Abstract
Contamination of estuarine system due to heavy metals is a severe issue in tropical countries, especially in India. For the evaluation of the risk due to heavy metals, the current study assessed spatial and temporal variation of acid-volatile sulfide (AVS), simultaneously extracted metal (SEM), and total metal concentration as toxicity indicator of aquatic sediments in Vembanad Lake System (VLS), India. Surface sediment samples collected from 12 locations from the northern portion of VLS for 4 years during different seasons. The results suggest, in post-monsoon season, 91% of the sampling locations possessed high bioavailability of metals and results in toxicity to aquatic biota. The average seasonal distribution of SEM during the period of observations was in the order post-monsoon > pre-monsoon > monsoon (1.76 ± 2.00 > 1.35 ± 0.60 > 0.80 ± 0.54 μmol/g). The concentration of individual metals on ∑SEM are in the order SEM Zn > SEM Cu> SEM Cd ≈ SEM Pb > SEM Hg. Considering annual ΣSEM/AVS ratio, 83% of the sites cross the critical value of ‘One,’ reveals that active sulfide phase of the sediment for fixing the metals is saturated. The molar ratio (differences between SEM and AVS) and its normalized organic carbon ratio reveals that in the post-monsoon season, about 42% of the sites are in the category of adverse effects are possible. The study suggests the toxicity and mobility of the metals largely depend on the available AVS, and the current situation may pose harm to benthic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lake sediments are considered to be a significant source of dissolved substances, and they act as essential regions playing the crucial role in biogeochemical cycling of nutrients and metals (Oehm et al. 1997). Among these, the sulfur species associated with sediments play a vital role in determining sediment toxicity. These compounds mainly produced by anaerobic decomposition of organic matter, which is one of the common, abundant constituents of aquatic sediments. In estuarine sediment, sulfur act as a vital redox element and may associate with the biogeochemical process of many elements (Shylesh et al. 2012).The diverse sulfur forms exist in sedimentary environment act as a primary controller for the trace metal mobility in anoxic-semi anoxic sediments. It acts as a critical binding phase for trace metals and controls metal mobility and availability. Many studies reveal, during the early sediment diagenesis, the redox cycling of the element sulfur affects sediment-bound metallic pollutants (Howarth 1984).
At the oxic-anoxic interface, a significant portion of the produced sulfide can be oxidized chemically or biologically (mediated by bacteria), and hence, regenerating the sulfate or may form intermediate sulfur species such as elemental sulfur, polysulfides, sulfite, thiosulfate, and polythionates (Thamdrup 1994). Acid-volatile sulfide (AVS) has a sedimentary property, which can control the biological impact of heavy metals. It is one of the chief chemical forms in aquatic sediments, which have a crucial role in controlling mobility and toxicity of metallic pollutants (Di Toro et al. 1990, 1991 and 1992; Ankley et al. 1991). Acid-volatile sulfide consists of more soluble sulfides, principally FeS, when present as a solid phase in the sediment. In an aquatic sedimentary environment, AVS exist in the form of FeS (Dilks et al. 1995). Both types of mercury sulfides—cinnabar (red HgS) and meta-cinnabar (black HgS)—are incredibly insoluble and this reason, HgS is considered to be an essential sink of mercury in anoxic sediments. However, the solubility of HgS strongly depends on the chemical composition of the sediment. Natural organic matter, especially humic acids, favors the dissolution or inhibits the precipitation of HgS (Fabbri et al., 2001, b; Waples et al., 2005). The ligand-metal interaction may promote the dissolution process, and the recent studies on complexes of Hg(II) with humic substances have demonstrated the relevance of reduced sulfide functional groups in the coordination of the metal.
Examination of the concentration of AVS in estuarine sediment is of considerable practical interest as it can immobilize toxic metals as insoluble sulfides. The concentration of AVS in metallic contaminated sediments has of immense significance as it reduces the metal toxicity. When the molar ratio of [SEM]/[AVS] ≥ 1 or [SEM]–[AVS] ≥ 1 0, shows acute toxicity is possible to such aquatic system (Di Toro et al., 1990; Ankley et al., 1991). Hence the AVS/SEM ratio widely uses as an index in the environmental quality of coastal systems. The AVS—trace metals interaction possibly creates thermodynamically stable metal sulfide precipitates, which can result in the decreased concentration of free metal ions, which negatively affects the metal mobility and availability in the sedimentary environment. The metals that are associated with AVS can be extracted simultaneously with the AVS extraction and is known as simultaneously extracted metal (SEM). The SEM is defined as the metal fraction that comprises the sum of molar concentrations of toxicologically significant, cationic metals that have solubility products lower than FeS and MnS. In both freshwater and marine environments, AVS measurements are increasingly being used in sediment quality studies to predict the absence of toxicity of Cd, Cu, Ni, Pb, and Zn (Di Toro et al. 1992; Chapman et al. 1998).
The fate of the pollutants depends mainly on various factors like oxic/anoxic environment, organic matter, sulfide chemistry, the presence of Fe species, and other physical parameters. Bioavailability and toxicity of metals in sediments were not well predicted by sediment metal concentrations alone. The analysis of the influence of geochemical factors like reduced sulfur species on the metal bioavailability has the potential to cause toxicity. By reviewing similar geochemical investigation, it is found that essential data on sulfur-metal interaction in aquatic sediments are scarce and absent in Indian estuarine environment. Hence, the present study aims (1) to characterize the spatial and temporal variation in concentration of heavy metals in the estuarine sediments in the northern portion of Vembanad Lake based on AVS-SEM analysis and (2) to determine the potential toxicity and ecological risk due to the heavy metals. The north part of the Vembanad Lake was chosen because it is regarded as one of the most severely threatened heavy metal polluted areas in the south-west coast of India (Ramasamy et al. 2017).
Materials and methods
Study area
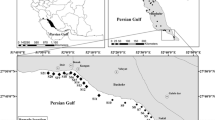
The Vembanad Lake System (VLS—9° 30′–10° 10′ N, 76° 10′–76° 25′ E), a tropical estuarine system, is one of the largest mixohaline systems (Menon et al. 2000; Segerstrale 1964) having an international status as Ramsar Site (No. 1214). It covers an area of over 1513 km2 thereby making it the largest wetland system of the country. This industrial belt accommodates around 247 chemical industries consists photochemical products, pesticides, rare earth element processing units, fertilizers, metallurgical industries, leather processing units, etc. The system supports a large number of people along the Kerala coast. The study was conducted on the northern limb of the estuary.
Sediment collection and preservation
Around 176 surface sediment samples from 12 locations (0–20 cm) were sampled from the year 2012–2014 on different seasons such as pre-monsoon, monsoon, and post-monsoon period of each year using Van Veen Grab sediment sampler (Fig. 1). The primary concern during sampling and handling was the oxidation of AVS to elemental sulfur. In order to avoid such interference, one set of the collected samples were immediately transferred to a nitrogen glove chamber and treated in situ by adding zinc acetate (ZnAc) to fix readily oxidizable sulfides. The other set of sediment samples devoid of ZnAc were taken for total metal analysis. The samples for AVS measurements were tightly sealed in self-sealed plastic bags and kept in ice box at a temperature around 4 °C and analyzed within 48 h of sampling.
Analysis of total metal concentration
Air-dried samples of sediments were ground and passed through ASTM sieve for < 63-μm fraction. For metals Cd, Cu, Pb, and Zn, about 0.2–0.25 g of these samples were taken and 7:1 ratio acid mixture (HNO3 and HCLO4) was added to the samples and digested at 1600C for 15 min in a microwave digester (CEM-MARS 6, USA). The digestate was then transferred to a 50-mL volumetric flask and made up to the mark by adding 18 Ω pure water (Cascada AN-Pall Corporation, UK). An aliquot of this consequential extract was injected to voltametric trace metal analyzer (797 VA Computrace, Metrohm, Switzerland). Quality controls were performed by including CRM and method blank in each batch of samples. Replicates of the samples were performed regularly. Spiked samples were also used for calculating the recoveries of procedures, and 93% of recovery was obtained.
For the metal mercury (Hg), an aliquot of sample (0.1–0.25 g) was subjected to microwave digestion with an acid mixture of HNO3/H2SO4 followed by bromine monochloride (BrCl) oxidation (USEPA, 2002). The digested solution was reduced with stannous chloride and detected with cold vapor atomic fluorescence spectrometer (CVAFS, Brooks Rand, USA). To check the validity of procedure, certified reference materials—CRM (European Reference Materials of the Institute of Reference Materials and Measurements) and ERM CC580 (Estuarine sediment) were used. A recovery of 98% was obtained for the ERM.
AVS and SEM analysis
AVS concentration in sediments was determined by standard cold-acid purge-and-trap method (Allen et al. 1993). Approximately 5 g of the wet sediment sample was taken into a reaction flask along with 100 ml of ultra-pure water of 18.2-MΩ cm resistivity. The system was purged with nitrogen gas for 10 min at 40 cm3/m before the introduction of reagents. Then 10 ml of 12 N HCl was added to the reaction flask, which has been bubbled with nitrogen gas for 1 h, simultaneously, the system was kept for boiling. The hydrogen sulfide (H2S) generated during the reaction will collect in an Erlenmeyer flask of 50 ml contains zinc acetate (ZnAc) trapping solution. After 1 h, the concentration of sulfide in the ZnAc trapping solution was determined using the iodometric back-titration method. After the generation of AVS has been completed, the sediment suspension remaining in the H2S generating flask was filtered through a 0.45-μ membrane filter paper. One portion of the filtrate was analyzed for total mercury using cold vapor atomic fluorescence spectrometer (CVAFS-Brooks Rand) and the other portion for metals such as Cd, Cu, Zn, and Pb with voltametric trace metal analyzer. The results obtained were computed for sulfide concentration/g dry weight of sediments. Validation of the AVS method was done using matrix spikes and apparatus blank. Standard sodium sulfide was also used for calculating the calibration curve, recovery percentage, and method validation. The analytical precision of AVS measurements estimated through duplicate measurements, and it demonstrated more than 10% accuracy.
Assessment of heavy metal toxicity
Sediment toxicity to benthic biota was predicted by a combined approach of chemical indices such as pollution load index (Chakravarty and Patgiri 2009), ΣSEM/AVS ratio, ΣSEM-AVS, and (∑SEM-AVS)/ƒOC. It is calculated using total metal concentration, simultaneously extracted metals and acid-volatile sulfide (USEPA 2004; Landner and Reuther 2004).
Physicochemical analysis
Samples were dried and ground using an agate mortar. The < 200-μm fraction of the dry and ground sediment was taken for the analysis of pH, conductivity, and organic carbon (Maiti 2003).
Results and discussion
Characterizing the study sites
The physiochemical properties of samples were depicted in Table 1. The annual pH value ranged from 4.61 to 6.18, with an average of 5.25 ± 0.41. Sediments collected during the pre-monsoon and post-monsoon seasons showed slight acidic character. However, during the monsoon season, the sediment showed near to neutral nature except for site 10–12, it may be by the terrigenous input from the nearest urban centers. The organic carbon (OC) content in surface sediments showed both the spatial and temporal variations. The annual OC ranged from 2.07–4.26 with an average of 3.11 ± 0.65 percentage. Monsoon season showed comparatively higher OC content than the pre-monsoon and post-monsoon seasons. Land runoff and terrigenous derivatives might have contributed to the monsoonal increase of organic carbon.
Spatial distribution of heavy metals
The total concentration of mercury (Hg), cadmium (Cd), copper (Cu), lead (Pb), and zinc (Zn) in sediment samples collected from Vembanad Lake are shown in Table 1, on milligram per kilogram dry weight basis. The values obtained were compared with the sediment quality criteria’s such as threshold effect level (TEL), probable effect level (PEL), and effects range low (ERL) described by Smith et al. (1996) and Long and Morgan (1990). The concentration of Hg ranged from 0 to 2.85 with a mean value of 3.11 ± 1.56 mg/kg. According to the sediment quality guidelines, 67–83% of the sites crossed TEL and PEL for the metal Hg (Fig. 2). In monsoon, 25% of the sites reached effect range median level. The results showed that the obtained concentration of Hg in this area was higher than those in sediments recorded from other Hg polluted estuarine and marine regions of the world like Arctic Ocean Basin (conc. 0.01–0.116 mg/kg), East China Sea (conc. 0.001–0.219 mg/kg), Malaysian coast (Conc.0.02–0.127 mg/kg), etc. (Gobeil et al. 1999; Kannan and Falandysz 1998). The presence of mercury may be attributed to the historic discharge from a chlor-alkali plant, which was closed in late 1990s (Mohan et al. 2014).
The concentration of the metal Cd in this region was ranged between 0.21 and 15.81 (average 6.35 ± 3.17) mg/kg. The concentration of Cd in sediments exhibited marginal seasonal fluctuation. In pre-monsoon, 83% of the location crosses the PEL limit (Fig. 3). Highest readings were observed at site six in pre- and post-monsoon period. The concentration obtained in all locations was higher than the environmental background value (0.136 mg/kg) of Bohai Bay (Shuyuan and Fengmin 1995).
The average Pb concentration was in the range of non-detectable (ND) to 69.04 with an average of 17.92 ± 7.05 mg/kg. This metal exhibited moderate seasonal variations. A higher level of Pb was observed during the post-monsoon season followed by monsoon (Fig. 4). Three locations in post-monsoon and on location in monsoon season cross the TEL. The study observed that 66% of location in post-monsoon and 41.6% samples in monsoon season were higher with respect to the background concentration of this metal (16.6 mg/kg, Shuyuan and Fengmin 1995). In post-monsoon concentration of Pb exhibits almost decreasing trend from location 1 to 12 pointing out the possibility of industrial discharges to upper stream area.
Among the studied metals, Zn was the predominant metal in spatial distribution and concentration. The metal was in a range of 10.7–2679.2 with an average concentration of 629.54 mg/kg. The higher average concentration was observed during post-monsoon, followed by monsoon and pre-monsoon period. It was also observed that the first five sampling points reached above PEL limit suggest that the continuous input of Zn in all the seasons (Fig. 5). In the case of metal, Cu post-monsoon season shows predominant concentration (Fig. 6). In this season, all the locations exceeded the background concentration. The concentration ranges from 9.80–203 with an average of 27 mg/kg. The higher concentration metals such as Cd, Pb, and Zn might be due to the influence of rapid urbanization, untreated effluent from chemical and metallurgical industries, and industrial discharges.
Spatial distribution of simultaneously extracted metals and acid-volatile sulfide
The AVS and SEM characteristic of the estuarine sediment from 12 locations during pre-monsoon (Fig. 7), monsoon (Fig. 8), and post-monsoon (Fig. 9) seasons were analyzed. AVS concentrations varied among sites and were ranged from 0.10–3.30 with an average 1.15 ± 0.97 μmol/g. The higher average concentration of AVS was observed in monsoon (1.658 ± 0.82 μmol/g) and pre-monsoon seasons (1.010 ± 0.60 μmol/g). The predominance of AVS during the monsoon season is a matter of high concern because it can pose toxicity to bottom sediments and thereby affects the benthic organisms. The other pyritic forms such as iron and manganese monosulfides in AVS group have higher solubility products and can be displaced by other metals to form more insoluble metal sulfides. It has also been noted that the concentration of AVS in sediments is the result of a balance between the generation of sulfides from a source of SO2 and the loss by oxidation or diffusion. The circumstances leading to the formation and the presence of AVS in aquatic sediments are very complicated due to the seasonal and spatial variations in physical and chemical properties of the pore water (Van Griethuysen et al. 2006).
The average seasonal distribution of SEM during the period of observations was in the order post-monsoon > pre-monsoon > monsoon (1.76 ± 2.00 > 1.35 ± 0.60 > 0.80 ± 0.54 μmol/g). The concentration of individual metals on ∑SEM are in the order SEM Zn > SEM Cu > SEM Cd ≈ SEM Pb > SEM Hg. SEM Zn contributes 85% of ∑SEM, and SEM Hg contributes the least.
The results of this study demonstrated that the AVS and SEM in sediment were strongly affected by the seasonal variations. During the post-monsoon season, the concentration of extractable metals and the ratio of SEM-AVS were observed much higher than the following other two seasons, which proved that the concentrations of AVS during monsoon and post-monsoon season were sufficient enough to scavenge the metals Hg, Cd, Pb, Cu, and Zn.
Evaluation of sediment toxicity
Pollution load index (PLI) is widely regarded as a comprehensive ecological index in assessing sediment quality. Hence, the PLI values are calculated and illustrated in Fig. 10. The PLI values range from 0.88–6.23 with an average of 2.98 ± 1.91. The rise in numerical PLI values suggests the progressive deterioration in sediment quality due to heavy metals. Progressive increase in PLI values observed for first 6 locations and higher values recorded stations 5 and 6. These values suggest 16% of the location have baseline levels of pollutants present and the rest of the stations indicate progressive deterioration of the site and estuarine quality (Tomlinson et al. 1980).
The concentration of AVS in the sedimentary environment is the result of the balance between the formation of this chemical entity and its loss due to oxidation or diffusion (Oehm et al. 1997). If the SEM/AVS ratio in the sediment exceeds one, metal availability/toxicity to surrounding sedimentary environment will be triggered, and the sediment is considered potentially toxic to aquatic ecosystems (Painuly et al. 2015). Seasonal distribution of ΣSEM/AVS ratio (Fig. 11) was in the order post-monsoon > pre-monsoon > monsoon. For post-monsoon, 92% of the sites possess a critical condition of contamination by heavy metals (bioavailability > 1), 67% for pre-monsoon and 9% for monsoon season. The annual average ΣSEM/AVS ratio ranged from 0.08 to 11.70 with an average of 2.3 ± 1.2. While considering yearly average ΣSEM/AVS ratio, 83% of the sites cross the critical value of “one”. According to Di Toro et al. (1992), acute metal toxicity could be expected from these sites. ΣSEM/AVS ratio in this estuary reveals that active sulfide phase of the sediment for fixing the metals is saturated.
According to Hansen et al. (1996), the difference between the molar concentrations of SEM and AVS ([SEM]–[AVS]) could provide valuable insights into the extent of additional available binding capacity. The differences between the molar concentrations of SEM and AVS in this study were shown in Fig. 12. According to McGrath et al. (2002), if SEM concentration is higher than that of AVS, toxicity could be anticipated by the divalent metals which exist as free metals. Based on this concept SEM-AVS ratio can categorize into three tiers, tier 1, SEM-AVS > 5; tier 2, SEM-AVS > 0–5; tier 3, SEM-AVS < 0 (USEPA 2004). In post-monsoon period 8% of stations fall under tier 1 category, and 91% of are in tier 2. Tier 1 represents “associated adverse effects are probable,” and tier 2 represents “associated adverse impacts are possible.” In pre-monsoon, 66% of the stations fall under tier 2 and in monsoon, all the locations were in tier 1, indicating no adverse impacts.
Organic carbon may act as a metal ligand and reduce metal availability/toxicity to sediments. The molar ratio/differences between SEM and AVS and its normalized organic carbon ratio (i.e., ∑SEM-AVS)/ƒOC) could provide better insights to the biological implication of metal toxicity of Vembanad Lake sediments. The organic carbon difference between SEM-AVS concentration to the concentration of organic carbon also accounted for the classification and prediction of metal toxicity to sediments (Chai et al. 2015). In this index, three-tier system proposed by USEPA (2005) was utilized. As per this categorization, during pre-monsoon and monsoon season, all the locations were fallen under tier 3 indicating no adverse effects are expected (Figs. 12, 13). While in the post-monsoon season, about 42% of the sites fall under tier 2 were associated adverse effects are possible.
Sediment toxicity imparted by heavy metal–sulfur interaction varied spatially and temporally. The blend of physical and biological factors as well as temperature, salinity, redox potential, oxygen, and organic content, the flow rate of surface water may be responsible for the various seasonal distributions of AVS in aquatic environments. The study suggests the ratio SEM/AVS > 1 in much of the location is a warning of heavy metal availability to benthic biota in this estuarine environment.
Statistical analysis
Statistical analysis techniques such as Pearson correlations analysis applied to observe possible correlations between the geochemical parameters and heavy metals. Pearson correlations among geochemical parameters and metals show significant correlation. The pH value shows significant correlation with organic carbon, iron, and SEM Cd, SEM Pb negatively correlated. Mercury shows significant positive correlation SEM Hg, and SEM Cu and Pb. Cadmium showed significant correlation with Pb and Zn. SEM Hg exhibited a high correlation with SEM Cd. The total metal concentration of Cu and Zn exhibits strong positive correlation with their SEM counterpart. Pb shows strong positive correlation with AVS.
Conclusion
Vembanad Lake is a designated Ramsar site having high ecological significance. The study suggests sulfur chemistry is highly significant as it is mainly linked to the transformation and mobility of heavy metals. Sediment toxicity imparted by heavy metal—sulfur interaction varied spatially and temporally. These values suggest 16% of the location have baseline levels of pollutants present and the rest of the stations indicate progressive deterioration of the site and estuarine quality. Any dredging operation or natural sediment resuspension activities can release metal sulfides from sediments to the water column and raise the AVS/SEM ratio to above “one”. The change in pH value to an acidic level, AVS fraction can release sulfide and may combine with hydrogen, and lead create toxicity to benthic organisms.
PLI values indicate that all the sediments of the three areas are contaminated with the heavy metals studied. According to the sediment quality guidelines, 67–83% of the sites crossed threshold effect level (TEL), and probable effect level (PEL) for the metal Hg is a significant alarm on anthropogenic metal pollution. The concentration of total metal reported in this study is comparable with varies polluted estuaries in the world. Among the reported metals, the concentration of Cadmium invites serious attention.
It may be considered that study area receives a continuous release of industrial and urban waste discharges along with dredging operations. More studies are required to understand the sulfur-metal interaction and their toxicity to aquatic organisms in tropical areas, especially in India. Hence, the present study draws a particular attention towards Cochin, one of the major fish-landing center in India and a considerable population exists in this region depending directly on this estuary for their livelihood.
References
Allen HE, Fu G, Deng B (1993) Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ Toxicol Chem 12(8):1441–1453. https://doi.org/10.1002/etc.5620120812
Ankley GT, Phipps GL, Leonard EN, Benoit DA, Mattson VR, Kosian PA, Cotter AM, Dierkes JR, Hansen DJ, Mahony JD (1991) Acid volatile sulfide as a factor mediating cadmium and nickel bioavail ability in contaminated sediments. Environ Toxicol Chem 10(10):1299–1307. https://doi.org/10.1002/etc.5620101009
Chai M, Shen X, Li R, Qiu G (2015) The risk assessment of heavy metals in Futian mangrove forest sediment in Shenzhen Bay (South China) based on SEM–AVS analysis. Mar Pollut Bull 97(1–2):431–439. https://doi.org/10.1016/j.marpolbul.2015.05.057
Chakravarty M, Patgiri AD (2009) Metal pollution and assessment in sediments of the Dikrong River N.E. India. J Hum Ecol 27(1):63–67. https://doi.org/10.1080/09709274.2009.11906193
Chapman PM, Wang F, Janssen C, Persoone G, Allen HE (1998) Ecotoxicology of metals in aquatic sediments: binding and release, bioavailability, risk assessment, and remediation. Can J Fish Aquat Sci 2001, 58(7):1442–1452. https://doi.org/10.1139/f98-145
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Hicks MB, Mayr SM, Redmond MS (1990) Toxicity of cadmium in sediments: the role of acid volatile sulfide. Environ Toxicol Chem 9(12):1487–1502. https://doi.org/10.1002/etc.5620091208
Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA, Paquin PR (1991) Technical basis for the equilibrium partitioning method for establishing sediment quality criteria. Environ Toxicol Chem 11:1541–1583
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Carlson AR, Ankley GT (1992) Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments.Environ. Sci Technol 26(1):96–101. https://doi.org/10.1021/es00025a009
Dilks DW, Helfand JS, Bierman Jr VJ (1995) Development and application of models to determine sediment quality criteria—driven permit limits for metals. Pub. Proc Toxic Subst Water Environ Assess Control
Fabbri D, Locatelli C, Snape CE, Tarabusi S (2001) Sulfur speciation in mercury-contaminated sediments of a coastal lagoon: the role of elemental sulfur. J Environ Monit 3(5):483–486. https://doi.org/10.1039/b104477j
Gobeil C, Macdonald RW, Smith JN (1999) Mercury profiles in sediments of the Arctic Ocean basins. Environ Sci Technol 33(23):4194–4198. https://doi.org/10.1021/es990471p
Hansen DJ, Berry WJ, Mahony JD, Boothman WS, Di Toro DM, Robson DL, Ankley GT, Ma D, Yan Q, (n.d.) Pesch https://doi.org/10.1007/s11356-017-0997-8
CE (1996) Predicting the toxicity of metal contaminated field sediments using interstitial concentration of metals and acid-volatile sulfide normalizations. Environ Toxicol Chem 15(12):2080. https://doi.org/10.1002/etc.5620151204
Howarth RW (1984) The ecological significance of sulfur in the energy dynamics of salt marsh and coastal marine sediments. Biogeochemistry 1(1):5–27. https://doi.org/10.1007/BF02181118
Kannan K, Falandysz J (1998) Speciation and concentrations of mercury in certain coastal marine sediments. Water Air Soil Pollut 103(1/4):129–136. https://doi.org/10.1023/A:1004967112178
Landner L, Reuther R (2004) Metals in society and in the environment: a critical review of current knowledge on fluxes, speciation, bioavailability and risk for adverse effects of copper, chromium, nickel and zinc. Environ Pollut 8
Long, E. R., Morgan, L. G. (1990) The potential for biological effects of sediments-sor bed contaminants tested in the National Status and trends program. National Oceanic and Atmospheric Administration. http://udspace.udel.edu/handle/19716/1562
Maiti SK (2003) Handbook of methods in environmental studies, vol. 2: air, noise, soil, overburden, solid waste and ecology. ABD Publishers, Japur
McGrath JA, Paquin PR, Di Toro DM (2002) Use of the SEM and AVS approach in predicting metal toxicity in sediments. Fact Sheet Environ Risk Assess 10:1–7
Menon NN, Balchand AN, Menon NR (2000) Hydrobiology of the cochin backwater system —a review. Hydrobiologia 430(1–3):149–183. https://doi.org/10.1023/A:1004033400255
Mohan M, Chandran MS, Jayasooryan KK, Ramasamy EV (2014) Mercury in the sediments of Vembanad Lake, western coast of India. Environ Monit Assess 186(6):3321–3336. https://doi.org/10.1007/s10661-014-3620-1
Oehm NJ, Luben TJ, Ostrofsky ML (1997) Spatial distribution of acid volatile sulfur in the sediments of Canadohta Lake, PA. Hydrobiologia 345(1):79–85. https://doi.org/10.1023/A:1002971130433
Painuly AS, Shrestha S, Hackney P (2015) Bioavailability of heavy metals using simultaneously extracted metal/acid volatile sulfide in the sediments of Lake Burragorang, NSW, Australia. J Environ Pollut Hum Health 3:12–17. https://doi.org/10.12691/jephh-3-1-3
Ramasamy EV, Jayasooryan KK, Chandran MS, Mohan M (2017) Total and methyl mercury in the water, sediment,and fishes of Vembanad, a tropical backwater system in India. Environ Monit Assess 189(3):130. https://doi.org/10.1007/s10661-017-5845-2
Segerstrale SG (1964) Marine zoology in the Baltic area in 1953-1962. Oceanogr Mar Biol Annu Rev 2:373–392
Shuyuan L, Fengmin M (1995) Study of the background values of heavy metals in the sediments of Bohai Sea. Acta Oceanol Sin 17(2):78–85
Shylesh Chandran MS, Sudheesh S, Ramasamy EV, Mohan M (2012) Sulphur fractionation in the sediments of cochin estuary. J Environ 01(01):1–6 ISSN 2049-8373
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG, Field LJ (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638. https://doi.org/10.1016/S0380-1330(96)70985-1
Thamdrup B, Fossing H, Jørgensen BB (1994) Manganese, iron and sulfur cycling in a coastal marine sediment, Aarhus Bay, Denmark. Geochim Cosmochim Acta 58:5115–5129
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun 33(1):566. https://doi.org/10.1007/BF02414780
USEPA (2004) The incidence and severity of sediment contamination in surface waters of the United States (National Sediment Quality Survey. EPA-823-R-04-007, second ed.). United States Environmental Protection Agency, Office of Science and Technology, Washington, DC
USEPA (2005) Procedures for the derivation of equilibrium partitioning sediment benchmarks (ESBs) for the protection of benthic organisms: metal mixtures (cadmium, copper, lead, nickel, silver, and zinc). United States Environmental Protection Agency, Office of Research and Development, Washington, DC. https://doi.org/10.1007/s11356-017-0997-8 EPA-600-R-02-011
Van Griethuysen C, De Lange HJ, Van den Heuij M, De Bies SC, Gillissen F, Koelmans AA (2006) Temporal dynamics of AVS and SEM in sediment of shallow freshwater floodplain lakes. Appl Geochem 30 21(4):632–642. https://doi.org/10.1016/j.apgeochem.2005.12.010
Waples JS, Nagy KL, Aiken GR, Ryan JN (2005) Dissolution of cinnabar (HgS) in the presence of natural organic matter. Geochim Cosmochim Acta 69(6):1575–1588. https://doi.org/10.1016/j.gca.2004.09.029
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• This study assessed spatial and temporal variation of acid-volatile sulfide (AVS), simultaneously extracted metal (SEM) and heavy metals in Vembanad Lake sediments.
• It is a first time report from an Indian estuarine system engaging SEM/AVS ratio as a toxicity index.
• Considering annual ΣSEM/AVS ratio, 83% of the sites cross the critical value of one, reveals that active sulfide phase of the sediment for fixing the metals is saturated.
• According to the sediment quality guidelines, 67–83% of the sites crossed threshold effect level (TEL) and probable effect level (PEL) for the metal Hg is a significant alarm on anthropogenic metal pollution.
• The molar ratio/differences between SEM and AVS and its normalized organic carbon ratio ((∑SEM-AVS)/ƒOC) reveals that in the post-monsoon season, about 42% of the sites are in the category of adverse effects are possible.
• Pollution load index revealed that 84% of sites belongs to the have anthropogenic metal contamination.
• The study suggests the ratio SEM/AVS > 1 in much of the location is a warning of heavy metal availability to benthic biota in this estuarine environment.
• The present study draws a special attention towards Cochin, one of the major fish-landing center in India and also a huge population exists in this region depending directly on this estuary for their livelihood.
Rights and permissions
About this article
Cite this article
Shyleshchandran, M.N., Mohan, M. & Ramasamy, E.V. Risk assessment of heavy metals in Vembanad Lake sediments (south-west coast of India), based on acid-volatile sulfide (AVS)-simultaneously extracted metal (SEM) approach. Environ Sci Pollut Res 25, 7333–7345 (2018). https://doi.org/10.1007/s11356-017-0997-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0997-8