Abstract
The study aimed to identify an effective phosphate-solubilizing and organochlorine pesticide-tolerant bacterial strain(s). A total of 50 phosphate-solubilizing bacterial (PSB) strains were isolated from pesticide-stressed soil. Ten isolates showing higher solubilization were selected for organochlorine pesticides (endosulfan, aldrin, and lindane) tolerance. The strain IITISM08 showed the maximum potential of phosphorous solubilization in Pikovaskya agar medium (solubilization index = 3.2) and in broth medium (348 ± 2 μg mL−1) and tolerated up to 250 μg mL−1 of organochlorine pesticides. During phosphorous solubilization, the presence of functional group and organic acid production were also observed using FT-IR and HPLC. The plant growth-promoting (PGP) traits of the strain IITISM08 was highly inhibited in presence of endosulfan among the three organochlroine pesticides. The strain IITISM08 degraded aldrin (79%), lindane (68%), and endosulfan (51%) at a concentration of 50 μg mL−1. The strain IITISM08 was identified using 16S rDNA gene sequencing as Paenibacillus sp. (IITISM08). The study revealed that the strain IITISM08 can be used as PGP candidate even under organochlorine pesticide-stressed condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorous (P) is a vital nutrient required for the growth and development of plants (Illmer and Schimmer 1992). The P available in the soil is lesser than 1 μmol L−1 due to the low solubility of mineral P and constant fixation of available P as iron and aluminum phosphates in acidic soils and calcium phosphates in alkaline soils (Goldstein 1986; Rajasankar et al. 2013). Some microorganisms are known to be involved in the conversion of insoluble form of P to a form accessible to plants, like orthophosphate. These phosphate-solubilizing microorganisms (PSB) play an important role in increasing the growth and yield of crop plants (Hariprasad and Niranjana 2009).
P solubilization by the PSB is mediated through their ability to decrease the pH of the medium, either by releasing organic acids or protons (Rajasankar et al. 2013). The organic acids secreted can either chelate iron and aluminum ions associated with phosphate or dissolve the mineral phosphate as a result of anion exchange (Bajpai and Rao 1971). Finally, the mineral phosphate is solubilized into soluble monobasic (H2PO4) and dibasic (HPO4 2−) ions by the process known as mineral phosphate solubilization. This leads to an increase in the available P to plants and in turn its uptake by plants (Gyaneshwar et al. 2002). Solubilization of the insoluble P by the rhizospheric soil bacteria have been reported in number of studies like, Aneurinibacillus aneurinilyticus strain CKMV1 (Chauhan et al. 2017), Burkholderia and Alcaligenes (Pande et al. 2017), Bacillus, Pseudomonas, Micrococcus, Staphylococcus, Microbacterium and Delftia (Panda et al. 2016), Pseudomonas rhizosphaerae (Kwak et al. 2015), Pantoea ananatis (M36), Rahnella aquatilis (M100) and Enterobacter sp. (M183) (Bakhshandeh et al. 2014), Enterobacter (Hwangbo et al. 2003), Azospirillum (Rodriguez et al. 2004), Pantoea (Son et al. 2006), Serratia (Hameeda et al. 2008), and Pseudomonas (Park et al. 2009; Vyas and Gulati 2009; Ahemad and Khan 2012).
Organochlorine pesticides are semivolatile organic compounds of global concern (Ali et al. 2014). The main reasons for environmental contamination by organochlorine pesticides are their long time use and their physical and chemical persistence in the natural environment (Mishra and Sharma 2011). However, increased use of both the organochlorine and organophosphorous pesticides may affect the PSB, which are valuable plant growth-promoting rhizobacteria (PGPR) of soil (Tripti and Kumar 2015; Ramani 2011). PGPR can affect growth and development of plant by several mechanisms such as biological nitrogen fixation, solubilization of phosphorous, production of plant hormones, iron sequestration by siderophores, and by decreasing the inhibitory effects of various pathogens in the forms of biocontrol agent (Rani and Kumar 2017; Glick 1995). Recently, few studies had reported the effect of pesticides on the bacterial growth and their role in P solubilization (Ramani 2011; Ahemad and Khan 2011, 2012).
In the present study, P-solubilizing and organochlorine pesticide-tolerating Paenibacillus sp. strain IITISM08 was isolated from rhizospheric soil of Solanum lycopersicum and Brassica compestris and its PGP traits were studied in the presence of varying concentrations of organochlorine pesticides (endosulfan, aldrin, and lindane). Furthermore, this is a significant study that illustrates the effect of organochlorine pesticides on the qualitative production of organic acids during P solubilization.
Materials and methods
Isolation and screening of PSB
Rhizospheric soil of S. lycopersicum and B. compestris, grown in pesticide-stressed agricultural fields of Dhanbad, India, were collected using sterile polypropylene zipper bags, thoroughly mixed, and transferred to the laboratory. The samples were stored at 4 °C for further analyses.
The PSB was isolated from the soil samples (10 g each) using serial dilution assay, carried out in 0.9% NaCl solution, and 10 μL of the diluted suspension was spread plated on Pikovskaya (PKV) agar medium (Pikovskaya 1948). The plates were incubated for 7 days at 28 ± 2 °C in a bacteriological incubator (CIS-24 Plus, REMI). The colonies with clear halo zones were considered as P solubilisers. The halo zone formed was measured as solubilization index determined by the following formula:
For the quantitative measurement of P solubilization, the inoculum was prepared by inoculating pure PSB strains in 100 mL of nutrient broth (Himedia, India) and incubated at 28 ± 2 °C for 48 h. The inoculum was centrifuged at 10,000 rpm for 20 min, washed twice with sterilized dH2O, and resuspended in sterilized dH2O such that the suspension contained 108 cells mL−1 (at 660 nm) (Ramani 2011). Briefly, 100 mL of PKV broth was inoculated with 1 mL of bacterial inoculum and incubated at 28 ± 2 °C for 6 days with intermittent shaking. The culture broth (10 mL) was collected and centrifuged at 10,000 rpm for 20 min. Then, 2.5 mL of Barton’s reagent was added to 10 mL of supernatant and the final volume was made up to 50 mL. The absorbance of the solution was read in a UV-Visible spectrophotometer (UV 1800, Shimadzu) at 430 nm and the amount of P solubilization was measured (Subba Rao 1982). Bacterial isolates showing substantially greater P solubilization on PKV (solid and broth) media were selected for further studies.
Pesticide tolerance
The selected bacterial strains were tested for tolerance to organochlorine pesticides (Dr. Ehrenstorfer, Germany) (Table 1) by an agar plate dilution method using minimal salt agar medium (g L−1 KH2PO4 1, K2HPO4 1, NH4NO3 1, MgSO4.7H2O 0.2, CaCl2.2H2O 0.02, FeSO4.7H2O 0.2, CaCl2.2H2O 0.01, pH 6.5). The freshly prepared agar plates were amended separately with varying concentrations (50, 100, 150, 200, 250, and 300 μg mL−1) of endosulfan, aldrin, and lindane. Plates were spot inoculated with five 10-fold dilution of 10 μL of 108 cells mL−1 of bacterial strains. Plates were incubated at 28 ± 2 °C for 72 h. The maximum concentration of organochlorine pesticides supporting bacterial growth was defined as the maximum tolerance level (MTL).
Bioassays of PGP traits
Among 10 bacterial isolates, bacterial strain showing higher MTL values and P solubilization was assayed for PGP traits in the presence of organochlorine pesticides (endosulfan, aldrin, and lindane) at different concentrations (50, 100, and 150 μg mL−1).
Bioassay of phosphate solubilization under organochlorine pesticides stress
Solubilization of phosphate by selected bacterial strain was studied qualitatively and quantitatively in the presence and absence of organochlorine pesticides. For qualitative analysis, 10 μL of inoculum was inoculated in PKV agar medium supplemented with different concentrations of organochlorine pesticides (50, 100, and 150 μg mL−1) and incubated at 28 ± 2 °C for 7 days (Jeenie and Khana 2011). A halo zone formed around the bacterial strain was measured as solubilization index.
For the quantitative measurement of P solubilization, different concentrations of organochlorine pesticides (50, 100, and 150 μg mL−1) were added separately to the 100-mL PKV broth inoculated with 1 mL of 108 cells mL−1 of bacterial culture and incubated at 28 ± 2 °C with intermittent shaking at 120 rpm. The available P was measured in the bacterial supernatant on the 3rd, 6th, 9th, and 12th day. The change in pH following phosphate solubilization was also recorded.
Screening of bacterial supernatant for organic acids by FT-IR and HPLC analysis
Screening of bacterial supernatant of PKV broth to test the type of organic acids produced during P solubilization in the presence and absence of organochlorine pesticides were analyzed using FT-IR. After 9 days, the PKV culture broth was centrifuged (10,000 rpm, 20 min) and the clear supernatant were finely ground with 300 mg of KBr using a pestle and mortar. The homogenized mixture was placed into a stainless steel holder and was made into pellets by applying pressure ranging from 7500 to 1500 cm−2 for 3 min. The infrared spectrum of each sample was recorded using ATR-FT-IR (Agilent) equipped with MCT detector and temperature control mechanism. High energy ceramic light source was employed and the acquisition parameters were within the range of 4000–400 cm−1 at a resolution of 4 cm−1 (Rajasankar et al. 2013).
The qualitative production of organic acids in the presence and absence of the organochlorine pesticides were validated by using HPLC. After 9 days of incubation, the broth was harvested and centrifuged at 10,000 rpm for 10 min. The supernatants were filtered through a 0.22 μm filter (Millipore) and the filtrate was injected to HPLC (Dionex UHPLC Ultimate 3000) equipped with UV detector (210 nm) and C18 column (dim. 150 mm × 3 mm, particle size. 6 μm, Thermo Scientific). The chromatograms were developed using a mobile phase consisting of 50 mM KH2PO4 moving at a constant flow rate of 0.7 mL min−1 in isocratic mode. The retention time of each signal was recorded at a wavelength of 210 nm (Rajasankar et al. 2013).
Bioassay of indole-3-acetic acid (IAA), ammonia, hydrogen cyanide (HCN), siderophore, and exo-polysaccharides (EPS) production under organochlorine pesticide stress. An Erlenmeyer flask containing 100 mL of Luria Bertini (LB) broth supplemented with 100 μg mL−1 of tryptophan and different concentration of organochlorine pesticides was inoculated with 1 mL of inoculum containing 108 cells mL−1 for quantitative assay of IAA. IAA concentration was measured by the method of Gordon and Weber (1951), later modified by Brick et al. (1991).
For the production of ammonia, bacterial culture was inoculated in peptone water supplemented with organochlorine pesticides. After incubation, 1 mL of Nesseler’s reagent was added to each flask. Development of yellow color indicated ammonia production (Dye 1962).
Production of HCN by bacterial strain was detected by the method of Bakker and Schipper (1987) using HCN induction medium plate. The plates were sealed with parafilm and incubated for 4 days at 28 ± 2 °C. Change of color of the filter paper from orange to red indicated HCN production.
Siderophore production [salicylic acid (SA) and 2,3-dihydroxybenzoic acid (DHBA)] by the bacterial strain was measured using chrome azurol S (CAS) agar medium (Himedia, India) and Modi medium supplemented with organochlorine pesticides following the method of Alexander and Zuberer (1991) and Reeves et al. (1983), respectively.
Production of EPS was determined using LB broth following the method of Mody et al. (1989). EPS was extracted by adding three volumes of chilled acetone to one volume of supernatant. The precipitated EPS was repeatedly washed three times alternatively with dH2O and acetone, transferred to a filter paper, and weighed after overnight drying.
Degradation of organochlorine pesticides by selected isolate
The degradation potential of bacterial strain IITISM08 was determined under optimized conditions (pH = 7, temperature = 30 °C and inoculum volume of 600 μL of 108 cells mL−1) in Erlenmeyer flasks containing 100 mL of non sulfur medium (NSM) broth (Sutherland et al. 2000) supplemented with organochlorine pesticides at different concentrations (50, 100, and 150 μg mL−1). Uninoculated NSM broth containing pesticides was also incubated under similar condition to check the abiotic degradation of pesticides (Rani and Kumar 2017). Extraction of endosulfan from NSM broth samples was carried out as described by Kumar et al. (2007). The concentration of organochlorine pesticides was measured by gas chromatography.
Morphological and biochemical characteristics
The bacterial strain was preliminarily identified by morphological and biochemical test. These tests were performed using standard methods outlined in Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994).
Molecular identification and phylogenetic analysis
The bacterial strain was further identified by using 16S rDNA gene sequencing. Genomic DNA of the bacterial strain was isolated using the Insta GeneTM Matrix Genomic DNA isolation kit. For this, pure bacterial isolate was picked and suspended in 0.5 mL of sterilized saline and centrifuged for 10 min at 10,000 rpm. The pellet was suspended in 0.5 mL of Insta-Gene Matrix (Bio-Rad, USA), incubated at 56 °C for 30 min, and finally heated at 100 °C for 10 min. After heating, the supernatant was used for polymerase chain reaction (PCR). Template DNA (1 mL) and PCR solution consisted of 5 μL 10× buffer (with Mg2+), 8 μL dNTP mixture (1.25 mM each) and 0.5 μL of each primer was prepared and subjected for amplification. Amplification was performed for 35 PCR cycles with denaturation at 94 °C for 45 s, annealing at 55 °C for 60 s, and extension at 72 °C for 60 s using the genomic DNA as template and bacterial primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′). The amplified DNA of approximately 1500 bp was purified by removing unincorporated PCR primers and deoxyribonucleoside triphosphates (dNTPs) by using a Montage PCR Cleanup kit (Millipore). The purified PCR products were sequenced using ABI PRISM® BigDyeTM Terminator Cycle Sequencing Kits with AmpliTaq® DNA polymerase (FS enzyme). Sequencing products were resolved on an Applied BioSystems-3730XL, an automated DNA sequencing system (Applied BioSystems).
All samples were analyzed in triplicates. Both the negative (distilled water + all PCR components) and positive (a DNA template + all PCR components) controls were included in each run. The 16S rDNA sequence obtained was compared against the sequence available from Gene bank using the NCBI Blast program for gene homology. A phylogenetic tree was constructed by a neighbor joining method using the MEGA 7 software.
Statistical analysis
All the experiments were conducted in triplicate, and mean values were used in the analysis of data. One-way analysis of variance (ANOVA) was performed to compare the mean value of the different treatments. The significant differences between the mean values (p < 0.05) were observed by Tukey’s test and Duncan’s multiple range test. All statistical analyses were performed using SPSS version 21.0.
Results
Isolation of PSB strains
A total of 50 PSB strains were initially isolated on the basis of zone formation around the colonies on PKV agar plate. Out of 50, 10 strains showing higher solubilization index (SI > 2.5) were selected for quantitative assay in PKV broth medium and organochlorine pesticides tolerance.
Organochlorine pesticides tolerance
Among 10 isolates, strain IITISM08 was assayed for PGP traits due to its maximum capability of phosphorous solubilization in PKV agar medium (SI = 3.2) and broth medium (348 ± 2 μg mL−1) and showed higher tolerance of endosulfan, aldrin, and lindane (up to 250 μg mL−1) (Table 2).
Bioassay for PGP traits
In this study, strain IITISM08 displayed PGP traits (phosphate solubilization, IAA, ammonia, HCN, siderophores, and EPS production) when tested both in the absence and presence of endosulfan, aldrin, and lindane.
The phosphate-solubilizing potential of strain IITISM08 in the presence of varying concentrations of organochlorine pesticides was assessed both qualitatively and quantitatively using PKV medium (Table 3). When the concentration of each pesticide was increased from 50 to 150 μg mL−1, the halo zone showing phosphate-solubilizing potency decreased considerably. The maximum zone was found at 50 μg mL−1 concentration, in order of lindane > aldrin > endosulfan. At 150 μg mL−1 concentration, the most adverse effect on halo formation in order of endosulfan > aldrin > lindane was observed.
Also, the amount of phosphate solubilization in PKV broth medium decreases with increase in concentration of each pesticides from 50 to 150 μg mL−1. Among all pesticides, the highest adverse effect was shown by endosulfan on day 12 which decreases phosphorous-solubilizing activity of strain IITISM08 by 75, 77, and 96% at a concentration of 50, 100, and 150 μg mL−1, respectively, over control. In 50 μg mL−1 concentration, lindane added broth showed maximum phosphorous availability (286 μg mL−1) on day 9 while, the least (85 μg mL−1) was obtained in endosulfan added broth on day 12. Additionally, in the 100 μg mL−1 concentration, a maximum of 272 μg mL−1 was obtained in lindane-added broth on day 9, while the least was recorded in endosulfan-added broth (81 μg mL−1) on day 12. Also, in the 150 μg mL−1 concentration of lindane-added broth, strain IITISM08 showed a maximum (176 μg mL−1) phosphorous availability on day 3 of incubation, while the least (15 μg mL−1) was recorded in endosulfan added broth on day 12.
During phosphorous solubilization, the presence of organic acid functional group in the supernatant of bacterial culture of PKV broth in the presence and absence of organochlorine pesticides was screened by FT-IR. In the pesticide-free broth, the strain IITISM08 showed the IR spectrum range of 1635.543 cm−1 for COOH group (Fig. 1a). However, the shift in the spectrum was observed in the endosulfan (1639.928 cm−1) (Fig. 1b), aldrin (1640.507 cm−1) (Fig. 1c), and lindane (1644.980 cm−1) (Fig. 1d) added PKV broth.
The presence of organic acid functional group in PKV broth inoculated with strain Paenibacillus sp. IITISM08 was identified using FT-IR. a Bacterial supernatant of pesticide free PKV broth. b Bacterial supernatant of endosulfan amended PKV broth at 50 μg mL−1 concentration. c Bacterial supernatant of aldrin-amended PKV broth at 50 μg mL−1 concentration. d Bacterial supernatant of lindane amended PKV broth at 50 μg mL−1 concentration
On qualitative production of organic acids in the PKV broth in the presence and absence of organochlorine pesticides, gluconic acid was found to be the dominant acid (Fig. 2a, d). In the endosulfan-amended broth, in addition to gluconic acid, a peak pertaining to acetic acid (Fig. 2b) was also observed. Similarly, in the aldrin and lindane-added broth in addition to gluconic acid, peaks of formic and citric acids (Fig. 2c, d) were also noticed.
HPLC analysis of organic acids in PKV broth inoculated with strain Paenibacillus sp. IITISM08. a Culture broth unamended with organochlorine pesticide showing the presence of gluconic, acetic, formic, and citric acids. b Culture broth supplemented with endosulfan showing the presence of gluconic and acetic acids. c Culture broth supplemented with aldrin showing the presence of gluconic, formic, and citric acids. d Culture broth supplemented with lindane showing the presence of gluconic, formic, and citric acids. e–h Standard chromatogram peak of gluconic, acetic, citric, and formic acids
Strain IITISM08 produced maximum (44 μg mL−1) IAA in LB broth medium in the absence of organochlorine pesticides which reduced substantially with the addition of increasing concentration of each pesticide. The order of toxicity of pesticides at 50 μg mL−1 (percent decrease in IAA production over control), was found as endosulfan (73%) > lindane (56%) > aldrin (36%). While comparing the effect at concentrations of 150 μg mL−1, aldrin showed least toxicity by 75%, whereas, endosulfan reduced the IAA production maximally by 89% (Table 4). In this study, the three concentrations of organochlorine pesticides did not suppress the ammonia and the HCN production by strain IITISM08.
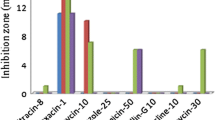
Strain IITISM08 showed siderophore producing ability by forming an orange-colored zone of 13 mm on CAS agar medium in the absence of pesticide. While comparing the effect of organochlorine pesticide, reduction in the siderophore zone size was prominent in the presence of all three concentrations of each organochlorine pesticide. The order of a percent decrease in zone diameter at 150 μg mL−1 concentration relative to control was endosulfan (38%) > lindane (31%) = aldrin (31%).
In the absence of pesticide, strain IITISM08 produced 34 μg mL−1 of SA and 15 μg mL−1 of DHBA. The siderophores (SA and DHBA) produced by the strain IITISM08 decreased consistently with the increasing concentration of each organochlorine pesticide. At 150 μg mL−1 concentration, endosulfan showed the highest toxic effect on the production of both SA and DHBA and decreased it maximally by 79 and 80%, respectively, as compared to control. The order of decline in SA synthesis was endosulfan (79%) > lindane (70%) > aldrin (68%) compared to control at a concentration of 150 μg mL−1. Moreover, the order of toxicity at 150 μg mL−1 concentration on DHBA production was endosulfan (80%) > aldrin (53%) = lindane (53%) (Table 4).
In contrast to other PGP substances, EPS synthesized by the strain IITISM08 increased progressively with consistent increase in concentrations of each organochlorine pesticide. The maximum stimulatory effect on EPS production was exhibited for endosulfan, which increased it by 17, 23, and 41% in 50, 100, and 150 μg mL−1 concentrations, respectively, compared to control. At 150 μg mL−1 concentration, maximum EPS secretion (percent increase over control) was found as endosulfan (41%) > lindane (35%) >aldrin (29%) (Table 4).
Degradation of organochlorine pesticides
Maximum degradation of endosulfan, aldrin, and lindane by Paenibacillus IITISM08 was observed at a concentration of 50 μg mL−1 with degradation potential of 51, 79, and 68%, respectively. However, the lowest degradation was 20% for endosulfan, 39% for aldrin, and 28% for lindane at a concentration of 150 μg mL−1, respectively (Fig. 3).
Degradation of organochlorine pesticides (endosulfan, aldrin, lindane) at different concentrations (50, 100, and 150 μg mL−1) by Paenibacillus sp. IITISM08 at optimized conditions (pH = 7, temperature = 30 °C, and inoculum volume of 600 μL of 108 cells mL−1). Values are in Mean ± SD, (n = 3); error bars denote standard deviation
Identification of the strain ISMIIT08
Strain IITISM08 was identified by a series of biochemical reactions as per the Bergey’s Manual of Systemic Bacteriology (Table 5) and later subjected to 16S rDNA sequencing using the BLAST program which showed its close relationship with the DNA sequence of Paenibacillus dendritiformis LT732643 with 99% similarity. Such high similar values confirmed the strain IITISM08 to be Paenibacillus sp. (Fig. 4).
Discussion
Tolerance to organochlorine pesticides
In the present study, the Paenibacillus sp. strain IITISM08 showed considerably higher MTL values to the three organochlorine pesticides (endosulfan, aldrin, and DDT). In agreement to this, Singh et al. (2009) reported that Paenibacillus sp. D1 have also exhibited resistance to fungicides, insecticides, and termiticides at concentrations higher than recommended for field applications. The development of resistance or tolerance capability of microorganisms against various pesticides is a complex process, regulated at the physiological and genetic level (Ahemad and Khan 2010). Since the medium used in the present study to assess the MTL values of the Paenibacillus sp. IITISM08 strain did not contain any carbon and nitrogen source except the tested organochlorine pesticides. The Paenibacillus sp. strain IITISM08 might have utilized these organochlorine pesticides as the only energy source.
Bioassay of PGP traits
Rhizospheric bacteria are able to promote plant growth by different mechanisms. Solubilization of mineral P in the rhizosphere and providing soluble P to plants is one of the important mechanisms. The property of phosphate solubilization is due to decrease in pH, which has been associated with the capability of microbes to secrete low molecular weight organic acids such as gluconic, oxalic, citric, malic, acetic, succinic acids, etc. (Zaidi et al. 2009). Soil inoculated with PSB has been displayed to improve solubilization of insoluble phosphates present in rhizosphere and provide soluble P to plants, resulting in a higher crop yield (Hameeda et al. 2008; Linu et al. 2009).
In endosulfan-amended broth, strain IITISM08 produced maximum soluble phosphorous (122 μg mL−1) at day 9. However, in the absence of pesticides, strain IITISM08 showed the maximum P solubility (348 μg mL−1), at day 6. The strain IITISM08 showed better P solubilization as compared to previously reported strains. Some recent studies reported that soluble phosphorous was produced 260 mg L−1 by A. aneurinilyticus (Chauhan et al. 2017), 305 μg mL−1 by Burkholderia cepacia C1 (Pande et al. 2017), 263 μg mL−1 by R. aquatilis M100 (Bakhshandeh et al. 2014), and 271 μg mL−1 by Pseudomonas plecoglossicida PSB-5 (Kaur and Reddy 2013), in pesticide-free broth. Similarly, Ramani 2011 reported that B. sphaericus and P. cepacia showed higher phosphate solubilization (40.78 and 158.99 mg % P2O5, respectively) at recommended dose of endosulfan, at day 15.
In the present study, strain IITISM08 solubilized the insoluble phosphates considerably both in the presence and absence of organochlorine pesticides. The incubation period also exhibited significant effects on the quantity of phosphate solubilization. In the culture filtrate, the amount of P solubilized increased significantly from the third day and remained high up to the ninth day. However, a significant decrease in amounts of soluble phosphorous was noticed on the 12th day. In a similar study, Chaiharn and Lumyong (2009) reported that the amount of P solubilized in the culture filtrate of strain Acinetobacter CR 1.8 increased significantly from the third day and remained high for 9 days. Walpola and Yoon 2013 also reported that the content of soluble phosphorus released by the isolates P. agglomerans and Burkholderi anthina in culture medium increased during the first 2 days of the incubation, then remained high for several days and decreased towards the end of the incubation. The decrease in the level of soluble phosphorous could be due to the availability of soluble form of phosphate, which has an inhibitory effect on further solubilization of phosphate (Varsha-Narsian et al. 1994) and the formation of an organophosphate compound induced by the release of organic metabolites which in turn decreases the amount of available phosphate (Chaiharn and Lumyong 2009). The decrease in pH of pesticide added and free broth during P solubilization may be attributed to the production of organic acids.
The FT-IR analysis showed the presence of COOH group in the culture supernatants; in addition, shift in the absorption spectrum was noticed in the culture supernatant of pesticide-added broth. The shift in FT-IR spectrum may be because of the additional binding of pesticide residues to free carboxyl terminal of the organic acids. In agreement to this, Rajasankar et al. (2013) reported a similar shift in the IR spectra denoting the addition of pesticide residues to COOH group, when mineral phosphorous was solubilized by Pseudomonas sp. strain SGRAJ09.
The possible mechanism of organochlorine pesticides to inhibit phosphate-solubilizing (PS) capacity may be attributed to the fact that the presence of organochlorine pesticides inhibits the growth of microorganisms, resulting in decrease in organic acid production. Madhaiyan et al. (2006) and Pham et al. (2004) reported that organochlorine pesticides may cause specific stresses in bacterial cells related to DNA, protein, and oxidative damages which may be a possible reason for inhibition of phosphate-solubilizing activity by the microorganisms. Several studies have also reported the reduction in phosphate-solubilizing activity of the microorganisms due to pesticides (Ramani 2011; Ghisalba et al. 1987; Siddaramappa et al. 1973).
Using HPLC, the production of organic acids in the pesticide supplemented and unsupplemented broth was analyzed. Gluconic acid was found major as well as common organic acid in all the pesticide added broth. Moreover, the presence of acetic, citric, and formic acids was also observed. Similarly, a study stated the possible production of organic acids during P solubilization under the stress of imidacloprid and monocrotophos constituting NBRIP broth (Rajasankar et al. 2013).
The IAA produced by the plant growth-promoting rhizobacteria controls many important physiological processes of plants such as cell elongation or cell division (Khan et al. 2009). In the present study, Paenibacillus sp. strain IITISM08 produced IAA even under pesticide stress, but decreased consistently with increasing pesticide concentration. In agreement to this, many researchers have reported reduction in IAA production by Achromobacter xylosoxidans JCp4 and Ochrobactrum sp. FCp1 (Akbar and Sultan 2016), Burkholderia sp. strain L2 (Tripti and Kumar 2015), Pseudomonas sp. SGRAJ09 (Rajasankar et al. 2013), Pseudomonas putida (Ahemad and Khan 2012), Enterobacter asburiae (Ahemad and Khan 2010), Gluconacetobacter diazotrophicus (Madhaiyan et al. 2006), Pseudomonas aeruginosa, Serratia sp., an Bacillus sp. (Wani et al. 2005) in the presence of pesticides.
Varying the concentrations of each pesticide did not inhibit the production of ammonia and HCN by Paenibacillus sp. strain IITISM08. The ammonia synthesized by the rhizobacteria may act as a signaling role in the interaction between rhizobacteria and plants and also enhance the glutamine synthetase activity (Sood et al. 2002). Wani et al. (2007) have also reported the ammonia production by rhizobacterial strains. HCN synthesized by the rhizobacteria shield the growing plants from pathogen attack by killing parasites (Kang et al. 2010). In agreement with our report, Devi et al. (2007) also reported the excretion of HCN by the rhizobacterial strains into the rhizosphere.
In the present study, Paenibacillus sp. strain IITISM08 produced siderophores on CAS agar plates as well as in Modi medium (SA and DHBA) both in the presence and absence of pesticides. Iron exists in the trivalent state as insoluble hydroxides and oxyhydroxides in the aerobic environment thus making it generally inaccessible to microorganisms (Ahemad and Khan 2011). Under iron-limiting conditions, many microorganisms exhibit a most common strategy involving the secretion of low molecular weight iron chelators with high association constants for complexion with iron called siderophores in order to maintain sufficient amounts of iron. Siderophores function as solubilizing agents for iron from minerals or organic compounds and make it available to plants (Miethke and Marahiel 2007). Siderophores may promote the biosynthesis of other antimicrobial compounds by increasing the availability of these minerals to the bacteria and may function in local and systemic host resistance in plants (Sinha and Mukherjee 2008). The ability of the strain Paenibacillus sp. strain IITISM08 to produce siderophores in CAS agar plates, SA, and DHBA suggests that the bacterial strain could also be used as a biological control agent against soil borne phytopathogens.
In contrast to other plant growth-regulating substances, the EPS synthesized by the strain Pseudomonas sp. IITISM08 increased progressively with gradual increase in pesticide concentration; this might be due to the fact that pesticide might have induced the EPS production by the strain (Ahemad and Khan 2010). Production of EPS is an important trait of bacteria as it protects the cell from phagocytosis, desiccation, phage attack, and also promote N2 fixation by preventing high oxygen tension (Tank and Saraf 2003). However, Madhaiyan et al. (2006) and Ahemad and Khan (2011, 2012) reported that PGP activities were decreased considerably in the presence of different pesticides. Similarly, in the present study also, reduced PGP activities were recorded in the presence of different organochlorine pesticides except for EPS, HCN, and ammonia production. This reduction in PGP activities could probably be due to the adverse effect of pesticide on the metabolic activities (Boldt and Jacobsen 1998; Kumar et al. 2010).
The present study reports that Paenibacillus sp. strain IITISM08 degraded 79% of aldrin, 68% of lindane, and 51% of endosulfan at a concentration of 50 μg mL−1. Degradation of endosulfan in aqueous medium by Klebsiella pneumoniae JAS8 was also studied by Abraham and Silambarasan (2014). Doolotkeldieva et al. (2017) reported that Bacillus polymyxa, Pseudomonas fluorescens, Flavobacterium sp., and Micrococcus has degraded 48.2, 43.2, 27.0, and 24.2% of aldrin, respectively.
Previously, the degradation of lindane by bacteria has been reported by several researchers such as Anupama and Paul (2009) (Azotobacter chroococcum JL 102), Manickam et al. (2006) (Microbacterium sp. ITRC1 strain), and Nawab et al. (2003) (Pseudomonas strains).
Conclusion
The organochlorine pesticides (endosulfan, aldrin, and lindane) at different concentrations displayed varying degrees of toxicity towards PGP traits of Paenibacillus sp. strain IITISM08 except EPS production, which increased progressively with increase in concentration of each pesticide. When this strain was added to PKV agar and broth medium, P solubilization was decreased with increase in pesticide concentration, considerably in a progressive order with some variations. On subjecting to FT-IR and HPLC analysis, the presence of organic acid functional group in the bacterial culture and production of gluconic acid as the dominant acid aiding the P solubilization were identified. The present study provides evidence that in the presence of higher concentrations of organochlorine pesticides, Paenibacillus sp. strain IITISM08 exhibited several PGPs traits and thus can be used as bioinoculant in organochlorine pesticide stressed soil.
References
Abraham J, Silambarasan S (2014) Role of novel fungus Lasiodiplodia sp. JAS12 and plant growth promoting bacteria Klebsiella pneumoniae JAS8 in mineralization of endosulfan and its metabolites. Ecol Eng 70:235–240. https://doi.org/10.1016/j.ecoleng.2014.05.029
Ahemad M, Khan MS (2010) Plant growth promoting activities of phosphate-solubilizing Enterobacter asburiae as influenced by fungicides. Eurasia J Biosci 4:88–95
Ahemad M, Khan MS (2011) Effects of insecticides on plant-growth promoting activities of phosphate solubilizing rhizobacterium Klebsiella sp. strain PS19. Pestic Biochem Phys 100(1):51–56. https://doi.org/10.1016/j.pestbp.2011.02.004
Ahemad M, Khan MS (2012) Effect of fungicides on plant growth promoting activities of phosphate solubilizing Pseudomonas putida isolated from mustard (Brassica compestris) rhizosphere. Chemosphere 86(9):945–950. https://doi.org/10.1016/j.chemosphere.2011.11.013
Akbar S, Sultan S (2016) Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz J Microbiol 47(3):563–570. https://doi.org/10.1016/j.bjm.2016.04.009
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12(1):39–45. https://doi.org/10.1007/BF00369386
Ali U, Syed JH, Malik RN, Katsoyiannis A, Li J, Zhang G, Jones KC (2014) Organochlorine pesticides (OCPs) in south Asian region: a review. Sci Total Environ 476:705–717
Anupama KS, Paul S (2009) Ex situ and in situ biodegradation of lindane by Azotobacter chroococcum. J Environ Sci Health B 45(1):58–66. https://doi.org/10.1080/03601230903404465
Bajpai PD, Rao WBS (1971) Phosphate solubilizing bacteria II. Extracellular production of organic acids by selected bacteria solubilizing insoluble phosphates. Soil Sci Plant Nutr 17(2):44–45. https://doi.org/10.1080/00380768.1971.10432852
Bakhshandeh E, Rahimian H, Pirdashti H, Nematzadeh GA (2014) Phosphate solubilization potential and modeling of stress tolerance of rhizobacteria from rice paddy soil in northern Iran. World J Microb Biol 30:2437–2447
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Boldt TS, Jacobsen CS (1998) Different toxic effects of the sulphonylurea herbicides metsulfuron methyl chlorsulfuron andthifensulfuron methyl on fluorescent Pseudomonads isolated from an agricultural soil. FEMS Microbiol Lett 161(1):29–35. https://doi.org/10.1111/j.1574-6968.1998.tb12925.x
Brick JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Chaiharn M, Lumyong S (2009) Phosphate solubilization potential and stress tolerance of rhizobacteria from rice soil in northern Thailand. World J Microbiol Biotechnol 25(2):305–314. https://doi.org/10.1007/s11274-008-9892-2
Chauhan A, Guleria S, Balgir PB, Walia A, Mahajan R, Mehta P, Shirkot CK (2017) Tricalcium phosphate solubilization and nitrogen fixation by newly isolated Aneurinibacillus aneurinilyticus CKMV1 from rhizosphere of Valeriana jatamansi and its growth promotional effect. Braz J Microbiol 48(2):294–304. https://doi.org/10.1016/j.bjm.2016.12.001
Devi KK, Seth N, Kothamasi S, Kothamasi D (2007) Hydrogen cyanide-producing rhizobacteria kill subterranean termite Odontotermes obesus (rambur) by cyanide poisoning under in vitro conditions. Curr Microbiol 54(1):74–78. https://doi.org/10.1007/s00284-006-0473-z
Doolotkeldieva T, Konurbaeva M, Bobusheva S (2017) Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bioremediation possibilities. Environ Sci Pollut Res:1–15
Dye DW (1962) The inadequacy of the usual determinative tests for the identification of Xanthomonas spp. Nat Sci 5:393–416
Ghisalba O, Kuenzi M, Tombo GM, Schar HP (1987) Organophosphorus microbial degradation and utilization of selected compounds strategies and applications organophosphorus compounds strategies and applications. Chem Aust 41:206–210
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117. https://doi.org/10.1139/m95-015
Goldstein AH (1986) Bacterial solubilization of mineral phosphates: historical perspective and future prospects. Am J Altern Agric 1(02):51–57. https://doi.org/10.1017/S0889189300000886
Gordon S, Weber RP (1951) The colorimetric estimation of IAA. Plant Physiol 26(1):192–195. https://doi.org/10.1104/pp.26.1.192
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245(1):83–93. https://doi.org/10.1023/A:1020663916259
Hameeda B, Harini G, Rupel OP, Wani SP, Reddy G (2008) Growth promotion of maize by phosphate solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163(2):234–242. https://doi.org/10.1016/j.micres.2006.05.009
Hariprasad P, Niranjana SR (2009) Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil 316(1-2):13–24. https://doi.org/10.1007/s11104-008-9754-6
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams & Wilkins, Baltimore, MD
Hwangbo H, Park RD, Kim YW, Rim YS, Park KH (2003) 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol 47(2):87–92
Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem 24(4):389–395. https://doi.org/10.1016/0038-0717(92)90199-8
Jeenie SP, Khanna V (2011) In vitro sensitivity of rhizobium and phosphate solubilising bacteria to herbicides. Indian J Microbiol 51(2):230–233. https://doi.org/10.1007/s12088-011-0145-y
Kang BG, Kim WT, Yun HS, Chang SC (2010) Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol Rep 4(3):179–183. https://doi.org/10.1007/s11816-010-0136-1
Kaur G, Reddy MS (2013) Phosphate solubilizing rhizobacteria from an organic farm and their influence on the growth and yield of maize (Zea mays L.) J Gen Appl Microbiol 59(4):295–303. https://doi.org/10.2323/jgam.59.295
Khan MS, Zaidi A, Wani PA, Ahemad M, Oves M (2009) Functional diversity among plant growth-promoting rhizobacteria: current status. In: Microbial strategies for crop improvement. Springer Berlin Heidelberg, pp 105–132. https://doi.org/10.1007/978-3-642-01979-1_6
Kumar K, Devi SS, Krishnamurthi K, Kanade GS, Chakrabarti T (2007) Enrichment and isolation of endosulfan degrading and detoxifying bacteria. Chemosphere 68(2):317–322. https://doi.org/10.1016/j.chemosphere.2006.12.076
Kumar N, Anubhuti Bora JI, Amb MK (2010) Chronic toxicity of the triazole fungicide tebuconazole on a Heterocystous, nitrogen fixing rice paddy field cyanobacterium, Westiellopsis prolific Janet. J Microbiol Biotechnol 20(7):1134–1139. https://doi.org/10.4014/jmb.0907.07013
Kwak Y, Jung BK, Shin JH (2015) Complete genome sequence of Pseudomonas rhizosphaerae IH5T (= DSM 16299T), a phosphate-solubilizing rhizobacterium for bacterial biofertilizer. J Biotechnol 193:137–138. https://doi.org/10.1016/j.jbiotec.2014.11.031
Linu MS, Stephen J, Jisha MS (2009) Phosphate solubilising Gluconacetobacter sp, Burkholderia sp. and their potential interaction with cowpea (Vigna unguiculata (L.) Walp.) Int J Agric Sci 4:79–87
Madhaiyan M, Poonguzhali S, Hari K, Saravanan VS, Sa T (2006) Influence of pesticides on the growth rate and plant-growth promoting traits of Gluconacetobacter diazotrophicus. Pestic Biochem Phys 84(2):143–154. https://doi.org/10.1016/j.pestbp.2005.06.004
Manickam N, Mau M, Schlomann M (2006) Characterization of the novel HCH-degrading strain, Microbacterium sp. ITRC1. Appl Microbiol Biotechnol 69(5):580–588. https://doi.org/10.1007/s00253-005-0162-z
Miethke M, Marahiel MA (2007) Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71(3):413–451. https://doi.org/10.1128/MMBR.00012-07
Mishra K, Sharma RC (2011) Assessment of organochlorine pesticides in human milk and risk exposure to infants from north-East India. Sci Total Environ 409(23):4939–4949. https://doi.org/10.1016/j.scitotenv.2011.07.038
Mody BR, Bindra MO, Modi VV (1989) Extracellular polysaccharides of cowpea rhizobia: compositional and functional studies. Arch Microbiol 1:2–5
Nawab A, Aleem A, Malik A (2003) Determination of organochlorine pesticides in agricultural soil with special reference to γ-HCH degradation by Pseudomonas strains. Bioresour Technol 88(1):41–46. https://doi.org/10.1016/S0960-8524(02)00263-8
Panda B, Rahman H, Panda J (2016) Phosphate solubilizing bacteria from the acidic soils of eastern Himalayan region and their antagonistic effect on fungal pathogens. Rhizosphere 2:62–71. https://doi.org/10.1016/j.rhisph.2016.08.001
Pande A, Pandey P, Mehra S, Singh M, Kaushik S (2017) Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J Genet Eng (In Press)
Park KH, Lee CY, Son HJ (2009) Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth promoting activities. Lett Appl Microbiol 49(2):222–228. https://doi.org/10.1111/j.1472-765X.2009.02642.x
Pham CH, Min J, Gu MB (2004) Pesticide induced toxicity and stress response in bacterial cells. Bull Environ Contam Toxicol 72(2):380–386. https://doi.org/10.1007/s00128-003-8845-6
Pikovskaya RI (1948) Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiologia 17:362–367
Rajasankar R, Gayathry GM, Sathiavelu A, Ramalingam C, Saravanan VS (2013) Pesticide tolerant and phosphorus solubilizing Pseudomonas sp. strain SGRAJ09 isolated from pesticides treated Achillea clavennae rhizosphere soil. Ecotoxicology 22(4):707–717. https://doi.org/10.1007/s10646-013-1062-0
Ramani V (2011) Effect of pesticides on phosphate solubilization by Bacillus sphaericus and Pseudomonas cepacia. Pestic Biochem Phys 99(3):232–236. https://doi.org/10.1016/j.pestbp.2011.01.001
Rani R, Kumar V (2017) Endosulfan degradation by selected strains of plant growth promoting Rhizobacteria. Bull Environ Contam Toxicol 99(1):1–8. https://doi.org/10.1007/s00128-017-2102-x
Reeves MW, Pine L, Neilands JB, Balows A (1983) Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol 154(1):324–329
Rodriguez H, Gonzalez T, Goire I, Bashan Y (2004) Gluconic acid production and phosphate solubilization by the plant growth promoting bacterium Azospirillum spp. Naturwissenschaften 91(11):552–555. https://doi.org/10.1007/s00114-004-0566-0
Siddaramappa R, Rajaram KP, Sethunathan N (1973) Degradation of parathion by bacteria isolated from flooded soil. Appl Microbiol 26(6):846–849
Singh AK, Ghodke I, Chhatpar HS (2009) Pesticide tolerance of Paenibacillus sp. D1 and its chitinase. J Environ Manag 91(2):358–362. https://doi.org/10.1016/j.jenvman.2009.09.001
Sinha S, Mukherjee SK (2008) Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56(1):55–60. https://doi.org/10.1007/s00284-007-9038-z
Son HJ, Park GT, Cha MS, Heo MS (2006) Solubilization of insoluble inorganic phosphates by a novel salt and pH tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour Technol 97(2):204–210. https://doi.org/10.1016/j.biortech.2005.02.021
Sood CR, Chanda SV, Singh YD (2002) Effect of different nitrogen sources and plant growth regulators on glutamine synthetase and glutamate synthase activities of radish cotyledons. Bulg J Plant Physiol 28:46–56
Subba Rao NS (1982) Phosphate solubilization by soil microorganisms. Advances in agricultural microbiology. Oxford and IBH Publishing, New Delhi
Sutherland TD, Horne I, Lacey MJ, Harcourt RL, Russell RJ, Oakeshott JG (2000) Enrichment of an endosulfan-degrading mixed bacterial culture. Appl Environ Microbiol 66(7):2822–2828. https://doi.org/10.1128/AEM.66.7.2822-2828.2000
Tank N, Saraf M (2003) Phosphate solubilization, exopolysaccharide production and indole acetic acid secretion by rhizobacteria isolated from Trigonella foenum-graecum. Indian J Microbiol 43:37–40
Tripti AK, Kumar V, Anshumali (2015) Effect of commercial pesticides on plant growth-promoting activities of Burkholderia sp. strain L2 isolated from rhizosphere of Lycopersicon esculentum cultivated in agricultural soil. Toxicol Environ Chem 97:1180–1189
Varsha-Narsian J, Thakkar J, Patel HH (1994) Inorganic phosphate solubilization by some yeast. Indian J Microbiol 35:113–118
Vyas P, Gulati A (2009) Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol 9(1):174–188. https://doi.org/10.1186/1471-2180-9-174
Walpola BC, Yoon MH (2013) Isolation and characterization of phosphate solubilizing bacteria and their co-inoculation efficiency on tomato plant growth and phosphorous uptake. Afr J Microbiol Res 7:266–275
Wani PA, Khan MS, Zaidi A (2007) Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 70(1):36–45. https://doi.org/10.1016/j.chemosphere.2007.07.028
Wani PA, Zaidi A, Khan AA, Khan MS (2005) Effect of phorate on phosphate solubilization and indole acetic acid releasing potentials of rhizospheric microorganisms. Ann Plant Protect Sci 13:139–144
Zaidi A, Khan M, Ahemad M, Oves M (2009) Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung 56(3):263–284. https://doi.org/10.1556/AMicr.56.2009.3.6
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Diane Purchase
Rights and permissions
About this article
Cite this article
Rani, R., Usmani, Z., Gupta, P. et al. Effects of organochlorine pesticides on plant growth-promoting traits of phosphate-solubilizing rhizobacterium, Paenibacillus sp. IITISM08. Environ Sci Pollut Res 25, 5668–5680 (2018). https://doi.org/10.1007/s11356-017-0940-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0940-z