Abstract
The impoundment of the Three Gorges Reservoir (TGR) in China influences the quality of the water supply. Surface sediment samples from the TGR mainstream and three tributaries were collected. Acid volatile sulfide (AVS), simultaneously extractable metals (SEMs), and the fraction of organic carbon (foc) were used to assess the toxicity of heavy metals. Sediment quality guidelines (SQGs) were established using the equilibrium partitioning approach. The results showed that the surface sediments were found to be oxic or suboxic. AVS concentrations in sediments were relatively low, below SEM concentrations. The [SEM] − [AVS] model indicated that all sediments possibly have adverse effects on aquatic life. However, ([SEM] − [AVS])/foc predicted no adverse biological effects in some areas of the Meixi and Caotang Rivers, while adverse effects to aquatic life were uncertain for the other sediments. The partitioning coefficients, water quality criteria, and residual metals in the sediments were the main factors influencing the SQGs for the TGR, while the metals bound to AVS had a negligible effect. The normalized TGR SQGs were all much higher than the existing standards except for cadmium and copper. The differences might be attributed to the approaches used for derivation of SQGs and the physical and chemical characteristics of the sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are an ecological concern in aquatic ecosystems because of their non-degradable, persistent nature, which causes them to accumulate in sediments (Gao et al. 2013; Fu et al. 2014). Hence, sediments have become the main reservoir for metal contaminants (Louriño-Cabana et al. 2011). However, when the aquatic environment changes (pH, Eh, etc.), sediments may become a source of heavy metals, impacting water quality and posing risks to aquatic organisms (Morgan et al. 2012; Zhuang and Gao 2013).
Several approaches have been developed to assess the impacts of heavy metals in sediments to the environment over the past 30 years (Wenning 2005). Traditionally, total concentrations of metals have been compared to numeric sediment quality guidelines (SQGs). However, studies have increasingly shown that the total heavy metal concentration alone may not accurately reflect ecological effects on aquatic organisms (Campana et al. 2013; Vink 2002). The chemical form of heavy metals in sediments determines their solubility and bioavailability in the aquatic environment (Yu et al. 2001).
As a major chemical component of sediments, acid volatile sulfide (AVS) plays a significant role in controlling the activity and bioavailability of metals in interstitial water (Gao et al. 2013; Prica et al. 2008). Several divalent transition metal cations (Cd, Cu, Ni, Pb, and Zn) react with sulfide to form highly insoluble metal sulfides that have minimal biological availability (Ankley et al. 1996; Di Toro et al. 1990; USEPA 2004). AVS is operationally defined as the amount of sulfide that can be extracted with cold hydrochloric acid (6 M HCl). Metals in the sediments extracted during this process are called simultaneously extractable metals (SEMs), including mainly Cd, Cu, Ni, Pb, and Zn (Di Toro et al. 1990). Comparison of the molar concentration of AVS ([AVS], μmol/g) to the sum of SEM molar concentrations ([SEM], μmol/g) has been used to evaluate the potential effects of metals on benthic species in anaerobic sediments. When [SEM]/[AVS] > 1 or [SEM] − [AVS] > 0, heavy metals may be toxic, whereas acute toxicity is less likely when [SEM]/[AVS] < 1 or [SEM] − [AVS] < 0 (Ankley et al. 1996; Di Toro et al. 1990, 2005). However, this approach has limitations. When [SEM]/[AVS] > 1 or [SEM] − [AVS] > 0, heavy metal toxicity can be overestimated. Other constituents of the sediments such as organic matter can also combine with free metal ions, thereby reducing their bioavailability and toxicity (Burton et al. 2005). Experimental results have shown that the fraction of organic carbon (foc) in sediments can be used to normalize the difference between SEM and AVS, i.e., ([SEM] − [AVS])/foc (Di Toro et al. 2005; Burton et al. 2005; Nguyen et al. 2011; Costello et al. 2012; USEPA 2005).
SQGs are a fundamental component of all sediment quality assessment frameworks (Simpson et al. 2011). A number of SQGs have been developed using various methods to predict toxicity in the aquatic environment under regulatory programs (USEPA 2004). These SQGs can be categorized into empirical and mechanistic groups (Zhong et al. 2013). Empirical SQGs have been derived from existing datasets of sediment chemistry and corresponding adverse biological effects (Chapman et al. 1999; Li et al. 2014b, c). Among the empirical SQGs, the threshold/probable effect levels (TELs/PELs) and the effects range low/median (ERLs/ERMs) are the most commonly used worldwide to assess sediment toxicity (Li et al. 2014b, c). China also established marine SQGs in 2002. Other SQGs have been developed using a mechanistic approach; the US Environmental Protection Agency (USEPA) equilibrium partitioning (EqP) approach is representative of these methods for selected metals (Ankley et al. 1994). The EqP model is based on the assumption that the toxicity of metals is associated with their interstitial water concentrations taking into account the influence of sulfides and organic carbon in sediments (Burton et al. 2005). Water quality criteria (WQC) can be applied to interstitial water contaminants, and SQGs can be calculated based on the concentration of contaminants in the sediments that is in equilibrium with the WQC concentration in the interstitial water (Chen et al. 2007). The ratio of the contaminant concentration in the sediment solid phase (C s) to that in interstitial water (C iw) is defined as the partitioning coefficient (K p). However, this method is less developed and few EqP-based SQGs for metals are currently available. SQGs derived using the EqP model depend on the K p and AVS, SEM, total organic carbon (TOC), and other binding factors (Chen et al. 2007).
The Yangtze River in China is the longest river in Asia and the third longest in the world (Yang et al. 2009). The Three Gorges Reservoir (TGR) is located at the outlet of the upper Yangtze River, with a surface area of 1080 km2, a drainage area greater than 1.0 × 106 km2 (Huang et al. 2006; Ye et al. 2011), and a storage capacity of 39.3 billion m3 in 2009 (Tang et al. 2014). The TGR is one of the largest man-made reservoirs in the world. As the most important freshwater resource in China, the TGR greatly affects the ecological environment of the Yangtze River system and the quality of the water supply. However, the aquatic environment downstream of the Yangtze River and its tributaries and heavy metal biological toxicity links to the highly dynamic hydrologic regime after impoundment are not well understood (Feng et al. 2013). In recent years, heavy metals in the TGR have caused widely public concern; traditional total heavy metal concentrations, the geoaccumulation index, and potential ecological risk index demonstrated the existence of potential risks to local residents (Tang et al. 2014; Wang et al. 2012). These studies have inadequate scientific evidence to evaluate toxicity and bioavailability. In this study, we collected surface sediment samples from the TGR and determined the heavy metal concentrations (Cu, Cd, Pb, Zn, and Ni) and the concentrations of AVS, SEM, and TOC. The objectives of this study were to (1) assess the toxicity of the heavy metals in sediments (Cu, Cd, Pb, Zn, and Ni) using AVS, SEM, and TOC and (2) develop SQGs using the USEPA EqP model.
Materials and methods
Sample collection
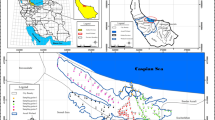
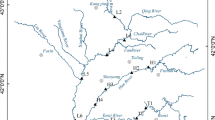
Sediments were collected from the Yangtze River mainstream and three tributaries (Zhuyi River (ZY), Meixi River (MX), and Caotang River (CT)) to the TGR in July 2014 (Fig. 1). At each sampling site, samples were taken using a core sampler (K-B type, Wildco, USA) near the middle of the stream. Surface sediment was collected into clean polyethylene bags and treated immediately on returning to the laboratory. The sediment samples were wet-sieved through an acid-cleaned 63-μm mesh nylon sieve to obtain the chemically active material, dried at 40 °C to a constant weight, and ground in an agate mortar to ensure homogeneity.
Acid volatile sulfide and simultaneously extracted metals analysis
AVS and SEM were analyzed within 2 weeks of sample collection by optimizing the methods described by Allen et al. (1993) and Brouwer and Murphy (1994). Briefly, sediments were homogenized gently in a N2-filled glove bag. The experimental setup involved a 250-mL round-bottom reaction flask sequentially connected to a scrubbing bottle containing 30 mL of 0.5 M NaOH solution to trap the evolved gases. The experimental apparatus was purified for 20 min with high-purity N2 at a flow rate of 60 mL/min to remove internal oxygen. About 5 g of the wet sediment sample was added to the reaction flask and sparged for another 10 min with N2 at a flow rate of 60 mL/min. Next, 20 mL of HCl (1 + 1) solution was quickly injected into the round-bottom reaction flask to release H2S. The sediment suspension was stirred for 50 min under continuous N2 at a flow rate of 40–60 mL/min. The H2S was subsequently collected in NaOH solution. The dissolved sulfide concentration in the NaOH solution was determined using the methylene blue colorimetric method at 665 nm (Cline 1969). The acidic extracts remaining in the flask after extraction were filtered through a 0.45-μm membrane for SEM analysis. SEM analyses for Cd, Cu, Ni, Pb, and Zn were carried out using inductively coupled plasma mass spectrometry (ICP-MS, Perkin Elmer Elan DRC-e). [SEM] was calculated as the sum of the concentrations of all simultaneously extracted metals, using the equation [SEM] = [SEMCu] + [SEMCd] + [SEMPb] + [SEMNi] + [SEMZn] (Di Toro et al. 2005). Quality control of AVS extraction was assured by adding known amounts of sulfide to wet sediments. The mean recovery of AVS was 90 %.
Metal and conventional analysis
The water content of the sediments was determined gravimetrically. About 5–10 g of wet sediment was dried at 105 °C to a constant weight. The granularity of each sample was analyzed using a particle size analyzer (Mastersizer 2000; Malvern, UK) with the ability to analyze a particle size range of 0.02–2000 μm. The particle size ranges used were <4 μm (clay), 4–63 μm (silt), and >63 μm (sand) (Gao and Chen 2012). TOC in decarbonated sediments was analyzed using an Elementar Vario MACRO Cube CHNS analyzer.
A modified European Community Bureau of Reference (BCR) sequential extraction method was used to characterize four metal fractions in sediments. Briefly, about 0.8 g of dried sediment was weighed into a set of centrifuge tubes and subjected to a series of extractions. The four fractions were acid-exchangeable (F1), reducible (F2), oxidizable (F3), and residual (F4) metals. The total metal concentration in the sediment was measured using acid digestion. Heavy metal concentrations in interstitial water were measured after centrifugation. Sediment samples were placed in centrifuge tubes, centrifuged at 4000 rpm for 20 min, and filtered through a 0.45-μm membrane. ICP-MS was used to determine the heavy metal concentrations, including Cu, Zn, Cd, Pb, and Ni in the digest, BCR extract, and interstitial water.
All results are expressed on a dry weight basis. All reagents were of analytical or guaranteed grade and all materials (bottles, tubes, etc.) were soaked in 20 % HNO3 (v/v) and cleaned with de-ionized water before use.
Water quality criteria
In accordance with the USEPA approach, the freshwater criteria for heavy metals were calculated as a function of hardness in the water column (USEPA 2009). There are two levels of criteria: the criterion maximum concentration (CMC) and the criterion continuous concentration (CCC). The CMC is the highest concentration of a substance that can be present in surface water for a short time without unacceptable adverse effects to the aquatic community. The CCC is the highest concentration of a substance that can be present in surface water over a long-term exposure without unacceptable adverse effects to aquatic organisms. The CCC is protective of most aquatic communities, so we selected the CCC as the WQC for derivation of SQGs. National quality standards (Grade I and II in GB3838-2002) for freshwater recommended by the State Environmental Protection Administration of China (SEPAC) (2002) were also adopted as protective levels for benthic organisms. The WQC formulas and related parameters are shown in Supplementary Table S1.
Sediment quality guideline calculation methods
Consistent with the EqP model, SQGs were calculated as follows (USEPA 1995):
where WQC is the selected water quality criterion (mg/L), K p is the partitioning coefficient, C s is the concentration of the metal in the sediment solid phase (mg/kg), C iw is the concentration of the metal in the interstitial water (μg/L), C T is the total concentration of the metal in the sediment (mg/kg), and A is the percentage of residual heavy metals in the sample (%).
Heavy metals in the residual state have very low bioavailability. According to Di Toro et al. (1992), AVS can bind several divalent metals and forms insoluble sulfide complexes with minimal biological availability. Therefore, the normalized SQGs for these metals can be calculated as follows:
where M Ri is the residual concentration of the metal in the sample (mg/kg), M AVSi is the concentration of the metal bound to AVS (mg/kg), [AVS] is the concentration of AVS (μmol/g), and M i is the atomic weight of the metal (Cu, Cd, Pb, Zn, or Ni).
Results and discussion
Acid volatile sulfide and simultaneously extractable metal concentrations
Concentrations of AVS, SEM, and acid-extractable metals in surface sediments are presented in Table 1. Generally, AVS concentrations at the river mouth were higher than upstream and concentrations in the mainstream were higher than in the tributaries. Relatively low AVS concentrations were found in the study area, within the range of 0.0034–0.0572 μmol/g, indicating oxic or suboxic environments in the surface sediments. Li et al. (2014b) and Ji et al. (2011) measured AVS in coastal sediments and found that large areas of sediments were oxic or suboxic, similar to our results. All of the surface sediments had relatively low AVS concentrations, suggesting [SEM] > [AVS].
Zn and Cu were highest among the acid-extractable metals in the samples (Table 1), contributing approximately 78.41–84.5 % to the total SEM. In contrast, the much more toxic element Cd accounted for <0.5 % of the SEM. Concentrations of acid-extractable trace metals fell in the following order: [SEMCd] < [SEMPb] ≈ [SEMNi] < [SEMCu] < [SEMZn]. These results were consistent with study of Bohai and the Yellow Sea (Gao et al. 2013).
Assessment of sediment heavy metal toxicity using equilibrium partitioning method
SEM can react with AVS to form insoluble metal sulfides that are not bioavailable to benthic organisms. According to the USEPA, the difference between the sum of the SEM concentrations of five heavy metals (Cu, Cd, Pb, Zn, and Ni) and the AVS concentration is one method for evaluating metal toxicity in sediments (USEPA 2004). Samples are classified into the following three tiers: Tier 1, [SEM] − [AVS] > 5, adverse effects on aquatic life are probable; Tier 2, 0 < [SEM] − [AVS] < 5, adverse effects on aquatic life are possible; and Tier 3, [SEM] − [AVS] < 0, no adverse effects are observed (USEPA 2004). All of the samples in this study were classified into Tier 2, except the Yangtze site, which was Tier 1 at 5.0691. Thus, heavy metals in surface sediments in all of the samples possibly have adverse effects on aquatic life.
However, considering that there are many heavy metal binding phases in sediments, not all sediments with [SEM] − [AVS] > 0 exhibit adverse effects to aquatic life (Burton et al. 2005; Brix et al. 2010; De Jonge et al. 2012). Therefore, USEPA has developed a complementary method for evaluating metal toxicity more accurately. This approach incorporates TOC content in the sediments using the formula ([SEM] − [AVS])/foc (μmol/g OC). The results are also classified into three tiers: Tier 1, adverse biological effects may occur when ([SEM] − [AVS])/foc > 3000; Tier 2, adverse effects are uncertain when ([SEM] − [AVS])/foc = 130–3000; and Tier 3, adverse effects are unlikely when ([SEM] − [AVS])/foc < 130 (USEPA 2005). According to this approach, the surface sediments at sites Meixi 1, 2, 3, and 5 and Caotang 1 fell into Tier 3 indicating no adverse biological effects, whereas the [SEM] − [AVS] criterion predicted possible adverse effects on aquatic life. Adverse effects to aquatic life were uncertain (Tier 2) for all of the other sites.
Analysis of variables used to derive sediment quality guidelines
Using the EqP method, the SQGs are affected by the K p, WQC, M AVS, and M R; of these, the K p is one of the most important (Chen et al. 2007). There are two methods for determining the K p. The K p can be calculated using a surface complexation model (Wang et al. 1997) or by directly measuring metal concentrations in interstitial water and the sediment solid phase. We selected the latter method because it minimizes potential errors by reflecting the actual physical and chemical conditions in the sediments (Huo and Chen 1997). C s and C iw are listed in Supplementary Table S2. Metal concentrations in interstitial water were low compared with sediment concentrations. The higher metal concentrations in the solid phase and lower concentrations in the interstitial water resulted in high K p values (Table 2). K p values for Pb and Zn were particularly high, 1–2 orders of magnitude larger than in previous studies (Chen et al. 2007; Deng et al. 2011). This higher K p can be explained by the distribution of particle sizes (Chen et al. 2007). Particles <63 μm (i.e., fines) consisting of silt and clay have a high surface area and are more likely to absorb heavy metals because of their surface chemistry. The percent of fines was 87.80–98.66 % of the sediments (Supplementary Table S3), indicating that the studied sediments in the TGR were primarily composed of silt and clay.
The WQC are also important in calculating SQGs. WQC determined by hardness (CaCO3, mg/L) and the Chinese Grade I and Grade II WQC are shown in Table 3. WQC based on hardness were lower in the mainstream than in the three tributaries; the Yangtze River had the lowest hardness of 52.03 mg/L (Supplementary Table S4). In the three tributaries, the river mouth area had relatively higher hardness. In general, the WQC based on hardness were significantly different from the Chinese Grade I and Grade II WQC. The WQC for Cu and Pb based on hardness were lower than the Chinese Grade I WQC. For Cd and Zn, the WQC were between Grade I and Grade II. The WQC for Ni could not be compared because of the lack of a WQC for Ni in China. These differences may arise from the geochemical properties of different regions (Fang and Xu 2007).
Residual heavy metals (M Ri) are present in chemically stable mineral forms or bound to non-labile soil fractions. They are less mobile and bioavailable and, hence, less toxic (Zhang et al. 2010). M Ri is also significant in calculating the SQGs. The proportion of residual metals varied for different metals (Supplementary Fig. S1). The mean proportion of M Ri for Cu, Cd, Pb, Zn, and Ni was 47.66, 16.00, 18.55, 54.78, and 76.53 %, respectively (Supplementary Fig. S1).
Heavy metals in the sediments that combine with AVS are expected to have minimal bioavailability (Li et al. 2014a). Therefore, in the modified EqP approach, M AVS are also components of calculating the SQGs. Very low M AVS were measured for all sediments (Table 2), because sediments in the TGR are primarily oxidized (Fang and Xu 2007).
Proportions of the metals in each of these forms are shown in Supplementary Fig S2. For Cu, K p × WQC i, M AVSi, and M Ri ranged over 29.85–65.80, 0.0450–0.1107, and 33.94–69.38% with mean values of 49.88, 0.3824, and 49.73 %, respectively. For Ni, the proportions of K p × WQC i were higher than for Cu (62.08–82.71 %, mean 74.92 %), and M AVSi and M Ri were lower (0.0130–0.1755 and 17.21–37.80 %, means 0.1051 and 24.97 %, respectively). Cd, Pb, and Ni had similar distributions with K p × WQC i accounting for 82.22–99.36 %; the percentages of M AVS and M Ri were 0.0024–0.76418 and 0.6167–17.05 %, respectively. On the whole, different components (i.e., binding phases) made different contributions to the SQGs. K p, WQC, and M R were the predominant factors affecting the SQGs. M AVS accounted for only 0.0024–0.7707 % of the metals in sediments. Therefore, under strongly oxidative conditions, the effect of AVS on the SQGs can be neglected.
Sediment quality guidelines for the Three Gorges Reservoir
We calculated SQGs for the TGR using the EqP approach recommended by USEPA (Table 4). The SQGs for Zn were 1–2 orders of magnitude higher than for the other elements. We also used the Chinese Grade I and Grade II WQC to calculate SQGs; however, Ni was not included because of the absence of a Chinese WQC. The SQGs were closely related to the WQC. The SQGs based on Grade I WQC for Cd and Pb at all sampling sites and Cu in the Caotang River were all lower than the values based on hardness. The SQGs based on the Grade II WQC all exceeded the criteria based on hardness.
ISQGlow/ISQGhigh, TEC/PEC, and ISQVlow/ISQVhigh are internationally used SQGs developed based on empirical relationships between heavy metal concentrations in freshwater sediments and toxic effects. The SQGs for Cd, Pb, Zn, and Ni we developed were all an order of magnitude higher than the ISQGlow/ISQVlow and somewhat higher than the ISQGhigh/ISQVhigh. However, the SQGs for Cu we established were lower than the ISQGlow and ISQVlow except for the Yangtze River and were between the most rigorous standards TEC and PEC (Table 4). These differences may be attributed to the derivation method, level of protection, screening of key environmental factors, and the characteristics of the sediments (TOC, AVS, and pH) (Chen et al. 2005), all of which increase the complexity of SQG development and use.
Previous studies providing SQGs for the Yangtze River are also listed in Table 4. The SQGs for Pb and Zn we developed were near the upper end of the range of previous values. This may be attributed to the high K p values and percent fines in the present study. The TGR slows the flow rates of the rivers, increasing sediment stability. Heavy metals adsorb onto the fine particles and are deposited on the bottom of the TGR, resulting in the relatively high SQGs. For Cu and Cd, the results were about the same order of magnitude as in previous studies. Studies of Ni in China are rare because of the absence of WQC; previously reported SQGs ranged widely over 143–12,900 mg/kg. In general, previously developed SQGs in China have been fairly rough estimates. The existing WQC for China may not represent the WQC that would be appropriate for a specific area, although they are good reference values. Similarly, the EqP model better reflects the actual conditions compared to empirical SQGs. Remaining inaccuracies may be attributed to other uncertainties in the SQGs; for example, a large variety of contaminants are present in sediments that may interact with each other. The SQGs we established were higher than the total concentrations of heavy metals (Supplementary Table S5), demonstrating that heavy metals have no potential risk to aquatic organisms except for Cu, whose concentrations were slightly larger than the SQGs in some areas.
Conclusions
The EqP model was used to assess heavy metal toxicity and establish SQGs for the TGR. The results indicate that the surface sediments in the TGR were oxic or suboxic. Sediments with [SEM] − [AVS] > 0 might have no biological toxicity based on ([SEM] − [AVS])/foc; [SEM] − [AVS] overestimated the heavy metal toxicity in the TGR. In this study, K p, WQC, and M R were the main factors affecting the SQGs, while M AVS could be neglected. The SQGs we established provide a scientific basis for further evaluation of heavy metal toxicity in the TGR. Although the EqP approach has been supported by a number of sediment toxicity tests in deriving SQGs, AVS and TOC used in this study provide a useful tool to improve the reliability of SQGs; the application of this approach was also confounded by the various components that ameliorate the bioavailability of metals. Therefore, much additional research is needed to improve the reliability of SQGs such as considering the influence of particle size and other factors.
References
Allen HE, Fu GM, Deng BL (1993) Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ Toxicol Chem 12:1441–1453
Ankley GT, Thomas NA, Di Toro DM, Hansen DJ, Mahony JD, Berry WJ, Swartz RC, Hoke RA, Garrison AW, Allen HE (1994) Assessing potential bioavailability of metals in sediments: a proposed approach. Environ Manag 18:331–337
Ankley GT, Di Toro DM, Hansen DJ, Berry WJ (1996) Technical basis and proposal for deriving sediment quality criteria for metals. Environ Toxicol Chem 15:2056–2066
Brix KV, Keithly J, Santore RC, DeForest DK, Tobiason S (2010) Ecological risk assessment of zinc from stormwater runoff to an aquatic ecosystem. Sci Total Environ 408:1824–1832
Brouwer H, Murphy TP (1994) Diffusion method for the determination of acid‐volatile sulfides (AVS) in sediment. Environ Toxicol Chem 13:1273–1275
Burton GA, Nguyen LTH, Janssen C, Baudo R, McWilliam R, Bossuyt B, Beltrami M, Green A (2005) Field validation of sediment zinc toxicity. Environ Toxicol Chem 24:541–553
Campana O, Blasco J, Simpson SL (2013) Demonstrating the appropriateness of developing sediment quality guidelines based on sediment geochemical properties. Environ Sci Technol 47:7483–7489
Chapman PM, Allard PJ, Vigers GA (1999) Development of sediment quality values for Hong Kong special administrative region: a possible model for other jurisdictions. Mar Pollut Bull 38:161–169
Chen YZ, Yang H, Zhang ZK, Qin MZ (2005) The difference and cause analyses of freshwater sediment quality guidelines. J Lake Sci 3:002 (in Chinese)
Chen YZ, Yang H, Zhang ZK, Qin MZ, Jin F, LüJJ (2007) Application of equilibrium partitioning approach to the derivation of sediment quality guidelines for metals in Dianchi Lake. Pedosphere 17:284–294
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Costello DM, Burton GA, Hammerschmidt CR, Taulbee WK (2012) Evaluating the performance of diffusive gradients in thin films for predicting Ni sediment toxicity. Environ Sci Technol 46:10239–10246
De Jonge M, Teuchies J, Meire P, Blust R, Bervoets L (2012) The impact of increased oxygen conditions on metal-contaminated sediments part I: Effects on redox status, sediment geochemistry and metal bioavailability. Water Res 46:2205–2214
Deng BL, Zhu LY, Liu M, Liu NN, Yang LP, Du Y (2011) Sediment quality criteria and ecological risk assessment for heavy metals in Taihu lake and Liao river. Res Environ Sci 24:33–42 (in Chinese)
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Hicks MB, Mayr SM, Redmond MS (1990) Toxicity of cadmium in sediments: The role of acid volatile sulfide. Environ Toxicol Chem 9:1487–1502
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Carlson AR, Ankley GT (1992) Acid volatile sulfide predicts the acute toxicity of cadmium and nickel in sediments. Environ Sci Technol 26:96–101
Di Toro DM, McGrath JA, Hansen DJ, Berry WJ, Paquin PR, Mathew R, Wu KB, Santore RC (2005) Predicting sediment metal toxicity using a sediment biotic ligand model: methodology and initial application. Environ Toxicol Chem 24:2410–2427
Fang T, Xu XQ (2007) Establishment of sediment quality criteria for metals in water of the Yangtze river using equilibrium partitioning approach. Resour Environ Yangtze Basin 16:525–531 (in Chinese)
Feng L, Hu CM, Chen XL, Zhao X (2013) Dramatic inundation changes of China’s two largest freshwater lakes linked to the Three Gorges Dam. Environ Sci Technol 47:9628–9634
Fu J, Zhao CP, Luo YP, Liu CS, Kyzas GZ, Luo Y, Zhao DY, An SQ, Zhu HL (2014) Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. J Hazard Mater 270:102–109
Gao XL, Chen C-TA (2012) Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res 46:1901–1911
Gao XL, Li PM, Chen C-TA (2013) Assessment of sediment quality in two important areas of mariculture in the Bohai Sea and the northern Yellow Sea based on acid-volatile sulfide and simultaneously extracted metal results. Mar Pollut Bull 72:281–288
Huang ZL, Li YL, Chen YC, Li JX, Xing ZG (2006) Water quality prediction and water environmental carrying capacity calculation for Three Gorges Reservoir. China Water Power Press, Beijing, China (in Chinese)
Huo WY, Chen JS (1997) Water-particulate distribution coefficient of heavy metal and application in sediment quality criteria in China River, China. J Environ Sci 18:10–13 (in Chinese)
Ji WD, Wang WQ, Chen HB, He JM, He Q (2011) The ocean environmental quality status and baseline study. Ocean Science Publisher, Beijing (in Chinese)
Li F, Lin JQ, Liang YY, Gan HY, Zeng XY, Duan ZP, Liang K, Liu X, Huo ZH, Wu CH (2014a) Coastal surface sediment quality assessment in Leizhou Peninsula (South China Sea) based on SEM–AVS analysis. Mar Pollut Bull 84:424–436
Li L, Wang XJ, Liu JH, Shi XF, Ma DY (2014b) Assessing metal toxicity in sediments using the equilibrium partitioning model and empirical sediment quality guidelines: A case study in the nearshore zone of the Bohai Sea, China. Mar Pollut Bull 85:114–122
Li L, Wang XJ, Zhu AM, Yang G, Liu JH (2014c) Assessing metal toxicity in sediments of Yellow River wetland and its surrounding coastal areas, China. Estuar Coast Shelf Sci 151:302–309
Louriño-Cabana B, Lesven L, Charriau A, Billon G, Ouddane B, Boughriet A (2011) Potential risks of metal toxicity in contaminated sediments of Deûle river in Northern France. J Hazard Mater 186:2129–2137
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
McCready S, Birch GF, Long ER, Spyrakis G, Greely CR (2006) An evaluation of Australian sediment quality guidelines. Arch Environ Contam Toxicol 50:306–315
Morgan B, Rate AW, Burton ED (2012) Trace element reactivity in FeS-rich estuarine sediments: Influence of formation environment and acid sulfate soil drainage. Sci Total Environ 438:463–476
Nguyen LTH, Burton GA, Schlekat CE, Janssen CR (2011) Field measurement of nickel sediment toxicity: role of acid volatile sulfide. Environ Toxicol Chem 30:162–172
Prica M, Dalmacija B, Rončević S, Krčmar D, Bečelić M (2008) A comparison of sediment quality results with acid volatile sulfide (AVS) and simultaneously extracted metals (SEM) ratio in Vojvodina (Serbia) sediments. Sci Total Environ 389:235–244
SEPAC (State Environmental Protection Administration of China) (2002) Environmental Quality Standard for Surface Water GB 3838-2002. China Environmental Science Press, Beijing
Simpson SL, Batley GE, Hamilton IL, Spadaro DA (2011) Guidelines for copper in sediments with varying properties. Chemosphere 85:1487–1495
Tang Q, Bao YH, He XB, Zhou HD, Cao ZJ, Gao P, Zhong RH, Hu YH, Zhang XB (2014) Sedimentation and associated trace metal enrichment in the riparian zone of the Three Gorges Reservoir, China. Sci Total Environ 479–480:258–266
USEPA (United States Environmental Protection Agency) (1995) An SAB Report: Review of the Agency's approach for developing sediment criteria for five metals, EPA-SAB-EPEC-95-020. U.S. Environmental Protection Agency, Washington DC
USEPA (United States Environmental Protection Agency) (2004) The Incidence and Severity of Sediment Contamination in Surface Waters of the United States, National Sediment Quality Survey, 2nd edn. US Environmental Protection Agency, Office of Research and Development, Washington
USEPA (United States Environmental Protection Agency) (2005) Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: Metal Mixtures (Cadmium, Copper, Lead, Nickel, Silver and Zinc), US Environmental Protection Agency, Office of Research and Development Washington, DC
USEPA (United States Environmental Protection Agency) (2009) National Recommended Water Quality Criteria, US Environmental Protection Agency, Office of Water, Office of Science and Technology (4304T)
Vink JPM (2002) Measurement of heavy metal speciation over redox gradients in natural water-sediment interfaces and implications for uptake by benthic organisms. Environ Sci Technol 36:5130–5138
Wang FY (1994) Environmental geochemistry of river particulate-metals in Eastern China. PhD thesis. Beijing University, Beijing, China (in Chinese)
Wang FY, Chen JS, Forsling W (1997) Modeling sorption of trace metals on natural sediments by surface complexation model. Environ Sci Technol 31:448–453
Wang JK, Gao B, Zhou HD, Lu J, Wang YC, Yin SH, Hao H, Yuan H (2012) Heavy metals pollution and its potential ecological riskof the sediments in Three Gorges Reservoir during its impounding period. Environ Sci 33:1693–1699 (in Chinese)
Wenning RJ (2005) Use of sediment quality guidelines and related tools for the assessment of contaminated sediments. SETAC Press, Pensacola
Yang ZF, Wang Y, Shen ZY, Niu JF, Tang ZW (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194
Ye C, Li SY, Zhang YL, Zhang QF (2011) Assessing soil heavy metal pollution in the water-level-fluctuation zone of the Three Gorges Reservoir, China. J Hazard Mater 191:366–372
Yu KC, Tsai LJ, Chen SH, Ho ST (2001) Chemical binding of heavy metals in anoxic river sediments. Water Res 35:4086–4094
Zhang WH, Huang H, Tan FF, Wang H, Qiu RL (2010) Influence of EDTA washing on the species and mobility of heavy metals residual in soils. J Hazard Mater 173:369–376
Zhong WJ, Zeng Y, Zhu LY (2013) Current research status of sediment quality criteria. Asian J Ecotoxicol 8:285–294 (in Chinese)
Zhuang W, Gao XL (2013) Acid-volatile sulfide and simultaneously extracted metals in surface sediments of the southwestern coastal Laizhou Bay, Bohai Sea: Concentrations, spatial distributions and the indication of heavy metal pollution status. Mar Pollut Bull 76:128–138
Acknowledgments
This work was jointly supported by the Special Scientific Fund sponsored by the Chinese Institute of Water Resources and Hydropower Research (Grant No. HJ1506), the Open Research Fund of State Key Laboratory of Simulation and Regulation of Water Cycle in River Basin, China Institute of Water Resources and Hydropower Research (Grant No. 2014QN02, 2014RC08), and the China Postdoctoral Science Foundation (Grant No. 2014 T70094, 2013 M530668).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Marcus Schulz
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1137 kb)
Rights and permissions
About this article
Cite this article
Gao, L., Gao, B., Wei, X. et al. Assessment of metal toxicity and development of sediment quality guidelines using the equilibrium partitioning model for the Three Gorges Reservoir, China. Environ Sci Pollut Res 22, 17577–17585 (2015). https://doi.org/10.1007/s11356-015-4959-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4959-8