Abstract
Anthropogenic nitrogen (N) deposition is significantly altering both community structure and ecosystem processes in terrestrial ecosystems across the globe. However, our understanding of the consequences of N deposition in dryland systems remains relatively poor, despite evidence that drylands may be particularly vulnerable to increasing N inputs. In this study, we investigated the influence of 7 years of multiple levels of simulated N deposition (0, 2, 5, and 8 kg N ha−1 year−1) on plant community structure and biological soil crust (biocrust) cover at three semi-arid grassland sites spanning a soil texture gradient. Biocrusts are a surface community of mosses, lichens, cyanobacteria, and/or algae, and have been shown to be sensitive to N inputs. We hypothesized that N additions would decrease plant diversity, increase abundance of the invasive annual grass Bromus tectorum, and decrease biocrust cover. Contrary to our expectations, we found that N additions did not affect plant diversity or B. tectorum abundance. In partial support of our hypotheses, N additions negatively affected biocrust cover in some years, perhaps driven in part by inter-annual differences in precipitation. Soil inorganic N concentrations showed rapid but ephemeral responses to N additions and plant foliar N concentrations showed no response, indicating that the magnitude of plant and biocrust responses to N fertilization may be buffered by endogenous N cycling. More work is needed to determine N critical load thresholds for plant community and biocrust dynamics in semi-arid systems and the factors that determine the fate of N inputs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities have roughly doubled the amount of reactive nitrogen (N), in turn modifying the global N cycle and altering ecosystem processes across the world (e.g., Vitousek et al. 1997, 2013; Fenn et al. 2003; Phoenix et al. 2006; Duce et al. 2009). Increasing N deposition has been shown to drive dramatic changes in plant community diversity and structure (Baer et al. 2003; Bobbink et al. 2010; Simkin et al. 2016), with downstream implications for a range of ecosystem functions—such as biomass production and nutrient cycling (Tilman et al. 2006; Isbell et al. 2011, 2013). Yet, while arid and semi-arid systems likely have different responses to N deposition than their mesic counterparts, dryland ecosystems have received relatively less research attention (Fenn et al. 2003). Despite growing interest in understanding how dryland systems will respond to increasing N deposition (e.g., Sinsabaugh et al. 2015), large knowledge gaps remain regarding the effects of increasing reactive N inputs on dryland organisms and their function.
Pulse-driven semi-arid systems generally have low soil-resource availability and often maintain high levels of plant species diversity (Hooper and Johnson 1999; Chesson et al. 2004; Yahdjian et al. 2011). Nitrogen deposition has the potential to release plants from N limitation, leading to increased productivity, competitive exclusion, and decreased plant diversity (Padgett and Allen 1999). In Mediterranean ecosystems of California, where the majority of dryland depositional studies have occurred, N deposition has been shown to drive drastic structural changes to plant community composition through increases in non-native plant invasion (Allen et al. 1998, 2016; Cox et al. 2014; Kimball et al. 2014; Ochoa-Hueso et al. 2011; Talluto and Suding 2008). However, anthropogenic N deposition in southern California can be orders of magnitude larger (20–45 kg N ha−1 year−1) than the deposition occurring in many other drylands (Fenn et al. 2003; Ochoa-Hueso et al. 2011; Vourlitis 2012). For example, semi-arid ecosystems on the Colorado Plateau commonly receive between 3 and 5 kg N ha−1 year−1 anthropogenic deposition (NPS ARD 2018) and responses to these lower level inputs could be different from those in southern California or near urban centers. While deposition rates are currently low, there is potential for downwind deposition to semi-arid ecosystems to increase with increasing urbanization and agriculture (Dentener et al. 2006), presenting novel challenges to land managers and policymakers.
Dryland ecosystems are believed to be extremely sensitive to increases in N deposition, maintaining lower critical load thresholds—defined as the amount of atmospheric pollutant required for an ecosystem response to be observed—than many mesic ecosystems (Fenn et al. 2003; Pardo et al. 2011; Blett et al. 2014; Sinsabaugh et al. 2015). Relative to mesic systems, drylands have low hydrological export, which, coupled with low denitrification rates and relatively low and sporadic demand of N by plants, can lead to the accumulation of inorganic N in the soil. These factors may help drive a higher sensitivity of dryland biota to anthropogenic N inputs (Walvoord et al. 2003). However, a previous study undertaken at one of our study sites in Arches National Park found that soil microbial communities were unresponsive to multiple years of N addition, due at least in part to a short residence time of N (McHugh et al. 2017), and another study in the Chihuahuan desert found that plant species richness was unchanged by experimental N additions (Ladwig et al. 2012). These findings indicate that more research is needed to understand both the individual and coupled controls on community and ecosystem responses to increasing N deposition in dryland systems.
The Colorado Plateau is a set of semi-arid ecosystems characterized by low winter temperatures, relatively frequent snowfall, and diverse plant communities with low overall cover (Comstock and Ehleringer 1992). The high density of national parks and other public lands, as well as the potential for high ecosystem vulnerability to N inputs in this region, make an improved understanding of how these systems respond to N deposition of particular importance (Pardo et al. 2011). Common to soil surfaces in the Colorado Plateau are biological soil crusts (biocrusts), which are a consortium of photosynthetic and heterotrophic organisms including cyanobacteria, algae, mosses, and lichens. Biocrusts fix substantial amounts of N2, emit N gases (Weber et al. 2015; Barger et al. 2016), exchange CO2 with the atmosphere (Housman et al. 2006; Elbert et al. 2012; Barger et al. 2013; Darrouzet-Nardi et al. 2015; Sancho et al. 2016), and are the dominant soil cover for much of the Colorado Plateau (e.g., Torres-cruz et al. 2018). Biocrusts have responded to experimental N additions with decreases in N2 fixation and photosynthesis, as well as shifts in constituent community composition (Sheridan 1979; Bobbink et al. 2010; Fenn et al. 2010; Ochoa-Hueso et al. 2014; Reed et al. 2016). Despite this apparent sensitivity, long-term observations of biocrust responses to N deposition are severely lacking, making it challenging to make predictions about the ecosystem-level effects of N deposition in these systems.
Here, we aimed to assess ecosystem- and community-level responses to long-term N deposition using a multi-level N fertilization experiment (0, 2, 5, and 8 kg N ha−1 year−1—split into equal fall and spring applications) that was established in 2011 in three semi-arid sites on the Colorado Plateau, USA. The three sites were adjacent to one another (< 5 km apart; Haight et al. 2019), but, due to differences in soil formation, varied in soil texture. This allowed us to keep climate consistent and to explore the role of soil characteristics in mediating the effects of N inputs. Specifically, we examined the responses of plant and biocrust communities to different experimental N additions using data from 3 years after the experiment was established (2014) until 2018. We hypothesized that experimental N inputs would (1) decrease plant diversity, (2) increase abundance of non-native plants—particularly the invasive annual grass Bromus tectorum, and (3) decrease the cover of biocrusts. Furthermore, we hypothesized that all of these trends would grow stronger through time due to the compounding effects of chronic N addition.
Methods
Study site
We established the field experiment in March 2011 at three sites along a soil texture gradient in Arches National Park (NP) (38° 47′ N, 109° 39′ W; Table 1) near Moab, UT, USA. All sites were located along the Salt Valley Road in Arches NP, near the Park’s northwest boundary and were < 5 km apart. Mean annual precipitation for our study period (2014–2018) was 252 mm and mean annual temperature was 12.6 °C, with a 30-year normal mean annual precipitation of 233 mm and mean annual temperature of 12 °C (data obtained from PRISM Climate Group at Oregon State University, USA; http://prism.oregonstate.edu). The dominant vegetation across all sites was a mix of C3 and C4 grasses. The most abundant plant species were Festuca octoflora (annual C3 grass), Sporobolus spp. (perennial C4 grasses), Hesperostipa comata (perennial C3 grass), Bromus tectorum (annual invasive C3 grass), Plantago patagonica (annual C3 forb), and Achnatherum hymenoides (perennial C3 grass). Mean cover by site can be found in Supplementary Table 1. Bromus tectorum was present at all sites and was highly abundant (77% of all occurrences) at the finest textured site (sandy loam; Table 1). Another invasive annual plant, Salsola tragus, was present at all sites in some years. Biocrust communities were characterized as early successional for the Colorado Plateau—predominately comprised of lightly pigmented cyanobacteria (likely Microcoleus spp.; Garcia-Pichel et al. 2013)—with darkly pigmented cyanobacteria, lichen, and moss present, but less abundant. There is no grazing currently at the site, but there is a significant history of grazing in the region with sheep grazing allowed within our study area until 1982 (National Park Service Southeast Utah Group archives). The dominance of cyanobacteria and scarcity of later-successional biocrust constituents (e.g., mosses and lichens) is likely due to the history of grazing.
Experimental design
The N-addition treatments were 0, 2, 5, and 8 kg N ha−1 year−1—henceforth referred to as ambient, low, medium, and high. There were five replicate blocks of each treatment at each site resulting in 60 total plots (20 plots per site). Treatments were applied to 1 m2 experimental plots, as well as to an additional 0.25 m plot buffer on the sides of each plot that was not sampled. Each plot was centered on a mature Achnatherum hymenoides individual that was sampled for foliar chemistry, leaving the majority of the plot area made up by other plant species and biocrusts. Annual N additions were split into two pulses delivered in March and September each year. Nitrogen was added as NH4NO3 dissolved in deionized water in a simulated 3 mm rainfall event (sensu Aber et al. 1989). The N-addition inputs used for this study were much lower than many N fertilization studies, but were selected using regional N deposition inputs as a guide, including the National Atmospheric Deposition Program (NADP) and Interagency Monitoring of Protected Visual Environments (IMPROVE) stations in Canyonlands National Park (approximately 40 km from our study site), which suggest regional wet and dry deposition to total 2–3 kg ha−1 year−1.
Precipitation data
We obtained monthly precipitation data for our study area from the PRISM Climate Group at Oregon State University, USA (http://prism.oregonstate.edu). These data are based on a 4 km grid interpolation using data from multiple weather stations (Daly et al. 2008). From these data, we calculated precipitation totals for cold months (defined as months when mean temperature was < 5 °C) and warm months (defined as months when mean temperature was > 5 °C). Precipitation data are provided in Table 2. Annual precipitation each year (2014–2018) except for 2016 was different (P < 0.05) from 30-year normal precipitation (1988–2018) determined using the ‘z.test’ function from the “BSDA” R package (Arnholt and Evans 2017).
Plant and biocrust community assessment
We measured vascular plant and biocrust cover annually during the growing season in March, by placing a point frame over each plot and lowering a pin through 50 different locations within the frame. For each interception above the soil surface, we recorded the plant species and/or biocrust functional group, that the pin contacted, including lightly pigmented cyanobacteria (e.g., Microcoleus sp.), darkly pigmented cyanobacteria (e.g., Scytonema sp.), or moss. More than one contact could be recorded per pin location (e.g., the pin could contact multiple plant species’ canopy cover and also a biocrust on the soil’s surface). We calculated percent cover by dividing the total number of hits for each species or functional group above or on the soil surface per point frame by 50.
Soil and plant inorganic N
We collected soil samples before treatments were applied annually in March using a 2.54 cm-diameter soil corer to collect soil from 0 to 10 cm depth at two locations within the plot. For each plot, we composited the cores into a single plot sample and sieved to 4 mm. We assessed soil inorganic N concentrations by extracting a subsample of field wet soil (immediately after collection) with 2 M KCl. Samples in solution were shaken for 11 h, left to settle for 18 h, and then filtered using Whatman #1 filter paper (GE Healthcare, Fairfield, CT, USA). Extracts were stored frozen before being analyzed using a Smartchem 200 Discrete Autoanalyzer from Unity Scientific (Brookfield, CT, USA) to determine extractable ammonium (NH4+) and nitrate (NO3−) concentrations via the Indophenol blue and Gries Ilosvay (following reduction by cadmium column) methods adapted to the machine. A subsample of soil was weighed, dried for 48 h at 105 °C, and reweighed to determine soil moisture. Inorganic N concentrations were calculated per unit soil dry mass.
We collected full sun, green tissue of a focal A. hymenoides in the spring of 2015 to assess treatment effects on foliar N. Focal plants died over the course of the experiment, and thus, sampling was not performed in later years. Leaf tissue was ground, dried at 60 °C, and measured for total N concentrations using an elemental analyzer (Vario Micro Cube, Elementar, Langenselbold, Germany).
Data analysis
We calculated Shannon’s diversity indices for the plant community using the “vegan” R package (Oksanen et al. 2008). We grouped plant species into four functional groups: cactus, forb, shrub, and grass. To assess plant community change over time, we used the ‘rate_change_interval’ function from the “codyn” R package (Hallett et al. 2016) to calculate Euclidean distances at each time interval. We tested response variables for normality and homoscedasticity using Levene’s test. We log-transformed data before analysis if either assumption was violated. We used repeated-measures multivariate analyses of variance (MANOVAs), with the independent variable measured across years, to assess whether N addition, site, cold, and/or warm months’ precipitation caused significant variation in overall plant Shannon’s diversity (H), as well as plant and biocrust cover. We used repeated-measures MANOVAs, with the independent variable measured across years, to assess whether N addition or site caused significant differences in soil inorganic N concentrations, and plant functional group cover and diversity. We were unable to test for differences in shrub and cactus cover or diversity, because occurrence of these plant groups was low across all sites. We used an MANOVA to test whether foliar N concentrations in 2015 differed by site or N-addition treatment. Each independent variable was a separate MANOVA, model information can be found in Supplementary Table 2. When statistical differences were detected within either factor or their interaction, we calculated least-square means to make pairwise comparisons and adjusted p values following Tukey tests using the “emmeans” R Package (Lenth et al. 2018) with experiment-wide α = 0.05. To determine the relationship between Euclidean distances and time intervals at each site, we built linear models with Euclidean distance as the response variable and time interval as the predictor variable using the “lm” function in R (Collins et al. 2000). All analyses were performed using R 3.6.1 (R Core Team 2019).
Results
Plant community
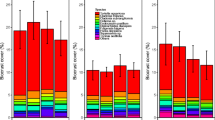
Nitrogen-addition treatments did not result in differences in vascular plant cover at any site (MANOVA, F3,48 = 0.356, P = 0.78 and F2,48 = 2.58, P = 0.09; Supplementary Fig. 1; Supplementary Table 2). However, precipitation during both the cold and warm months were significantly related to plant cover at all sites (MANOVA, F1,228 = 32.44, P = 0.0003 and F1,228 = 95.46, P = 0.0002; Supplementary Fig. 1; Supplementary Table 2). Forb and grass cover did not vary with N-addition treatments (MANOVA, F3,46 = 3.9, P = 0.07 and F3,48 = 0.89, P = 0.45; Fig. 1; Supplementary Table 2). Grass cover varied by site, but forb cover did not (MANOVA, F2,48 = 2, P = 0.02 and F2,46 = 0.23, P = 0.11; Supplementary Figs. 2, 3, 4).
Plant cover aggregated across sites for the years of the study used in this analysis (2014–2018; treatments began in 2011). Each panel represents a plant functional group: a cactus, b forb, c grass, or d shrub. Different colors represent the different N-addition treatments (ambient = 0 kg N ha−1; low = 2 kg N ha−1; medium = 5 kg N ha−1; high = 8 kg N ha−1). Values represent means ± SE
Shannon’s diversity indices of the plant community did not vary by site or treatment (MANOVA, F2,48 = 2.43, P = 0.1 and F3,48 = 0.964, P = 0.42; Supplementary Figs. 5, 6, 7, 8). Yet, cold months’ precipitation was positively related to Shannon’s diversity (MANOVA, F1,228 = 6.76, P = 0.01; Supplementary Fig. 5). Furthermore, we did not find differences in forb or grass diversity among treatments (MANOVA, F3,45 = 0.67, P = 0.6 and F3,45 = 1.1, P = 0.37; Fig. 2). The plant community remained relatively stable over time across sites in both the ambient and high N treatments (Fig. 3a, b). The muted response of plant community structure to the high N treatment is apparent in our assessment of plant community change over time, where the slopes of Euclidean distance through time are very similar at each site between the ambient and high N treatments. The slopes of linear models, with the exception of the site with finest textured soil, are relatively flat, indicating a relatively stable plant community (Fig. 3a, b; Collins et al. 2000). At the finest textured site, time interval was related to Euclidean distance in the ambient and low N (ambient linear model, P = 0.02, R2 = 0.11; Fig. 3a and low N linear model, P = 0.02, R2 = 0.09; Supplementary Fig. 9a). Yet, in the medium and high N plots at the finest textured site, there was not a significant relationship between time interval and Euclidean distance (medium N linear model, P = 0.05, R2 = 0. 06; Supplementary Fig. 9b and high N linear model, P = 0.32, R2 = 0.02; Fig. 3b).
Shannon’s diversity indices of the plant community aggregated across sites for the years of the study used in this analysis (2014–2018; treatments began in 2011). Each panel represents a plant functional group: a cactus, b forb, c grass, or d shrub. Different colors represent the different N-addition treatments (ambient = 0 kg N ha−1; low = 2 kg N ha−1; medium = 5 kg N ha−1; high = 8 kg N ha−1). Values represent means ± SE
Community change over time for plant species in the ambient (a–c) and high N (d–f) treatments, shown by regressing Euclidean distances versus increasing time lags. Positive slope indicates a directional change of the community. Flat slope indicates stability of the community. Negative slope implies that species composition is converging on a community-type characteristic of one of the early sample periods
Bromus tectorum cover was not affected by N addition at any site (MANOVA, F3,42 = 0.59, P = 0.63; Fig. 4). However, B. tectorum was primarily found at the finest textured site (MANOVA, F3,42 = 4.83, P = 0.01, Fig. 4). Additionally, B. tectorum increased in abundance during the years of our observation, but cover was highly variable (Fig. 4).
Bromus tectorum cover across the years of the study (2014–2018). Each panel represents a different site along a soil texture gradient: finer-textured soil (a), mid-textured soil (b), and the coarser-textured soil (c, Table 1). Different colors represent the different N-addition treatments (ambient = 0 kg N ha−1; low = 2 kg N ha−1; medium = 5 kg N ha−1; high = 8 kg N ha−1). Values represent means ± SE
Biocrust cover
Biocrust cover was negatively affected by N-addition treatments (MANOVA, F3,56 = 5.03, P = 0.004; Fig. 5). There were no differences among sites (MANOVA, F2,48 = 1.83, P = 0.17; data not shown). In 2014, biocrust cover declined in high N treatments relative to the control plots (post-ANOVA emmeans, P = 0.002; Fig. 5). In 2017, the medium N treatment had lower biocrust cover than controls (post-ANOVA emmeans, P = 0.05; Fig. 5). We did not observe differences among treatments during the other years of the experiment (Fig. 5). Precipitation during warm months was significantly related to biocrust cover (MANOVA, F1,228 = 65.54, P = 0.0002; Fig. 5; Table 2). Cold months’ precipitation did not relate to biocrust cover (MANOVA, F1,228 = 0.19, P = 0.67; Fig. 5; Table 2). The effect of N addition on biocrust cover was amplified during years with lower warm and cold months’ precipitation (MANOVA, F3,228 = 3.66, P = 0.01 and F3,228 = 5.23, P = 0.002; Fig. 5; Table 2). Interestingly, 2014 had the lowest precipitation for cold months (44.99 mm) and 2017 had the lowest annual precipitation during the duration of the experiment (183.45 mm; Table 2).
Biocrust cover aggregated across sites for the years of the study used in this analysis (2014–2018; treatments began in 2011). Different colors represent the different N-addition treatments (ambient = 0 kg N ha−1; low = 2 kg N ha−1; medium = 5 kg N ha−1; high = 8 kg N ha−1). Values represent means ± SE. *Indicates significant differences at p < 0.05 between ambient and high treatments
Soil inorganic N and foliar N
The interaction between N-addition treatments and year affected soil inorganic N concentrations (MANOVA, F5,273 = 3, P = 0.0003; Fig. 6a). Yet, this was driven solely by differences between ambient and medium N additions in 2018 (post-ANOVA emmeans, P = 0.008; Fig. 6a) and between ambient and medium N additions in 2018 (post-ANOVA emmeans, P = 0.001; Fig. 6a). Foliar N concentrations of A. hymenoides varied among sites (MANOVA, F2,47 = 4.19, P = 0.02; Fig. 6b), but did not vary significantly with N-addition treatment (MANOVA, F3,47 = 0.86, P = 0.49; Fig. 6b). Foliar N concentrations were significantly higher at the mid-textured site compared with coarser-textured site (post-ANOVA emmeans, P = 0.03), but not the finer-textured site (post-ANOVA emmeans, P = 0.05). Furthermore, foliar N concentrations did not differ between the finer- and coarser-textured site (post-ANOVA emmeans, P = 0.98).
Soil inorganic N concentrations aggregated across sites from 0 to 10 cm depth soil samples taken annually in the spring (a) and foliar N from 2015 from A. hymenoides (b). Colors represent the different N-addition treatments (ambient = 0 kg N ha−1; low = 2 kg N ha−1; medium = 5 kg N ha−1; high = 8 kg N ha−1). Values are means ± SE. *Indicates significant differences at p < 0.05 between ambient and high treatments
Discussion
In contrast to our hypotheses, long-term N additions had no effect on functional plant community composition or diversity, including cover of the exotic invasive grass B. tectorum, regardless of the quantity of N added or soil texture (Figs. 1, 2, 3, 4). These results diverge from the idea that dryland plant communities are exceptionally vulnerable to structural changes and invasion by non-native plants under increased N deposition (Fenn et al. 2003; Cox and Allen 2005; Pardo et al. 2011; Blett et al. 2014; Valliere et al. 2020). Yet, our study is not the first to observe muted impacts of N addition on plant species composition over time in a dryland system (Ladwig et al. 2012). Additionally, it is important to note that by only using measurements from the spring, we are unable to capture likely seasonal variation. Our results also demonstrated a lack of soil inorganic N accumulation in response to N additions and a lack of changes to foliar N concentrations (Fig. 6b), which may help explain the absence of structural changes in the plant community. Some studies suggest that desert ecosystems have low N retention (Peterjohn and Schlesinger 1990; McCalley and Sparks 2009) and results from these sites suggest that N is rapidly leaving the system (Fig. 6a). While N additions did not affect plant community structure, we found some support for our hypothesis that biocrust cover would decrease in response to additional N (Fig. 5). Overall, our work supports the hypothesis that biocrusts are vulnerable to N deposition, perhaps experiencing a higher effective dose of incoming N than underlying soils (Sinsabaugh et al. 2015), while also contributing to a growing body of research that suggests some dryland plant communities are relatively resistant to modest levels of N deposition (Ladwig et al. 2012). Surprisingly, we did not observe changes in overall or functional plant diversity in response to N-addition treatments (Fig. 2)—rather, the plant community remained relatively stable between the high and ambient N-addition plots regardless of soil texture (Fig. 3). We also observed no changes in foliar N concentrations with N-addition treatments at any of our sites in 2015 (Fig. 6). These findings suggest that soil texture did not interact with N inputs to differentially affect the plant community or chemistry. Furthermore, the lack of response to N inputs across sites with differing soil texture suggests that this plant community may be more resilient to N deposition in this dryland system than we hypothesized, and then, some literature would predict (Fenn et al. 2003; Pardo et al. 2011; Blett et al. 2014; Sinsabaugh et al. 2015). Although numerous studies in semi-arid systems have observed decreases in plant diversity in response to N addition (Allen et al. 1998, 2016; Kimball et al. 2014; Valliere et al. 2017, 2020; Vourlitis 2012; Vourlitis et al. 2009), our study is one of a limited number to occur in North American drylands outside of California (but see Ladwig et al. 2012; Sinsabaugh et al. 2015; McHugh et al. 2017), and plant diversity remain unchanged in our experiment. Future research assessing the consequences of the lower (i.e., non-urban) N inputs common for vast dryland areas would allow for a more predictive understanding of dryland thresholds and responses to increasing anthropogenic N deposition.
One possible explanation for the differences between our results and those previously observed is that we used application rates that are an order of magnitude lower than the N deposition rates used in many other semi-arid and arid experiments, the majority of which take place in areas adjacent to highly urbanized systems (Allen et al. 2009; Pardo et al. 2011; Vourlitis 2012; Valliere et al. 2017). The N inputs that we used are informed by deposition rates for the study area (ca. 3 kg N ha−1; Pardo et al. 2011; Reed et al. 2013) and thus represent an important addition to the wider body of work. There are some contemporary studies outside of urbanized systems that use similar rates to our study (Kox et al. 2016; Tulloss and Cadenasso 2016) and the latter also found limited impacts of N additions on plants. Thus, while we may have observed responses with larger inputs, those inputs would be outside the bounds of current and predicted N deposition for the area.
We did not observe any decrease in the abundance of slow-growing native species or increase in fast-growing invasive annual grasses following N addition. Often, when N availability increases in a system, there are accompanying shifts in plant community composition toward nitrophilous species with losses of slower-growing species (Tilman 1987; Vitousek and Howarth 1991). In other cases, N deposition has facilitated the establishment of invasive annual grasses as they are able to rapidly use available nutrients and outcompete native plants that employ stress-tolerating strategies (Allen et al. 1998; Kimball et al. 2014). Our results show a lack of such changes. There are multiple reasons this could occur. In addition to low retention of added N (Fig. 6b), limitation by other resources could dampen the effects on community composition. While N is widely regarded as the nutrient most limiting to dryland systems (Hooper and Johnson 1999), water availability and phosphorus could also constrain community responses and, in particular, non-native plant invasion (Padgett and Allen 1999; Chesson et al. 2004; Morillas et al. 2017).
The decrease in biocrust cover we observed in response to N addition could be the effect of high organismal sensitivity to N pollution relative to plants (e.g., Branquinho et al. 2010), but may also be a result of biocrusts receiving a higher effective N dose than the underlying soil (Sinsabaugh et al. 2015). If true, the biocrust layer could act as a buffer from the initial effects of N addition (Benvenutto‐Vargas and Ochoa‐Hueso 2019) by reducing the amount of added N that reaches the underlying soil and plant community. However, the treatment effects on biocrust cover did not increase through time and occurred only in years when annual or cold months precipitation was low. This suggests that low precipitation may interact with N addition to decrease biocrust cover. In particular, 2014 (the year we observed the strongest effect of the high N treatment on biocrust cover) was the year with the lowest precipitation during cold months, suggesting higher amounts of snowfall may help buffer the effects of N deposition on biocrusts. More work is needed to disentangle how biocrusts interact with climate, N inputs, and soil N cycling to determine the fate and impact of N deposition. Nevertheless, the ability of biocrusts to modulate the effects of N addition on ecosystem processes is likely affected by climate stressors (Reed et al. 2012). Additionally, biocrusts can decrease N2 fixation in response to N additions (Sheridan 1979; Reed et al. 2007; Ochoa-Hueso and Manrique 2014). We did not measure N2 fixation in our study, but if fixation does decrease in response to increasing N inputs, it could contribute to the lack of differences in soil inorganic N among the N addition treatments (Fig. 6). In other words, if N inputs from one source go up (e.g., via fertilization) but another N source declines (N2 fixation), then we might observe no change in available N. It is important to highlight that biocrust communities at our sites were primarily early successional cyanobacteria and, therefore, we cannot speak to how simulated N addition would affect lichens, mosses, or other later-successional biocrust organisms.
We found no consistent or cumulative treatment effects on soil inorganic N concentrations in our annual measurements or foliar N concentrations in 2015, which supports the idea that N retention is low in desert soils (Peterjohn and Schlesinger 1990; McCalley and Sparks 2009; Ladwig et al. 2012). However, it is important to note that a significant difference in soil inorganic N between some treatments did emerge in 2018, suggesting that there may be a potential lag effect. Furthermore, we applied N treatments as aqueous solutions, thus coupling N and water pulses, which could stimulate the rapid dissipation of N. Additionally, there is evidence suggesting a substantial increase in production of reactive N gas following wetting in deserts, which could be in part attributed to abiotic reactions regulated by temperature and N availability (Homyak et al. 2016; Leitner et al. 2017). The ephemeral effects of our N addition on soil inorganic N pools suggest that the N we added is rapidly leaving the system via dissolved and/or volatile loss pathways (Fig. 6a; Peterjohn and Schlesinger 1990; Homyak et al. 2017; McHugh et al. 2017). Such N losses have important implications for air and water quality (Peterjohn and Schlesinger 1990; Schlesinger 2009 Reed et al. 2013). Nitrous oxide is a strong greenhouse gas with a long atmospheric lifetime that can be destructive to ozone (Dickinson and Cicerone 1986; Cicerone 1987; Mooney et al. 1987). Additionally, N oxides can form O3 (i.e., smog) at Earth’s surface, thereby increasing air pollution (Williams et al. 1992). In addition to volatile losses, N may leach and accumulate beneath the biologically active soil zone. Nitrogen leaching was originally thought to be quite low in arid and semi-arid environments; however, further investigation has found that NO3− below 1 m in the soil profile can be considerable (Walvoord et al. 2003). Nitrate leaching can affect ground and stream water quality (Canter 1997). Therefore, quantifying leaching and gaseous losses of N is essential in building a comprehensive understanding of the consequences of dryland system responses to intensifying N deposition. Future efforts to study N deposition would greatly benefit from measuring N oxide (N2O and NO2) gas fluxes and subsurface NO3− following N addition to quantify leaching and volatile N losses.
Our study and Ladwig et al.’s in the Chihuahuan desert (Ladwig et al. 2012) illustrate the need for an improved understanding of how increasing N inputs affect different community members (e.g., plants vs. biocrusts) and suggest that such an understanding could help us to refine critical load estimates for drylands around the world. Critical load values can vary with target organisms or “receptors” (Fenn et al. 2010; Pardo et al. 2011). In our study, we observed significant effects on biocrust cover during 2017 at 5 kg ha−1 year−1, which is slightly higher than the estimated critical load for this region—3 kg ha−1 year−1 (Pardo et al. 2011). Yet, we observed no effect on plant community composition or diversity (Figs. 1, 2, 3, 4), suggesting the critical load for at least some vascular plants on the Colorado Plateau exceeds 8 kg ha−1 year−1. Much of the focus on assessing N deposition effects and critical loads in arid and semi-arid ecosystems is centered around plant community structure (Báez et al. 2007; Vourlitis and Pasquini 2009; Rao et al. 2010; Vourlitis 2012; Simkin et al. 2016; Valliere et al. 2017, 2020). Including a focus on other ecosystem responses (e.g., biocrusts and N losses) could increase the accuracy with which we categorize and manage for increasing anthropogenic N inputs. Accordingly, future desert N deposition research that takes a comprehensive approach to assessing impacts by including ecosystem responses in addition to changes in plant community structure will be of great value.
Conclusions
Our results indicate that plant community structure is resistant to chronic N additions at and above estimated critical load values for the region, suggesting a relatively low sensitivity of these dryland plants to added N (Peterjohn and Schlesinger 1990; McCalley and Sparks 2009; Ladwig et al. 2012). Our results illustrate that added N is quickly lost from the system, likely via heightened gaseous and dissolved N losses, which could help explain the lack of plant responses (i.e., if N is leaving the system quickly than it would not be expected to have large effects). Increases in volatile N losses and nitrate leaching (Peterjohn and Schlesinger 1990; Walvoord et al. 2003) would negatively impact water and air quality. Biocrust abundance—which is understudied in the context of N deposition—appears more sensitive to N additions than vascular plants, and, in the long-term, decreases in biocrust cover have the potential to affect plant community structure (Weber et al. 2016), soil stability and hydrology (Reed et al. 2012; Eldridge et al. 2020), and a range of coupled biogeochemical cycles (e.g., Sancho et al. 2016). Drylands make up over 40% of Earth’s terrestrial surface and their spatial extent is on the rise (Huang et al. 2016). Teasing apart the ecosystem effects of N deposition on these vast and heterogenous systems is critical, yet the majority of dryland N deposition research focuses on systems with a Mediterranean climate and highly urbanized areas with large rates of deposition downwind. Additional studies in rural areas with modest N application rates could provide a more complete and predictive understanding of the impacts of N deposition on drylands in the American Southwest and beyond.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo J (1989) Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–386. https://doi.org/10.2307/1311067
Allen EB, Padgett PE, Bytnerowicz A, Minnich R (1998) Nitrogen Deposition Effects on Coastal Sage Vegetation of Southern California. Proc Int Symp Air Pollut Clim Chang Eff For Ecosyst, 1996, Riverside, CA 131–140
Allen EB, Rao LE, Steers RJ, et al (2009) Impacts of atmospheric nitrogen deposition on vegetation and soils at Joshua Tree National Park. Mojave Desert Ecosyst Process Sustain 78–100
Allen EB, Egerton-Warburton LM, Hilbig BE, Valliere JM (2016) IInteractions of arbuscular mycorrhizal fungi, critical loads of 2 nitrogen deposition, and shifts from native to invasive species in a 3 southern California shrubland 4. Botany 94:1–22
Arnholt AT, Evans B (2017) R Package ‘BSDA’: Basic Statistics and Data Analysis
Baer SG, Blair JM, Collins SL, Knapp AK (2003) Soil resources regulate productivity and diversity in newly established tallgrass prairie. Ecology 84:724–735. https://doi.org/10.1890/0012-9658(2003)084[0724:SRRPAD]2.0.CO;2
Báez S, Fargione J, Moore DI et al (2007) Atmospheric nitrogen deposition in the northern Chihuahuan desert: temporal trends and potential consequences. J Arid Environ. https://doi.org/10.1016/j.jaridenv.2006.06.011
Barger NN, Castle SC, Dean GN (2013) Denitrification from nitrogen-fixing biologically crusted soils in a cool desert environment, southeast Utah, USA. Ecol Process 2:1–9. https://doi.org/10.1186/2192-1709-2-16
Barger NN, Weber B, Garcia-Pichel F, et al. (2016) Patterns and controls on nitrogen cycling of biological soil crusts. In: Biological soil crusts: an organizing principle in drylands. pp 257–285
Benvenutto-Vargas VP, Ochoa-Hueso R (2019) Effects of nitrogen deposition on the spatial pattern of biocrusts and soil microbial activity in a semiarid Mediterranean shrubland. Funct Ecol 1365–2435:13512. https://doi.org/10.1111/1365-2435.13512
Blett TF, Lynch JA, Pardo LH et al (2014) FOCUS: a pilot study for national-scale critical loads development in the United States. Environ Sci Policy. https://doi.org/10.1016/j.envsci.2013.12.005
Bobbink R, Hicks K, Galloway J et al (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59. https://doi.org/10.1890/08-1140.1
Branquinho C, Pinho P, Dias T, Cruz C (2010) Lichen transplants at our service for atmospheric NH3 deposition assessments. Bibl Lichenol J Cramer Gebrüder Borntraeger Verlagsbuchhandlung 105:103–112
Chesson PL, Gebauer RLE, Schwinning S et al (2004) Pulse events and arid ecosystems resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141:236–253. https://doi.org/10.1007/s00442-004-1551-1
Cicerone RJ (1987) Changes in stratospheric ozone. Science 237:35–42
Collins SL, Micheli F, Hartt L (2000) A method to determine rates and patterns of variability in ecological communities. Oikos 91:285–293. https://doi.org/10.1034/j.1600-0706.2000.910209.x
Comstock JP, Ehleringer JR (1992) Plant adaptation in the great-basin and Colorado plateau. Gt Basin Nat 52:195–215
Cox RD, Allen EB (2005) Stability of exotic annual grasses following restoration efforts in southern California coastal sage scrub. Reprod Biomed Online 11:674–675
Cox RD, Preston KL, Johnson RF et al (2014) Influence of landscape-scale variables on vegetation conversion to exotic annual grassland in southern California, USA. Glob Ecol Conserv 2:190–203. https://doi.org/10.1016/j.gecco.2014.09.008
Daly C, Halbleib M, Smith JI et al (2008) Physiographically sensitive mapping of climatological temperature and precipitation across the conterminous United. Int J Climatol 28:2031–2064. https://doi.org/10.1002/joc
Darrouzet-Nardi A, Reed SC, Grote EE, Belnap J (2015) Observations of net soil exchange of CO2 in a dryland show experimental warming increases carbon losses in biocrust soils. Biogeochemistry 126:363–378. https://doi.org/10.1007/s10533-015-0163-7
Dentener F, Drevet J, Lamarque JF et al (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cycles. https://doi.org/10.1029/2005GB002672
Dickinson RE, Cicerone RJ (1986) Future global warming from atmospheric trace gases. Nature 319:109–115. https://doi.org/10.1038/319109a0
Duce R, Galloway J, Liss P (2009) The impacts of atmospheric deposition to the ocean on marine ecosystems and climate. WMO Bull 61–66
Elbert W, Weber B, Burrows S et al (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5:459–462. https://doi.org/10.1038/ngeo1486
Eldridge DJ, Reed S, Travers SK, et al (2020) The pervasive and multifaceted influence of biocrusts on water in the world’s drylands. https://doi.org/10.1111/gcb.15232
Fenn ME, Baron JS, Allen EB et al (2003) Ecological effects of nitrogen deposition in the western United States. Bioscience 53:404. https://doi.org/10.1641/0006-3568(2003)053[0404:EEONDI]2.0.CO;2
Fenn ME, Allen EB, Weiss SB et al (2010) Nitrogen critical loads and management alternatives for N-impacted ecosystems in California. J Environ Manage 91:2404–2423. https://doi.org/10.1016/j.jenvman.2010.07.034
Garcia-Pichel F, Loza V, Marusenko Y et al (2013) Temperature drives the continental-scale distribution of key microbes in topsoil communities. Science 243:1574–1577
Haight JD, Reed SC, Faist AM (2019) Seed bank community and soil texture relationships in a cold desert. J Arid Environ 164:46–52. https://doi.org/10.1016/j.jaridenv.2019.01.008
Hallett LM, Jones SK, MacDonald AAM et al (2016) codyn: an r package of community dynamics metrics. Methods Ecol Evol 7:1146–1151. https://doi.org/10.1111/2041-210X.12569
Homyak PM, Blankinship JC, Marchus K et al (2016) Aridity and plant uptake interact to make dryland soils hotspots for nitric oxide (NO) emissions. Proc Natl Acad Sci U S A 113:E2608–E2616. https://doi.org/10.1073/pnas.1520496113
Homyak PM, Kamiyama M, Sickman JO, Schimel JP (2017) Acidity and organic matter promote abiotic nitric oxide production in drying soils. Glob Chang Biol 23:1735–1747. https://doi.org/10.1111/gcb.13507
Hooper DU, Johnson L (1999) Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–293. https://doi.org/10.1007/BF01007582
Housman DC, Powers HH, Collins AD, Belnap J (2006) Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J Arid Environ 66:620–634. https://doi.org/10.1016/j.jaridenv.2005.11.014
Huang J, Yu H, Guan X et al (2016) Accelerated dryland expansion under climate change. Nat Clim Chang. https://doi.org/10.1038/NCLIMATE2837
Isbell F, Calcagno V, Hector A et al (2011) High plant diversity is needed to maintain ecosystem services. Nature 477:199–202. https://doi.org/10.1038/nature10282
Isbell F, Reich PB, Tilman D et al (2013) Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci U S A 110:11911–11916. https://doi.org/10.1073/pnas.1310880110
Kimball S, Goulden ML, Suding KN, Parker S (2014) Altered water and nitrogen input shifts succession in a southern California coastal sage community
Kox MAR, Lüke C, Fritz C et al (2016) Effects of nitrogen fertilization on diazotrophic activity of microorganisms associated with Sphagnum magellanicum. Plant Soil 406:83–100. https://doi.org/10.1007/s11104-016-2851-z
Ladwig LM, Collins SL, Swann AL et al (2012) Above- and belowground responses to nitrogen addition in a Chihuahuan Desert grassland. Oecologia 169:177–185. https://doi.org/10.1007/s00442-011-2173-z
Lenth R, Singmann H, Love J et al (2018) Package ‘emmeans.’ R Packag version 115–15(34):216–221. https://doi.org/10.1080/00031305.1980.10483031%3e.License
McCalley CK, Sparks JP (2009) Abiotic gas formation drives nitrogen loss from a desert ecosystem. Science 326:837–841
McHugh TA, Morrissey EM, Mueller RC et al (2017) Bacterial, fungal, and plant communities exhibit no biomass or compositional response to two years of simulated nitrogen deposition in a semiarid grassland. Environ Microbiol 19:1600–1611. https://doi.org/10.1111/1462-2920.13678
Mooney HA, Vitousek PM, Matson PA (1987) Exchange of materials between terrestrial ecosystems and the atmosphere. Science 238:926–932
Morillas L, Roales J, Portillo-Estrada M, Gallardo A (2017) Wetting-drying cycles influence on soil respiration in two Mediterranean ecosystems. Eur J Soil Biol 82:10–16. https://doi.org/10.1016/j.ejsobi.2017.07.002
Ochoa-Hueso R, Allen EB, Branquinho C, et al (2011) Nitrogen deposition effects on Mediterranean-type ecosystems: an ecological assessment. https://doi.org/10.1016/j.envpol.2010.12.019
Ochoa-Hueso R, Bell MD, Manrique E (2014) Impacts of increased nitrogen deposition and altered precipitation regimes on soil fertility and functioning in semiarid Mediterranean shrublands. J Arid Environ 104:106–115. https://doi.org/10.1016/j.jaridenv.2014.01.020
Oksanen AJ, Blanchet FG, Friendly M, et al (2008) Vegan: community ecology package : Available from Package ‘ vegan ’
Padgett PE, Allen EB (1999) Differential responses to nitrogen fertilization in native shrubs and exotic annuals common to mediterranean coastal sage scrub of California. Plant Ecol 144:93–101. https://doi.org/10.1023/A:1009895720067
Pardo LH, Fenn ME, Goodale CL et al (2011) Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol Appl 21:3049–3082. https://doi.org/10.1890/10-2341.1
Peterjohn WT, Schlesinger WH (1990) Nitrogen loss from deserts in the southwestern United States
Phoenix GK, Hicks WK, Cinderby S et al (2006) Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts. Glob Chang Biol 12:470–476. https://doi.org/10.1111/j.1365-2486.2006.01104.x
Rao LE, Allen EB, Thomas M (2010) Risk-based determination of critical nitrogen deposition loads for fire spread in southern California deserts. Ecol Appl 20:1320–1335. https://doi.org/10.1890/09-0398.1
Reed SC, Coe KK, Sparks JP et al (2012) Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat Clim Chang 2:752–755. https://doi.org/10.1038/nclimate1596
Reed SC, FT Maestre, R Ochoa-Hueso, CR Kuske, A Darrouzet-Nardi, M Oliver, B Darby, LG Sancho, RL Sinsabaugh, J Belnap (2017) Biocrusts in the context of global change. In: Biological soil crusts: an organizing principle in drylands pp. 451–476. Springer
Sancho LG, Belnap J, Colesie C, et al (2016) Carbon budgets of biological soil crusts at micro-, meso-, and global scales. In: Biological soil crusts: an organizing principle in drylands. pp 287–304
Schlesinger WH (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci U S A 106:203–208. https://doi.org/10.1073/pnas.0810193105
Sheridan RP (1979) Effects of airborne particulates in nitrogen fixation in legumes and algae. Am Phytopathol Soc 69:1011–1018
Simkin SM, Allen EB, Bowman WD, et al (2016) Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. https://doi.org/10.5061/dryad.7kn53
Sinsabaugh RL, Belnap J, Rudgers J et al (2015) Soil microbial responses to nitrogen addition in arid ecosystems. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00819
Talluto MV, Suding KN (2008) Historical change in coastal sage scrub in southern California, USA in relation to fire frequency and air pollution. Landsc Ecol 23:803–815. https://doi.org/10.1007/s10980-008-9238-3
Tilman D (1987) Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecol Monogr 57:189–214
Tilman D, Reich PB, Knops JMH (2006) Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441:629–632. https://doi.org/10.1038/nature04742
Torres-cruz TJ, Howell AJ, Reibold RH, et al (2018) Species-specific nitrogenase activity in lichen-dominated biological soil crusts from the Colorado Plateau, USA. Plant Soil
Tulloss EM, Cadenasso ML (2016) Using realistic nitrogen deposition levels to test the impact of deposition relative to other interacting factors on the germination and establishment of grasses in the California oak savanna. Plant Ecol 218:43–55
Valliere JM, Irvine IC, Santiago L, Allen EB (2017) High N, dry: experimental nitrogen deposition exacerbates native shrub loss and nonnative plant invasion during extreme drought. Glob Chang Biol 23:1–13. https://doi.org/10.1111/gcb.13694
Valliere JM, Bucciarelli GM, Bytnerowicz A et al (2020) Declines in native forb richness of an imperiled plant community across an anthropogenic nitrogen deposition gradient. Ecosphere. https://doi.org/10.1002/ecs2.3032
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115. https://doi.org/10.1007/BF00002772
Vitousek PM, Aber JD, Howarth RW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750. https://doi.org/10.1890/1051-0761(1997)007[0737:HAOTGN]2.0.CO;2
Vitousek PM, Menge DNL, Reed SC, Cleveland CC (2013) Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2013.0119
Vourlitis GL (2012) Aboveground net primary production response of semi-arid shrublands to chronic experimental dry-season N input. Ecosphere 3:art22. https://doi.org/10.1890/ES11-00339.1
Vourlitis GL, Pasquini SC (2009) Experimental dry-season N deposition alters species composition in southern Californian mediterranean-type shrublands. Ecology 90:2183–2189. https://doi.org/10.1890/08-1121.1
Walvoord MA, Phillips FM, Stonestrom DA et al (2003) A reservoir of nitrate beneath desert soils. Science 302:1021–1024
Weber B, Wu D, Tamm A et al (2015) Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. Proc Natl Acad Sci U S A 112:15384–15389. https://doi.org/10.1073/pnas.1515818112
Weber B, Bowker M, Zhang Y, Belnap J (2016) Natural recovery of biological soil crusts after disturbance. In: Biological soil crusts: an organizing principle in drylands. pp 479–498
Yahdjian L, Gherardi L, Sala OE (2011) Nitrogen limitation in arid-subhumid ecosystems: a meta-analysis of fertilization studies. J Arid Environ 75:675–680. https://doi.org/10.1016/j.jaridenv.2011.03.003
Acknowledgements
We thank many people who helped establish this experiment and collect data, especially Erika Geiger, Cara Lauria, Armin Howell, Jenny Shostrand, Nick Melone, Carla Roybal, and all the previous USGS technicians who helped collect data over the years. We also thank Matt Van Scoyoc, Laura Martin, and Paula (Peekay) Briggs of the National Park Service Southeast Utah Group for help with permits, logistics, and archived information about the sites' history. We gratefully acknowledge support from the National Park Service Air Resources Division and the Ecosystems Mission Area of the U.S. Geological Survey. We thank Edith Allen for comments on earlier versions on this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
SCR conceived the ideas and designed methodology; RHR collected the data; DEW, RHR, and BBO assisted with data organization and cleaning; MLP analyzed the data; MLP led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Additional information
Communicated by Duncan Menge.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Phillips, M.L., Winkler, D.E., Reibold, R.H. et al. Muted responses to chronic experimental nitrogen deposition on the Colorado Plateau. Oecologia 195, 513–524 (2021). https://doi.org/10.1007/s00442-020-04841-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04841-3