD in rosemary plants (Fig. 4c), and NH4 + measures from spring 2015 and autumn 2015, but not spring 2014, were significant covariates in the two best-fitting models (Table 2 and Suppl. Table 3). Finally, N:P stoichiometry was not significantly affected by N and P additions (F statistic = 1.947 on 4 and 31 df, p value = 0.13). The N:P ratio was on average 13.14 ± 1.4SE, and it was not significantly altered by N nor by P addition treatments (Fig. 4d).
Discussion
In this study, we have shown that increased N deposition has the potential to reduce rosemary cover due to deleterious effects of excessive N on rosemary annual growth. Rosemary is a widespread shrub in the Mediterranean basin that is capable of colonizing nutrient-poor calcareous soils (Sardans et al. 2005), which makes observations from our site transferable to other similar locations throughout the Mediterranean Basin. Therefore, based on our local results, we predict that a small further increase in chronic N deposition levels in the Mediterranean basin could lead to a widespread decay of this type of shrublands. Shrub cover in semiarid ecosystems influences soil moisture via shading (Valladares et al. 2016) and nutrient availability due to litter accumulation (Maestre and Cortina 2005; Gimeno-García et al. 2001). By means of these mechanisms, short shrubs act as nurse plants in Mediterranean systems making easier the growth and survival of herbaceous species (Pugnaire and Lázaro 2000; Brooker et al. 2008, McIntire and Fajardo 2014) and increasing the productivity and the phylogenetic diversity of the plant community (Pugnaire et al. 1996b; Moro et al. 1997; Valiente-Banuet and Verdú 2007). Consequently, a reduction in shrub cover may indirectly enhance the loss of ecosystem services that is directly attributable to N deposition (Bobbink et al. 2010; Ochoa-Hueso et al. 2011). Furthermore, shrub facilitation makes also possible the recruitment of seedlings and saplings of trees and bigger woody species whose long-term success often relies on facilitation interactions (Rousset and Lepart 1999; Gómez-Aparicio et al. 2005a, b; Kunstler et al. 2006; Cuesta et al. 2010; Puértolas et al. 2010); hence, a reversion in the cover dynamic of shrub cover might impede the recruitment of kermes oak and threat this chaparral system. Finally, both the decay in rosemary expansion and the likely consequential limitation of kermes oak encroachment may result in an acceleration of desertification (Maestre et al. 2009).
We have shown that total levels of N deposition over 6–16 kg N ha−1 year−1 (i.e., 0–10 kg N ha−1 year−1 + background deposition) have the potential to revert the trend of expansion of the dominating woody vegetation. The observed shift in rosemary cover corresponds to a population dynamic change from encroachment to stabilization or likely recession (Fig. 6). Our results demonstrate that there is a natural expansion trend of the rosemary shrubland over the bare soil where biological crust and some annual forbs develop in control plots, whereas this trend would be at least neutralized, if not reverted if N deposition increases in our study sit—our 10 N treatment shows a clear negative Δcover, whereas the 20 N and 50 N treatments show negative mean values but the deviations are too high as to allow us to state they are nonnegative (Fig. 4a). This range of N deposition values coincides with the range of the average critical load (10 to 15 kg N ha−1 year−1) recently published in a study conducted with several plant species (Payne et al. 2013). The expansion trend found under the control conditions was reinforced by observations of recruitment of new rosemary individuals in our control plots and in the wider study area (personal observations). Furthermore, rosemary is an obligate seeder (Sardans et al. 2005), and we have observed rosemary seed activity and a healthy soil seedbank at the study site (unpublished data).
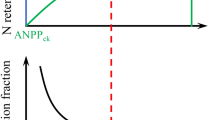
Schematic display of our main conclusions. a Background N deposition and experimental levels of N fertilization and the response of shrub cover over the 7-year experiment. b Defoliation levels scaled to the N addition gradient scale above. Note that at the control treatment, there is defoliation in lower than in the experimental fertilization treatments. c Growth of annual shoots scaled to the same N addition gradient scale. Note that there are no differences between the four experimental treatments because the background deposition is over the N-limiting load, and there is no growth decrease observed due to toxicity
To better understand the processes causing this N deposition-dependent cover change, we studied how N fertilization affected some leaf physiological parameters of rosemary shrub individuals. The N:P ratio value estimated for all our plots without significant differences between treatments is around 13, a level indicating that P and N are co-limiting nutrients (Tessier and Raynal 2003; Güsewell 2004), meaning either that both are equally limiting plant growth or that nutrient is actually a limiting factor. Our N and P fertilization experiments show no detectable effects on rosemary NPP; therefore, the N:P ratio indicates no N-P-limitation in El Regajal at the current background deposition level. We can conclude that, above the level at which N is a limiting nutrient, N deposition has no detectable deleterious effect on NPP and on the growth of new bourgeons. However, despite not affecting new leaf tissues, chronic N addition increased the VD of plants reducing the plant’s photosynthetic material. Along with the NPP results, we deduce that the higher VD of N-fertilized plants indicate premature leaf fall and therefore a shorter LL. Thus, the recession of shrub cover not related to a decrease in plant growth is caused by adult individuals’ death due to defoliation (Fig. 6).
Our VD best-fitting models show a significant effect of ammonium nitrate measured on spring 2015 and autumn 2015 on the defoliation measured by the end of the spring 2016, but previous soil measures of NH4 + and the NO3 − measures do not have any effect. The presence of high levels of NH4 + in soil, despite apparently not affecting the new twigs NPP, causes long-term harms that are detectable in leaves from the former activity peaks. NH4 + measures prior to spring 2015 may have had an effect on leaves from older cohorts, but we might have been unable to detect this effect due to the fact that these cohorts of leaves did already reach the end of their natural life cycle even in the control plots. That being so, the results suggest that rosemary leaves have a maximum LL of 3 activity peaks (1.5 years) in our study site, a value not unreasonable given that rosemary is a semideciduous species with a mean LL of ∼11 months (Villar and Merino 2001; Granati and Varone 2004). While growing bourgeons may have a control over the use of N, chronic exposition to sap with high concentration of NH4 + may accumulate this element in tissues over time, driving them to reach toxic levels. Other studies have reported similar N accumulation processes in mosses (Pesch et al. 2008) and coniferous trees (Blanes et al. 2013). For example, Blanes et al. (2013) observed that, in N-limited sites, N content in leaves typically decreases in older needles of Abies pinsapo, whereas it increases with leaf age in N-saturated sites; the overload of N in trees at N-saturated sites exceeded the homeostatic regulation capacity in terms of foliar stoichiometry.
Our results show that long-term N fertilization experiments are essential to assess the actual effects of N deposition on shrub dynamics. In short-term experiments, in which accumulation processes may not occur, the response of woody vegetation to chronic N deposition might be hidden. We also suggest the importance of accounting for the response of older plant organs—as opposed to only new ones—to better characterize the ecophysiological response of woody plants as a diagnostic tool in chronic N deposition/fertilization studies. Neglecting them may hinder the finding of evidences of effects mediated by chemical accumulation processes, even in long-term experiments.
Conclusions
We have shown that long-term reactive-N enhanced availability in soils damages old leaves of rosemary shrubs and reduces LL, not affecting NPP during the plant activity peaks. These findings point to the fact that N deposition may harm rosemary plants due to its accumulation in old leaf tissues by reaching toxic concentrations. Nitrogen deposition is a global change driver that may drive shrub cover, particularly in rosemary populations, to decay in Mediterranean ecosystems. The effects of N deposition on shrub cover may also enhance the loss of herbaceous plant biodiversity and productivity, reversing the chaparral-thicket encroachment and, therefore, accelerating the desertification due to climate change. Further ecosystem-level studies that confirm these hypotheses are strongly desirable and would allow shaping a general model of how N deposition in semiarid shrublands alters the spatial and temporal dynamic response of the plant community. Finally, future empiric studies approaching N deposition effects on woody vegetation may consider accumulation processes by means of studying long-term effects of N addition on woody plants’ old tissues.
References
Alcaraz F, Peinado M, Rivas-Martínez S et al (1987) La vegetación de España. Universidad de Alcala de Henares, Secretaria General, Servicio de Publicaciones
Austin AT, Yahdjian L, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia 141:221–235
Balaguer L, Pugnaire FI, Martínez-Ferri E, Armas C, Valladares F, Manrique E (2002) Ecophysiological significance of chlorophyll loss and reduced photochemical efficiency under extreme aridity in Stipa tenacissima L. Plant Soil 240:343–352
Basto S, Thompson K, Phoenix G, Sloan V, Leake J, Rees M (2015) Long-term nitrogen deposition depletes grassland seed banks. Nature. doi:10.1038/ncomms7185
Blanes A, Viñegla B, Merino J, Carreira AJ (2013) Nutritional status of Abies pinsapo forests along a nitrogen deposition gradient: do C/N/P stoichiometric shifts modify photosynthetic nutrient use efficiency? Oekologia 171(4):797–808
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman JW, Fenn M, Gilliam F, Nordin A, Pardo L, de Vries W (2010) Global assessment of N deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20(1):30–59
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres L, Kunstler G et al (2008) Facilitation in plant communities: the past, the present and the future. J Ecol 96:18–34
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Burriel F, Hernando V (1950) El fósforo en los suelos españoles V Nuevo método para determinar el fósforo asimilable en los suelos. Anales de Edafología y Agrobiología 9:611–622
Bussotti F, Ferrini F, Pollastrini M, Fini A (2014) The challenge of Mediterranean sclerophyllous vegetation under climate change: from acclimation to adaptation. Environ Exp Bot 103:80–98
Chapin FS III, Bloom AJ, Field CB, Waring RH (1987) Plant-responses to multiple environmental factors. Boscience 37:49–57
Crespo A, Barreno E (1975) Ensayo florístico y ecológico de la vegetación liquénica de los yesos del centro de España (Fulgensietalia desertori). Anal Inst Bot Cavanilles 32:873–908
Cuesta B, Villar-Salvador P, Puértolas J, Rey Benayas JM, Michalet R (2010) Facilitation of Quercus ilex in Mediterranean shrubland is explained by both direct and indirect interactions mediated by herbs. J Ecol 98:687–696
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Horowitz LW, Krol M, Kulshrestha UC, Lawrence M, Galy-Lacaux C, Rast S, Shindell D, Stevenson D, Van Noije T, Atherton C, Bell N, Bergman D, Butler T, Cofala J, Collins B, Doherty R, Ellingsen K, Galloway J, Gauss M, Montanaro V, Müller JF, Pitari G, Rodriguez J, Sanderson M, Solmon F, Strahan S, Schultz M, Sudo K, Szopa S, Wild O (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Glob Biogeochem Cycles. doi:10.1029/2005GB002672
Dias T (2012) Effects of increased nitrogen availability on the structure and functioning of a Mediterránean Basin maquis. Doctoral Thesis. Universidade de Lisboa, Faculdade de Ciências, Departamento de Biologia Vegetal. Lisboa, Portugal
Dias T, Clemente A, Martins-Loução MA, Sheppard L, Bobbink R, Cruz C (2014) Ammonium as a driving force of plant diversity and ecosystem functioning: observations based on 5 years’ manipulation of N dose and form in a Mediterranean ecosystem. PLoS One. doi:10.1371/journal.pone.0092517
Fenn ME, Baron JS, Allen EB, Rueth HM, Nydick KR, Geiser L (2003) Ecological effects of nitrogen deposition in the western United States. Bioscience 53(4):404–420
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DMA, Michaels F, Porter JH, Townsend AR, Vöosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Garbulsky MF, Filellab I, Vergerb A, Peñuelas J (2014) Photosynthetic light use efficiency from satellite sensors: from global to Mediterranean vegetation. Environ Exp Bot 103:3–11
Gimeno-García E, Andreu V, Rubio JL (2001) Influence of Mediterranean shrub species on soil chemical properties in typical Mediterranean environment. Commun Soil Sci Plant Anal 32:1885–1898
Gómez-Aparicio L, Gomez JM, Zamora R, Bottinguer JL (2005a) Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J Veg Sci 16:191–198
Gómez-Aparicio L, Valladares F, Zamora R, Quero JL (2005b) Response of tree seedlings to the abiotic heterogeneity generated by nurse shrubs: an experimental approach at different scales. Ecography 28(6):757–768
Granati L, Varone L (2004) Leaf key traits of Erica arborea L., Erica multiflora L. and Rosmarinus officinalis L. co-occurring in the Mediterranean maquis. Flora 199:58–69
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Izco J (1972) Coscojares, romelares y tomillares de la Provincia de Madrid. Anal Inst Bot Cavanilles 29:69–108
Kanzler M, Böhm C, Freese D (2015) Impact of P fertilisation on the growth performance of black locust (Robinia pseudoacacia L.) in a lignite post-mining area in Germany. Annals of Forest Research 58:39–54
Kunstler G, Curt T, Bouchaud M, Lepart J (2006) Indirect facilitation and competition in tree species colonization of sub-Mediterranean grasslands. J Veg Sci 17:379–388
Maestre FT, Cortina J (2005) Remnant shrubs in Mediterranean semi-arid steppes: effects of shrub size, abiotic factors and species identity on understorey richness and occurrence. Acta Oecol 27:161–169
Maestre FT, Bowker MA, Puche MD, Hinojosa MB, Martínez I, García-Palacios P, Castillo AP, Soliveres S, Luzuriaga AL, Sánchez AM, Carreira JA, Gallardo A, Escudero A (2009) Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol Lett 12(9):930–941
Matesanz S, Valladares F (2014) Ecological and evolutionary responses of Mediterranean plants to global change. Environ Exp Bot 103:53–67
McIntire EJB, Fajardo A (2014) Facilitation as a ubiquitous driver of biodiversity. New Phytol 201:403–416
Moro MJ, Pugnaire FI, Haase P, Puigdefábregas J (1997) Effect of the canopy of Retama sphaerocarpa on its understorey in a semiarid environment. Funct Ecol 11:425–431
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nardini A, Lo Gullo MA, Trifilò P, Salleo S (2014) The challenge of the Mediterranean climate to plant hydraulics: responses and adaptations. Environ Exp Bot 103:68–79
Navarro T, Cabezudo B (1998) Estrategias Fenomorfológicas de Especies de un Matorral Mediterráneo (Andalucía, España). Acta botánica malacitana 23:133–148 ISSN 0210-9506
Ochoa-Hueso R, Allen EB, Branquinho C, Cruz C, Dias T, Fenn ME, Manrique E, Pérez-Corona ME, Sheppard LJ, Stock WD (2011) N deposition effects on Mediterranean-type ecosystems: an ecological assessment. Environ Pollut 159:2265–2279
Ochoa-Hueso R, Mejías-Sanz V, Pérez-Corona ME, Manrique E (2013a) Nitrogen deposition effects on tissue chemistry and phosphatase activity in Cladonia foliacea (Huds.) Willd., a common terricolous lichen of semi-arid Mediterranean shrublands. J Arid Environ 88:78–81
Ochoa-Hueso R, Stevens CJ, Ortiz-Llorente MJ, Manrique E (2013b) Soil chemistry and fertility alterations in response to N application in a semiarid Mediterranean shrubland. Sci Total Environ. doi:10.1016/j.scitotenv.2013.02.049
Ochoa-Hueso R, Maestre FT, de los Ríos A, Valea S, Theobald MR, Vivanco MG, Manrique E, Bowker MA (2013c) N deposition alters N cycling and reduces soil carbon content in low-productivity semiarid Mediterranean ecosystems. Environ Pollut 179:185–193
Ochoa-Hueso R, Manrique E (2014) Impacts of altered precipitation, nitrogen deposition and plant competition on a Mediterranean seed bank. J Veg Sci 25(5):1289–1298
Padgett PE, Allen EB (1999) Differential responses to nitrogen fertilization in native shrubs and exotic annuals common to Mediterranean coastal sage scrub of California. Plant Ecol 144:93–101
Payne RJ, Dise NB, Stevens CJ, Gowing DJ, BEGIN Partners (2013) Impact of N deposition at the species level. PNAS 110(3):984–987
Pesch R, Schröder W, Schmidt G, Gensslerb L (2008) Monitoring N accumulation in mosses in central European forests. Environ Pollut 155(3):528–536
Phoenix GK, Hicks WK, Cinderby S, Kuylenstierna JCI, Stock WD, Dentener FJ, Giller KE, Austin AT, Lefroy RDB, Gimeno BS, Ashmore MS, Ineson P (2006) Atmospheric nitrogen deposition in world biodiversity hotspots: the need for a greater global perspective in assessing N deposition impacts. Glob Chang Biol 12:470–476
Puértolas J, Rey Benayas JM, Michalet R (2010) Facilitation of Quercus ilex in Mediterranean shrubland is explained by both direct and indirect interactions mediated by herbs. J Ecol 98:687–696
Pugnaire FI, Haase P, Incoll LD, Clark SC (1996a) Response of the tussock grass Stipa tenacissima to watering in a semi-arid environment. Funct Ecol 10:265–274
Pugnaire FI, Haase P, Puigdefábregas J, Cueto M, Clark SC, Incoll LD (1996b) Facilitation and succession under the canopy of a leguminous shrub, Retama shaerocarpa, in a semi-arid environment in southeast Spain. Oikos 76:455–564
Pugnaire FI, Lázaro R (2000) Seed bank and understorey species composition in a semi-arid environment: the effect of shrub age and rainfall. Ann Bot 86:807–813
Qian YL, Fry JD, Upham WS (1997) Rooting and drought avoidance of warm-season turfgrasses and tall fescue in Kansas. Crop Sci 37:905–910
Rivas-Martínez S (1987) Memoria del mapa de series de vegetación de España. ICONA, Madrid
Rousset O, Lepart J (1999) Shrub facilitation of Quercus humilis (downy oak) dynamics on calcareous grasslands. J Veg Sci 10:493–502
Sala OE, Chapin FS III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB et al (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sardans J, Rodà F, Peñuelas J (2005) Effects of water and a nutrient pulse supply on Rosmarinus officinalis growth, nutrient content and flowering in the field. Environ Exp Bot 53:1–11
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The C:N:P stoichiometry of organisms and ecosystems in a changing world: a review and perspectives. Perspectives in Plant Ecology, Evolution and Systematics 14:33–47
Tomaselli R (1977) The degradation of the Mediterranean Maquis. Ambio 6(6) SI The Mediterranean:356–362
Tessier JT, Raynal DJ (2003) Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J Appl Ecol 40:523–534
Valiente-Banuet A, Verdú M (2007) Facilitation can increase the phylogenetic diversity of plant communities. Ecol Lett 10:1029–1036
Valladares F, Laanisto L, Niinemets Ü, Zavala MA (2016) Shedding light on shade: ecological perspectives of understorey plant life. Plant Ecology & Diversity. doi:10.1080/17550874.2016.1210262
Van der Waal C, de Kroon H, de Boer WF, Heitkönig IMA, Skidmore AK, de Knegt HJ, van Langeveldem F, van Wieren SE, Grant RC, Page BR, Slotow R, Kohi EM, Mwakiwa E, Prins HHT (2009) Water and nutrients alter herbaceous competitive effects on tree seedlings in a semi-arid savannah. J Ecol 97:430–439
Villar R, Merino J (2001) Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytol 151:213–226
Vivanco MG, Palomino I, Vautani R, Bessaget B, Martín F, Menut L, Jimenez S (2009) Multi-year assessment of photochemical air quality simulation Spain. Environ Model Softw 24:63–73
Wagenmakers EJ, Farrell S (2004) Notes and comments: AIC model selection using Akaike weights. Psychon Bull Rev 11(1):192–196
Werner C, Correia O, Beyschlag W (1999) Two different strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought. Acta Oecol 20:15–23
Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol 75(5):1182–1189
Acknowledgements
This research was financially supported by a research grant of the Spanish Ministerio de Economía y Competitividad (BIOMOD CGL-2013-44661-R). Comunidad de Madrid also funded this work through REMEDINAL-3 project (S2013/MAE-2719). The authors are very thankful to the owners of “Finca El Regajal” for their kind permission to work in their property. They are also very thankful to Lucas Lecha for helping during the field and lab work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Cabal, C., Ochoa-Hueso, R., Pérez-Corona, M.E. et al. Long-term simulated nitrogen deposition alters the plant cover dynamics of a Mediterranean rosemary shrubland in Central Spain through defoliation. Environ Sci Pollut Res 24, 26227–26237 (2017). https://doi.org/10.1007/s11356-017-8879-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8879-7