Abstract
The oxidation of chloramphenicol (CAP) by potassium ferrate (VI) in test solution was studied in this paper. A series of jar tests were performed at bench scale with pH of 5–9 and molar ratio [VI/CAP] of 16.3:1–81.6:1. Results showed that raising VI dose could improve the treatment performance and the influence of solution pH was significant. VI is more reactive in neutral conditions, presenting the highest removal efficiency of CAP. The rate law for the oxidation of CAP by VI was first order with respect to each reactant, yielding an overall second-order reaction. Furthermore, five oxidation products were observed during CAP oxidation by VI. Results revealed that VI attacked the amide group of CAP, leading to the cleavage of the group, while benzene ring remained intact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chloramphenicol (CAP) is a broad spectrum antibiotic which is widely used throughout the world since 1950s (Sanchez-Fortun et al. 2009). CAP is applied in swine, cattle, poultry, and fish husbandry as feed additives and veterinary drugs for prophylactic, as well as therapeutic purposes (Yan et al. 2013). However, a series of environmental issues and human health problems caused by the widespread use of CAP have drawn people’s attention in recent decades (Marx et al. 2015; Li et al. 2015; Burkina et al. 2015). Previous study showed that CAP residues were found in animal foods and environmental pollution (Wang et al. 2011; Gao et al. 2012; Hanekamp and Bast 2015; Liu et al. 2016). Most of CAP were excreted unmetabolized from human or animal body via feces and urine (Kramer et al. 1984), causing its extensive distribution in soil, groundwater, surface water, seawater, and sediment worldwide (Jiang et al. 2011; Lou et al. 2012; Bayen et al. 2014). Moreover, the nature of CAP is stable, which made it difficult to degrade; therefore, it could exist in natural environment for a long time (Xue et al. 2015). Its presence in natural environment poses threat on human health and ecosystem through the development of drug resistance among pathogens and bacteria (Wang et al. 2011; Gao et al. 2012). Therefore, removing CAP from wastewater before discharged into the environment represents an important challenge. Several studies have shown that CAP can hardly be degraded through conventional wastewater treatment process due to its stable chemical properties (Peng et al. 2006; Badawy et al. 2009). Recently, many new processes, such as photocatalytic, Fenton process, and thermally activated persulfate, were developed to degrade CAP (Xia et al. 2014; Nie et al. 2014; Giri and Golder 2014; Giusy et al. 2016). However, these processes were difficult to apply in wastewater treatment process and led to secondary pollution during oxidation (Giusy et al. 2016).
Potassium ferrate (VI) is a strong oxidant in aqueous media with a reduction potential of 2.20 and 0.70 V in acidic and alkaline, respectively (Jiang et al. 2006). Under acidic conditions, the redox potential of VI ion is strongest among all oxidants/disinfectants used for water and wastewater treatment (Jiang 2014). It is also known as an environment-friendly chemical for coagulation, disinfection, and oxidation for multipurpose treatment of water and wastewater. During the oxidation/disinfection process, VI ions are reduced to Fe (III) or ferric hydroxide and simultaneously generate a coagulant in a single-dosing and mixing unit process (Jiang et al. 2012). Furthermore, the application of VI can improve the removal of natural organic matter or disinfection by-product (DBP) precursors (Sharma et al. 2006). However, no study on the removal of CAP using VI, as well as the degradation process has yet been reported.
In this study, batch tests were performed to analyze the influence factors of CAP oxidation, and the degradation products were identified. The objectives of this study were to (i) find the optimal react condition for the removal of CAP; (ii) evaluate the rate constants of the CAP oxidation, proposing the reasonable CAP degradation pathways and possible oxidation products; and (iii) advance mechanistic understanding of CAP degradation by VI oxidation.
Materials and methods
Chemicals and reagents
The CAP was purchased from Aladdin (Shanghai, China), methanol (high-performance liquid chromatography (HPLC) grade) was purchased from Tianjin Shiyou Chemical Reagent Factory (Tianjin, China), and potassium hydroxide (AR) was purchased from Hangzhou Xiaoshan Chemical Reagent Factory (Hangzhou, China). VI solution of high concentration was synthesized by the electro-chemical method, and then, solid VI was obtained after various purification steps (Li et al. 2005). The VI strength of the resulting dry product was measured by chromite method and direct spectrophotometric method using a wavelength of 505 nm and an absorbance coefficient of 1100 M−1 cm−1 (Jiang et al. 2006). The purity of VI was continuously monitored daily. The other reagents (AR) were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Water used in the experiments was generated by ultra-pure water supplier (UPHW1-90T, Sichuan Europtronic Ultrapure Technology Co. Ltd., China). Stock solutions of CAP were prepared in ultra-pure water at 100 mg L−1 for the use of jar test experiments.
Experimental procedure
Influence factor of CAP oxidation
The batch tests were performed using a magnetic stirrer (HJ-6, Jintan Jiang Nan Instrument Factory), with a mixing speed of 500 rpm. CAPs were sampled at given reaction time intervals, and sodium thiosulfate (0.1 mol L−1) was used as a quencher.
To identify the effect of VI dosage on CAP oxidation, the initial concentration of CAP was 1000 μg L−1 with solution pH of 6. VI dosage increased from 16.3:1 to 81.6:1 gradually in molar ratio (VI/CAP) to identify the effect of VI dosage on CAP oxidation.
The influence of pH on CAP degradation was studied as follows: with initial concentration of CAP of 1000 μg L−1, pH was adjusted from 5 to 9, and the dosage of [VI]/[CAP] was 40.8:1 in molar ratio.
Batch tests were carried out for the kinetic experiments. The initial concentration of CAP was 1000 μg L−1 and the solution pH was 6, and the dosage of VI was 50.5, 75.8, 126.3, and 252.5 μM at room temperature, respectively.
Degradation product identification
To identify degradation products of CAP, solutions with initial concentration of 1000 μg L−1 CAP were prepared. The VI powder was added to the solution of CAP with the molar ratio of 50:1. The samples were obtained with time intervals of 0, 1, 5, 15, 30, 45, and 60 min.
Analytical methods
The quantification of CAP was measured by an Agilent 1200 Series HPLC system equipped with a UV detector. A C18 Eclipse XDB (Supelco) column (150 × 4.6 mm, 5-μm particle size) was used as a stationary phase. The mobile phase for CAP was a mixture of ultra-pure water and methanol (v:v = 2:3) at a flow rate of 0.8 mL min−1. The detection wavelength was set at 278 nm, the sample injection volume was 10 μL, and the column temperature was kept at 30 °C. The oxidation products of CAP were analyzed by a GC-MS system (SHIMADZU, Japan) equipped with an SE-54 capillary column (Chrome, UK). For each GC-MS injection, samples were pretreated by hydrochloric acid and then extracted with chloroform. The GC conditions were given as follows: a sample volume of 1 μL injected in the splitless mode at 280 °C and the oven temperature programmed from 50 °C (5 min) to 250 °C at 10 °C min−1 followed by a 5-min hold at 250 °C and helium used as the carrier gas at a flow rate of 0.8 mL min−1. Mass spectrometer was operated under electron ionization mode at 70 eV with mass scan range of 40–500.
Results and discussion
Effect of VI dosage on CAP oxidation
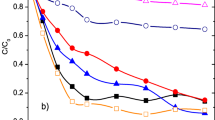
VI dosage was increased from 16.3:1 to 81.6:1 (in molar ratio) to identify its effect on CAP oxidation. The CAP concentration decreased rapidly in first 15 min. Along with the time, the concentration of CAP decreased slowly, and at the time of 30 min, the reaction had reached to a dynamic equilibrium (see Fig. 1). From 30 to 60 min, the concentration of CAP changed slightly. When the dosage of VI increased, higher removal rate was achieved. However, the removal rate increased slowly when [VI]/[CAP] range from 16.3:1 to 81.6:1. This indicates that the dose of VI did not exert significant influence on the oxidation of CAP.
Influence of pH on CAP oxidation
Batch test was conducted to explore the influence of pH on CAP degradation. Results revealed that neutral conditions (with pH of 7) presented the highest removal efficiency of CAP, while CAP oxidation was significantly suppressed in alkaline condition. The removal efficiency of CAP decreased significantly from 25 ± 0.4% (pH = 7) to 8 ± 0.5% (pH = 9) (see Fig. 2). Previous studies suggested redox potential of VI decreased with the increase of pH, restraining the reaction and removal of CAP (Jiang 2007). Ferrate with low oxidation potential under alkaline conditions led to restricted redox reactions. Studies have proven that with the increasing of pH, the mainly existed form of VI changed from H2FeO4 to FeO4 2−, which presented much lower oxidation capacity (Eqs. (1), (2), and (3)).
However, too low pH also seemed to restrain the removal efficiency of CAP. In this study, with the decrease of pH, the removal efficiency of CAP decreased from 25 ± 0.4% (pH = 7) to 21 ± 0.6% (pH = 5). Previous studies suggested that when pH <6, the ferrate became highly unstable, reducing rapidly within minutes, which decreased oxidation capacity (Graham et al. 2004; Jiang 2007).
Kinetic experiments
The rate expression for the reaction of Fe (VI) and CAP can be expressed as follows:
where [CAP] and [Fe(VI)] are the concentrations of CAP and Fe(VI), m and n are the orders of the reaction, and k is the overall reaction rate constant. Previous studies have shown that this reaction was first order with respect to each reactant, yielding an overall second-order reaction (Jiang et al. 2006; Jiang 2007). Therefore, the reaction of Fe (VI) and CAP can be expressed as follows:
When the kinetic studies were carried out with Fe (VI) in excess (dosage of [VI]/[CAP] greater than 10:1), reaction changed into pseudo-order conditions, and Eq. (5) can be rewritten as
where k ′ is the pseudo-order reaction rate constant (min−1).
The kinetic experiments were carried out to further understand the CAP degradation process. Results showed that points lie satisfactorily around straight lines (with correlation coefficients higher than 0.94), and ln [C 0/C] represents a linear relationship with reaction time, which confirmed that oxidation of CAP by VI can be accepted as pseudo-first-order reaction (see Fig. 3).
The pseudo-first-order reaction kinetic parameters of different doses of VI are shown in Table 1. Results revealed that reaction kinetic increased with the VI concentration (see Table 1), presenting a linear relationship with the concentration of the VI (k′ = 0.0093[Fe(VI)] + 6.843), which confirmed that oxidation of CAP by VI can be accepted as an overall second-order reaction. Previously, studies suggested that electron transfer rate was the controlling factors in oxidation, which increased with oxide concentration, and simultaneously improved the reaction rate (Graham et al. 2004; Jiang 2014). With pseudo-order reaction rate constant (k ′) and VI concentration, the overall rate constants (k) were calculated and depicted in Table 1.
Oxidation products
A number of oxidation products (OPs) formed during the CAP degradation were separated and detected by LC-MS, and their structures were presented (see Table 2). The possible pathways of CAP degradation during the treatment by VI were shown in Fig. 4. The attack on amide group of CAP by VI led to the cleavage of the group, and the oxidation products were dichloroacetic acid (CAP-1) and CAP-2. Then, the hydroxyl of CAP-2 with the addition of one oxygen atom formed CAP-3. Further oxidation of CAP-3 could lead to the loss of the nitro group and latter formation of CAP-4. In addition, the attack on the amino group and the hydroxyl of CAP-4 by VI formed CAP-5 (see Fig. 4).
Conclusions
CAP was degraded rapidly in the first 15 min, and with the dosage of VI increased, higher removal rate was achieved. The influence of solution pH was significant. Fe (VI) was more reactive in neutral conditions, presenting the highest removal efficiency of CAP, while redox potential of VI decreased in alkaline condition, which restrained the reaction and removal of CAP. The oxidation of CAP by VI presented a linear relationship with the concentration of the VI (k′ = 0.0093[Fe(VI)] + 6.843), which confirmed that oxidation of CAP by VI can be accepted as an overall second-order reaction. Five oxidations were detected during reaction process. The attack on the amide group of the CAP by VI led to the cleavage of the group, while benzene ring remained intact.
References
Badawy MI, Wahaab RA, EI-Kalliny AS (2009) Fenton-biological treatment processes for the removal of some pharmaceuticals from industrial wastewater. J Hazard Mater 167(1–3):567–574
Bayen S, Yi XZ, Segovia E, Zhou Z, Kelly BC (2014) Analysis of selected antibiotics in surface freshwater and seawater using direct injection in liquid chromatography electrospray ionization tandem mass spectrometry. J Chromatogr A 1338:38–43
Burkina V, Zlabek V, Zamaratskaia G (2015) Effects of pharmaceuticals present in aquatic environment on phase I metabolism in fish. Environ Toxicol Pharmacol 40(2):430–444
Gao P, Mao D, Luo Y, Wang L, Xu B, Xu L (2012) Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res 46:2355–2364
Giri AS, Golder AK (2014) Chloramphenicol degradation in Fenton and photo-Fenton: formation of Fe2+ chloramphenicol chelate and reaction pathways. Ind Eng Chem Res 53(42):16196–16203
Giusy L, Giovanni L, Roberta A, Antonietta S, Patrizia I, Marco G, Maurizio G, Annamaria VG, Maurizio C (2016) Photocatalytic degradation of the antibiotic chloramphenicol and effluent toxicity effects. Ecotoxicol Environ Saf 123:65–71
Graham N, Jiang CC, Li XZ, Jiang JQ, Ma J (2004) The influence of pH on the degradation of phenol and chlorophenols by potassium ferrate. Chemosphere 56:949–956
Hanekamp JC, Bast A (2015) Antibiotics exposure and health risks: chloramphenicol. Environ Toxicol Pharmacol 39(1):213–220
Jiang JQ, Wang S, Panagoulopoulos A (2006) The exploration of potassium ferrate (VI) as a disinfectant/coagulant in water and wastewater treatment. Chemosphere 63(2):212–219
Jiang JQ (2007) Research progress in the use of ferrate (VI) for environmental remediation. J Hazard Mater 146(3):617–625
Jiang JQ, Zhou ZW, Pahl O (2012) Preliminary study of ciprofloxacin (cip) removal by potassium ferrate (VI). Sep Purif Technol 88:95–98
Jiang JQ (2014) Advances in the development and application of ferrate (VI) for water and wastewater treatment. J Chem Technol Biotechnol 89(2):165–177
Jiang L, Hu XL, Yin DQ, Zhang HC, Yu ZY (2011) Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere 82(6):822–828
Kramer WG, Rensimer ER, Ericsson CD, Pickering LK (1984) Comparative bioavailability of intravenous and oral chloramphenicol in adults. J Clin Pharmacol 24(4):181–186
Li C, Li XZ, Graham N (2005) A study of the preparation and reactivity of potassium ferrate. Chemosphere 61(4):537–543
Li Z, Xiang X, Li M, Ma YP, Wang JH, Liu X (2015) Occurrence and risk assessment of pharmaceuticals and personal care products and endocrine disrupting chemicals in reclaimed water and receiving groundwater in China. Ecotoxicol Environ Saf 119:74–80
Liu YH, Lin SL, Fuh MR (2016) Determination of chloramphenicol, thiamphenicol and florfenicol in milk and honey using modified QuEChERS extraction coupled with polymeric monolith-based capillary liquid chromatography tandem mass spectrometry. Talanta 150:233–239
Lou J, Lu N, Wang TC, Wu Y (2012) Remediation of chloramphenicol-contaminated soil by atmospheric pressure dielectric barrier discharge. Chem Eng J 180:99–105
Marx C, Mühlbauer V, Krebs P, Kuehn V (2015) Environmental risk assessment of antibiotics including synergistic and antagonistic combination effects. Sci Total Environ 524:269–279
Nie MH, Yang Y, Zhang ZJ, Yan CX, Wang XN, Li HJ, Dong WB (2014) Degradation of chloramphenicol by thermally activated persulfate in aqueous solution. Chem Eng J 246:373–382
Peng XZ, Wang ZD, Kuang WX, Tan JH, Li K (2006) A preliminary study on the occurrence and behavior of sulfonamides, ofloxacin and chloramphenicol antimicrobials in wastewaters of two sewage treatment plants in Guangzhou, China. Sci Total Environ 371(1–3):314–322
Sanchez-Fortun S, Marva F, Rouco M, Costas V, Lopen R (2009) Toxic effect and adaptation in Scenedesmus intermedius to anthropogenic chloramphenicol contamination: genetic versus physiological mechanisms to rapid acquisition of xenobiotic resistance. Ecotoxicology 18(50):481–487
Sharma VK, Mishra SK, Nesnas N (2006) Oxidation of sulfonamide antimicrobials by ferrate (VI)[(FeVIO4 2−)]+. Environ Sci Technol 40(23):7222–7227
Wang H, Zhou XJ, Liu YQ, Yang MH, Guo QL (2011) Simultaneous determination of chloramphenicol and aflatoxin m residues in milk by triple quadrupole liquid chromatography-tandem mass spectrometry. J Agric Food Chem 59(8):3532–3538
Xia SQ, Gu ZL, Zhang ZQ, Zhang J, Hermanowicz SW (2014) Removal of chloramphenicol from aqueous solution by nanoscale zero-valent iron particles. Chem Eng J 257:98–104
Xue Q, Qi YJ, Liu F (2015) Ultra-high performance liquid chromatography-electrospray tandem mass spectrometry for the analysis of antibiotic residues in environmental waters. Environ Sci Pollut Res 21:16857–16867
Yan CX, Yang Y, Zhou JL, Liu M, Nie MH, Shi H, Gu LJ (2013) Antibiotics in the surface water of the Yangtze estuary: occurrence, distribution and risk assessment. Environ Pollut 175:22–29
Zhang HC, Chen WR, Huang CH (2008) Kinetic modeling of oxidation of antibacterial agents by manganese oxide. Environmental Science & Technology 42(15):5548–5554
Acknowledgment
The study was supported by National Natural Science Foundation of China (No. 21376219).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Zhou, Jh., Chen, Kb., Hong, Qk. et al. Degradation of chloramphenicol by potassium ferrate (VI) oxidation: kinetics and products. Environ Sci Pollut Res 24, 10166–10171 (2017). https://doi.org/10.1007/s11356-017-8656-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8656-7