Abstract
Degradation of total phenol (TPh) and organic matter, (expressed as total organic carbon TOC), of a simulated olive mill wastewater was evaluated by the Fenton oxidation process under batch and continuous mode conditions. A mixture of six phenolic acids usually found in these agro-industrial wastewaters was used for this purpose. The study focused on the optimization of key operational parameters of the Fenton process in a batch reactor, namely Fe2+ dosage, hydrogen peroxide concentration, pH, and reaction temperature. On the assessment of the process efficiency, > 99% of TPh and > 56% of TOC removal were attained when [Fe2+] = 100 ppm, [H2O2] = 2.0 g/L, T = 30 °C, and initial pH = 5.0, after 300 min of reaction. Under those operational conditions, experiments on a continuous stirred-tank reactor (CSTR) were performed for different space-time values (τ). TOC and TPh removals of 47.5 and 96.9%, respectively, were reached at steady-state (for τ = 120 min). High removal of COD (> 75%) and BOD5 (> 70%) was achieved for both batch and CSTR optimum conditions; analysis of the BOD5/COD ratio also revealed an increase in the effluent’s biodegradability. Despite the high removal of lumped parameters, the treated effluent did not met the Portuguese legal limits for direct discharge of wastewaters into water bodies, which indicates that coupled chemical-biological process may be the best solution for real olive mill wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The olive oil industry plays a major economic, social, and environmental role in the Mediterranean region, where countries like Spain, Italy, Greece, and Portugal—who are among the world’s biggest producers of olive oil—face serious waste management problems related to this industry (IOOC 2017). In particular, this agro-industrial activity is responsible for the generation of large amounts of liquid effluents, commonly referred to as olive mill wastewater (OMW). It is estimated that the worldwide production of OMW can be as high as 20–30 million m3/year (Niaounakis and Halvadakis 2006). Treatment of these effluents is a complex subject, not only due to the seasonality and geographically diffuse nature of olive oil production, but also because of the toxicity and high pollution potential of such wastewaters. Physicochemical characteristics of OMW such as color, turbidity, pH, COD, or BOD5 vary widely, depending on numerous factors (e.g., olive variety, harvest season, olive oil extraction process, etc.). Generically speaking, OMW is mostly comprised of water (~ 83%) and organic compounds (~ 15%), with the remaining percentage corresponding to the inorganic chemicals. The organic fraction consists primarily of polyphenols, sugars, polysaccharides, proteins, organic acids, and lipids (McNamara et al. 2008; Lucas and Peres 2009; Ochando-Pulido 2016). Of particular interest is the total phenolic content (TPh), as the presence of such recalcitrant compounds has been linked to the toxicity of these effluents toward microorganisms and plants, often hindering the application of biological treatment processes (Silva et al. 2007; Fajardo et al. 2015).
Environmental legislation is increasingly restrictive and the disposal and/or discharge of untreated OMW to the soil and water bodies is currently prohibited in Spain, while in Italy and Portugal (as well as in other European countries) only partial discharge on suitable grounds is permitted (Kapellakis et al. 2008; Ochando-Pulido 2016). Unfortunately, the lack of economically efficient solutions for proper olive mill wastewater treatment makes dumping these effluents in evaporation ponds a common practice in most Mediterranean countries (Nieto et al. 2011).
In this context, advanced oxidation processes (AOPs), and particularly Fenton’s oxidation, may play an important role in wastewater treatment, either to accomplish the environmental parameters to safe disposal or to minimize the effluent’s toxicity and consequently enhance its biodegradability to make it suitable for further depuration (e.g., biological processes) (Ahmadi et al. 2005; El-Gohary et al. 2009; Martins et al. 2010).
AOPs are based on the generation of highly reactive and non-selective hydroxyl radicals (HO·), and are among the most promising techniques for the abatement of recalcitrant organic compounds. The generation of HO· in Fenton’s process is achieved by the decomposition of hydrogen peroxide catalyzed by ferrous ions, according to Eq. (1), in acidic environment, under mild conditions of pressure and temperature, which will non-selectively react with the organic matter (Walling 1975).
In addition to the main reaction, Eq. (2) shows that the catalyst regeneration that results from the reaction between hydrogen peroxide and ferric iron also follows a radical mechanism involving hydroperoxyl radicals (HO2 ·), less reactive than the HO· ones.
Moreover, undesired scavenger effect of hydroxyl radicals can be attributed to the parallel reactions between the Fe2+ ion (Eq. (3)) and the hydrogen peroxide (Eq. (4)); therefore, dosage of both reactant species has to be optimized for every effluent to avoid predominance of such undesired reactions.
The use of relatively high concentration of iron ions in the homogeneous Fenton process (typically 50–80 ppm) and the strict acidic conditions required (pH in the 2–4 range) implies the subsequent treatment of the iron sludge formed and also the increase of the final effluent’s pH. Downstream processes such as precipitation, re-dissolution, and neutralization are usually necessary to meet EU directives regarding iron concentration and pH of treated water to be discharge directly into the environment (Sabhi and Kiwi 2001). Nonetheless, Fenton’s process is still one of the most interesting AOPs due to its low investment cost, easiness of implementation, low toxicity of reagents, and capacity to mineralize a wide range of organic compounds (Bautista et al. 2008).
The goal of this work is to evaluate the effectiveness of Fenton’s process on the abatement of the organic load (expressed as TOC and TPh) of a synthetic effluent containing six phenolic acids typically present in OMWs. First, batch experiments were conducted to determine the operational conditions (pH, temperature, Fe2+ and H2O2 doses) that maximized the mineralization degree and the efficiency of H2O2 consumption. Then, the best experimental conditions found on the batch experiments were used for further investigation on a reactor operating continuously (continuous stirred-tank reactor—CSTR), which entails a large number of advantages for industrial application as compared to the classical batch reactors addressed in the large majority of research studies. Therefore, the effect of space-time (τ) was assessed for the CSTR operation. Finally, the efficiency of the process was also addressed in terms of COD and BOD5 reduction, biodegradability (BOD5/COD ratio), and toxicity assessment of the treated effluent, to analyze the possibility of safe disposal/use after Fenton’s oxidation, according to Portuguese legislation (e.g., washing and irrigation activities within the olive mills).
Lab-scale study/optimization of Fenton’s process for the treatment of OMW has already proved its relevance, because the industrial application of this technology as a stage unit in a wastewater treatment plant (located in Badajoz, Spain) was successfully implemented (Amaral-Silva et al. 2016). Moreover, the acidic pH of such effluents makes the application of the Fenton process economically attractive, as little to no previous acidification may be required. Up to the authors knowledge, few works reported in literature deal with the application of the homogeneous Fenton process in a CSTR (Zhang et al. 2006; Ramirez et al. 2009; Hodaifa et al. 2013), and none addresses the treatment of a synthetic olive mill wastewater.
Materials and methods
Synthetic wastewater preparation
The synthetic effluent used in this study consists in the mixture of six different phenolic acids typically present in most abundance in real olive mill wastewaters. The phenolic compounds concentrations used for simulating the wastewater was adjusted according to works reported in literature (cf. Table S1 of the Supporting Information section) (Allouche et al. 2004; El-Abbassi et al. 2012; Daâssi et al. 2014; Aggoun et al. 2016) as follows: 50 mg/L of vanillic, caffeic, gallic, and 3,4-dihyroxyphenlyacetic acids, 100 mg/L of p-coumaric acid, and 200 mg/L of tyrosol (all compounds were purchased from Alfa Aesar except the caffeic acid, from Acros Organics). The concentrations used were selected to meet TPh values within the range 300–500 mg CAeq/L, commonly found in real OMW. The compounds were dissolved in distilled water and then the solution was submitted to ultrasounds (Sonorex Super RK255H) for 15 min, to ensure full dissolution, and no further purification was applied before use. The main characteristics of the synthetic effluent are summarized in Table 1.
Experimental procedure
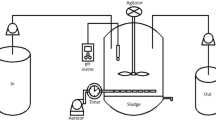
Fenton’s oxidation of the synthetic OMW was carried out in two distinct cylindrical jacketed reactors: the first one was a batch reactor, while the second was a CSTR, with 300 and 920 mL of capacity, respectively. For both cases, the reaction’s medium temperature was kept constant during the experiments by recycling water from a thermostatic bath (VWR International, model 89202-912) through the reactor’s jacket. The reactors were also equipped with a VWR VS-CT magnetic stirrer, keeping agitation at ca. 200 rpm. The pH of the solution was constantly measured by a pH meter (Inolab, WTW) and adjusted by adding NaOH 2.5 M (Merck) and/or H2SO4 1 M (Fluka) when needed. Figure 1 shows the Fenton’s oxidation experimental set-up for the batch (Fig. 1a) and the CSTR (Fig. 1b) operation.
For the batch experiments, the reactor was initially loaded with the synthetic OMW (250 mL) and the selected catalyst concentration, after pH adjustment to the desired value. Three different iron salts were tested as catalysts in this work: FeCl3·6H2O (LabChem), FeSO4·7H2O (Panreac), and Fe(NO3)3·9H2O (Riedel-de Haën). Fenton’s oxidation runs started when the hydrogen peroxide (30% w/v, Fisher Chemical) was added to the previous mixture (this induced a dilution < 2%).
For the operation of the CSTR, the synthetic OMW (containing the desired dissolved iron catalyst concentration) and the hydrogen peroxide solutions were pumped (Gibson M312 peristaltic pump) through two distinct streams to the reactor, allowing the use of total flow rates between ca. 2 and 26 mL/min−1. To avoid working with very concentrated H2O2 solutions, a single peristaltic pump was used for both solutions fed. Therefore, the effect of the 1:2 dilution that occurs at the reactor’s inlet, where the two streams are combined (see Fig. 1b), was taken into account; this implied to experimentally prepare more concentrated solutions, to have at the reactor entrance concentrations similar to those used in the batch experiments and that simulate a real OMW. For the %TOC and TPh removal determinations, Eq. (5) was used:
where X stands for conversion, C 0 for the inlet concentration, and C for the concentration of either TOC or TPh at reactor outlet, in a given instant. The vials containing the feed solutions were immersed in a Huber Polystat thermostatic bath for better temperature control, and set at a maximum of 50 °C to prevent possible thermal decomposition of the hydrogen peroxide. For each experiment in continuous mode, the reactor was initially half filled with the synthetic wastewater and half filled with distilled water.
During both sets of experiments, samples of the effluent were taken at regular intervals to assess the evolution of the following parameters: TOC and TPh (after stopping the possible reaction in the sampling flask by adding NaOH until pH > 10 and immediate cooling of the sample at 4 °C), and residual H2O2 (to determine the oxidant consumption efficiency). For the optimum operating conditions found on the batch and CSTR experiments, a larger sample of the final treated effluent was collected to posterior determination of COD, BOD5, and toxicity (for the CSTR operation, the sample was collected whenever steady-state was reached). For those determinations, pH of the samples was raised to ~ 10 by the addition of 10 M NaOH, in order to stop the possible homogeneous reaction with residual H2O2; then, the samples were neutralized to pH ~ 7.0 with 0.5 M H2SO4.
Analytical methods
The following analytical determinations were performed according to Standard Methods (APHA, AWWA, WEF 1998): TOC was determined by catalytic oxidation at 698 °C (method 5310 D) with a TC/TOC analyzer (Shimadzu TOC-L apparatus equipped with auto-sampler, which was operated at a maximum coefficient of variation ≤ 2%); COD was assessed by the closed reflux method (method 5220 D); (BOD5) was measured according to method 5210 B, which is based on the difference between the initial and final (after 5 days incubation at 20 °C in a Memmert, Model 854 oven) dissolved oxygen concentration (assessed with a Crison OXI 45 meter and WTW CellOx 325 electrode).
The total phenolic content (TPh) of the samples was determined by spectrophotometry using the Folin-Ciocalteu reagent (Panreac): 1 mL of the sample supernatant and 5 mL of the Folin-Ciocalteu reagent (1:10 dilution) were mixed together and vortexed for 3 min, then, 4 mL of Na2CO3 7.5% (w/v) was added. The color intensity, developed for 2 h in the dark, was measured at 765 nm using a Helios ϒ spectrophotometer (Thermo Electron Corporation) against a blank, prepared in the same way with distilled water. A calibration curve (R2 = 0.9984) was defined using caffeic acid as standard, therefore TPh values are reported as milligram/liter of equivalent caffeic acid (mg CAeq/L).
Hydrogen peroxide concentration was evaluated following the method developed by Sellers (1990), in virtue of its simplicity and accurate measurement. It quantifies the yellow-orange color of the complex formed from the reaction of hydrogen peroxide with titanium oxalate, measured by a spectrophotometer at 400 nm.
Acute toxicity values were obtained from the inhibition of Vibrio fischeri bacteria, following the standard DIN/EN/ISO 11348-3 (ISO 11348-3 2007). Bioluminescence of the bacteria was measured in a Microtox model 500 apparatus at different contact times (5, 15, and 30 min) with the effluent samples at 15 °C.
With the exception of TOC, the remaining analytical determinations were only performed in duplicate in order to avoid excessive experimental efforts. The coefficients of variation were less than 2% for TOC, 6% for BOD5, 4% for COD, and 5% for the remaining parameters.
Results and discussion
Batch catalytic tests
A parametric study on the influence of the main operational conditions affecting Fenton’s process is presented in the following sections.
Selection of iron salt
The presence of inorganic substances (e.g., Cl−, PO4 3−, NO3 −, or SO4 2−) in wastewaters or added as reagents (iron salt counter ion) is reported to influence the overall efficiency of Fenton and Fenton-like processes. Possible consequences include the scavenging of HO· and formation of less reactive species (e.g., SO· 4 −, Cl· 2 −), and/or complexation reactions with ferrous or ferric iron (De Laat et al. 2004; Siedlecka et al. 2007; Ratanatamskul et al. 2010). Since different inorganic ions in solution may influence the rate of reaction between H2O2 and ferrous/ferric ions (and thus the rate of oxidation of organic species), a preliminary study was performed using three commercially available iron salts: FeCl3·6H2O, FeSO4·7H2O, and Fe(NO3)3·9H2O.
Figure 2 reports the evolution of TOC and TPh removal along time for the three catalysts tested under identical experimental conditions based on similar reports (Lucas and Peres 2009; Martins et al. 2010). For the conditions tested, TPh removal was always greater than 97% after 300 min of reaction and no significant performance differences were found between the three iron salts. In respect to TOC degradation, a slightly higher efficiency was observed for the iron(II) sulfate salt (ca. 44%), when compared to the iron(III) chloride and iron(III) nitrate (ca. 41 and 39%, respectively).
In the work of Deng et al. (2012), the authors reported that the presence of SO4 2− and particularly Cl− hinders Fenton’s oxidation of a landfill leachate, while the presence of NO3 − in solution did not impact the COD removal rate. Similar results were found by Siedlecka et al. (2007) regarding the inhibition effect of chloride ions, particularly in the degradation of MTBE by Fenton’s oxidation. Nonetheless, under the operational conditions tested in these experiments, the slightly lower TOC degradation efficiencies observed when using chloride and nitrate salts may be simply related to the fact that the ferric to ferrous reconversion route (Eq. (2)) is a slower step to initiate Fenton’s process. Based on these results, and because industrial prices of such salts are similar, subsequent experiments were carried out with the iron sulfate.
Influence of H2O2 and Fe2+ doses
In Fenton’s oxidation, H2O2 and Fe2+ concentrations are closely related and have a large influence on the efficiency of the process, both in terms of mineralization degree of the organic load and overall cost of the process (Bautista et al. 2008; Martins et al. 2010). The selection of the H2O2 dose relies on the response of specific contaminants to oxidation as well as on the ultimate goal or strategy—i.e., the partial oxidation of the pollutants into intermediate compounds, instead of their full mineralization till CO2 and H2O, often leads to significant abatement of the organic load and/or toxicity reduction of the effluent, with lower operating costs (represented here by the costs of chemical reagents). Given the complexity of many agro-industrial effluents, it is common to report the oxidant dose as the theoretical stoichiometric weight ratio between H2O2 and the effluent’s initial COD (2.125 g H2O2/g COD) (Lucas and Peres 2009). Since hydrogen peroxide is also consumed in side reactions (see Eqs. (2) and (4)) that do not produce the highly reactive hydroxyl radicals, the dose of oxidant required is usually higher than the stoichiometric one (Zazo et al. 2009; Martins et al. 2010).
For this set of experiments, H2O2 doses corresponding to 0.6, 1.2, 1.8, and 2.4 times the stoichiometric amount where tested (corresponding to [H2O2] = 1.0, 2.0, 3.0, and 4.0 g/L, respectively), varying the concentration of the iron catalyst ([Fe2+] = 25, 50, and 100 mg/L). Experimental conditions for this set of trials were as follows: unadjusted initial pH (pH0 = 3.7), T = 30 °C, and reaction time of 300 min. As an example, Fig. 3 shows the evolution of TOC removal (%) during the runs when [Fe2+] = 50 mg/L. The figure shows that most of TOC reduction occurs at the beginning of the reaction (within the first 30 min of reaction) and from that point on, the TOC removal profile develops more slowly, approaching a plateau when t ≥ 180 min. A similar trend on the TOC removal curves was obtained for the other ferrous ion concentrations tested (see Fig. S1-a and S1-b of the Supporting Information section for [Fe2+] = 25 and 100 mg/L, respectively). Similar TOC and/or COD degradation profiles were observed by other authors in their experiments on organic matter reduction of simulated (Martins et al. 2010) and real olive mill wastewaters (Lucas and Peres 2009; Nieto et al. 2011) by homogeneous Fenton/Fenton-like processes.
Results shown in Fig. 4 report performances for TPh and TOC removal at the end of Fenton’s oxidation runs. For the conditions used, Fenton’s process proved to be very efficient in the depletion of the phenolic character of the wastewater, as ≥ 99% of TPh removal was observed in all experiments, with the exception of the runs where [H2O2] = 1.0 g/L. The lower TPh removal achieved while using a H2O2 dose below the stoichiometric one could be explained by the results found in the work of De Heredia et al. (2001). The authors established kinetic models for the oxidation of phenolics by Fenton’s process, showing that low values of the ratio between the initial concentrations of hydrogen peroxide and phenolic compounds (R = [H2O2]0/[TPh]0) lead to slower degradation rates of those compounds. Also, Kang et al. (2002) suggested that the availability of Fe2+ plays an important role in improving the degradation performance of phenolic compounds, which is in accordance to the higher TPh removal when Fe2+ doses go from 25 to 100 mg/L, for [H2O2] = 1.0 g/L (81.3 to 88.6%).
Regarding the TOC abatement, an increase in the H2O2 amount added to the reaction medium led to the expected enhancement of the mineralization degree (Rivas et al. 2001). Thus, for each Fe2+ concentration tested, the increase of the hydrogen peroxide dose from 1.0 to 4.0 g/L improved the organic matter degradation efficiency (e.g., Fig. 4b highlights this trend, as TOC removal efficiencies were 26.0, 44.1, 47.9, and 50.4% when [H2O2] = 1.0, 2.0, 3.0, and 4.0 g/L, respectively). Despite this improvement, mineralization levels maintained below the TPh removal ones (maximum TOC removal of 57.8% when [Fe2+] = 100 mg/L—see Fig. 4c), which can be explained by the decomposition of the parent phenolic compounds into refractory low molecular weight compounds (mainly carboxylic acids) that are difficult to be further oxidized by HO· (Kallel et al. 2009; Martins and Quinta-Ferreira 2011).
Moreover, for the same fixed amount of H2O2, mineralization degree is also positively affected by the increase of the Fe2+ dose, as it accelerates H2O2 conversion into hydroxyl radicals. However, this is only true up to some extent, as an additional experiment was performed with [Fe2+] = 200 mg/L using [H2O2] = 4.0 g/L (see Fig. S2) and TOC removal efficiency was found to be lower (53.3%) than the one achieved when [Fe2+] = 100 mg/L for the same H2O2 dose (57.8%). A similar behavior was found by Zazo et al. (2009) in the study of phenol degradation in aqueous solution by semi-continuous Fenton oxidation. Such trend can be related with the following: (1) the scavenging reactions that are promoted in the presence of either excess oxidant or catalyst (cf. Eqs. (3)–(4)); (2) the higher concentration of sulfate ions in solution (added as iron salt counter ion) that may act as a scavenger of HO· and/or compete with H2O2 to form Fe3+ complexes, thus inhibiting the Fe3+ regeneration cycle (mechanism described on Eqs. (I)–(XI) of Table 2) (Truong et al. 2004; Deng et al. 2012).
The potential application of the Fenton process for treating highly organic-loaded effluents also depends on the efficient use of the oxidant, since it plays a key role on the operating costs. To assess the H2O2 consumption efficiency, a normalized H2O2 consumption yield (η H2O2) was defined as shown in Eq. (6):
where the theoretical TOC removed (expressed in mg C/L) per H2O2 (expressed in g H2O2/L) is the maximum theoretical TOC conversion assuming complete oxidation until CO2, with the O2 resulting from the H2O2 decomposition (ca. 177 mg TOC/g H2O2) (Zazo et al. 2016) and R corresponds to the ratio between the H2O2 (in g/L) feed and the theoretical stoichiometric dose (ca. 1.82 g/L for this effluent). Values for η H2O2 vary from 0 to 1 (being 1 the maximum H2O2 consumption efficiency). Results shown in Fig. 4 indicate that H2O2 consumption efficiency decreases while increasing the oxidant dose, unless TOC removal overcomes the increase in the oxidant dose, which only occurs when [Fe2+] = 100 mg/L and [H2O2] = 2.0 g/L (Fig. 4c). The drop of the H2O2 consumption efficiency when using higher oxidant doses might be attributed to the radical scavenging effect (Eq. (4)), which reduces the amount of HO· available to oxidize the organic matter (Zazo et al. 2009; Martins et al. 2010). To confirm this, an additional experiment was performed with [H2O2] = 6.0 g/L using [Fe2+] = 100 mg/L; as expected, a drop in the TOC removal efficiency to 52.8% and η H2O2 to 0.06 was observed (results not shown). Taking into account all aspects mentioned so far, the best operating conditions found were [H2O2] = 2.0 g/L and [Fe2+] = 100 mg/L and further experiments were performed under those conditions.
Additionally, a blank experiment using [H2O2] = 2.0 g/L without the presence of Fe2+ was performed for the same operational conditions of the previous runs. TOC removal of approximately 8% and TPh of 6% (data not shown) was observed at the end of the 300 min of reaction. The low oxidation percentages observed are related to the lower oxidative potential of H2O2 when compared to HO· (Bigda 1995). Moreover, a blank experiment using [Fe2+] = 100 ppm (without the addition of H2O2) was also performed (initial pH of 3.7 and T = 30 °C). No significant TOC or TPh degradation was observed (< 2% for both cases—data not shown, which is in accordance with the experimental error for TOC and TPh determinations).
Influence of pH
To evaluate the pH influence on the oxidation process, experiments were carried out at different initial pH values (3.0, natural = 3.7, 5.0, and 7.0). It is worth mentioning that the pH of the reaction medium usually dropped by 1–2 pH unit (depending on the initial pH of the solution) after only 5–15 min, and maintained practically constant from that point on (see Fig. S3). This acidification of the solution can be explained by the partial oxidation of the phenolic compounds into low molecular weight carboxylic acids (Rivas et al. 2001), which predominantly occurs within the first stage of the Fenton process, as above mentioned.
Figure 5 shows the effect of the medium’s pH on TOC and TPh degradation after 300 min of reaction. A slight improvement on TOC mineralization was observed when initial pH increased from 3.0 to natural (~ 3.7) and then to 5.0 (52.8, 54.3, and 56.6%, respectively), while TPh removal was always > 99% for this range of pH values. It is well documented in the literature that the performance of Fenton’s process is highly affected by the reaction medium pH, and values in the range 2.5–4.0 favor the overall oxidation efficiency of the process (Rivas et al. 2001; Ramirez et al. 2009). As expected, TOC and TPh conversion percentages dropped significantly when initial pH = 7.0. In fact, under neutral-to-alkaline conditions, inhibition of the process may be attributed to the following: (1) the formation of ferric hydroxide complexes (cf. Eq. (7)), resulting in the decline of Fe2+ available to react with the hydrogen peroxide (Xu and Wang 2011); (2) the lower H2O2 stability, catalyzed by Fe(HO)3, which self-decomposes into molecular oxygen and water (cf. Eq. (8)), hindering the generation of hydroxyl radicals; and (3) the overall reduction of HO· oxidation potential under basic conditions (e.g., E0 = 2.65–2.80 V at pH = 3.0 and 1.90 V at pH = 7.0) (Chen et al. 2008). The first hypothesis was reinforced by the high concentration of unreacted hydrogen peroxide observed at the end of that run ([H2O2] unreacted = 1.47 g/L—corresponding to 73.5% of the initial dose applied).
For this set of experiments, maximum TOC removal was achieved at pH = 5.0 (56.6%), bearing in mind that the reaction medium pH dropped to ~ 3.2 after only a few minutes (known to be in the range of optimum values for Fenton’s oxidation, as previously mentioned). Oxidant use efficiency was also higher for pH = 5.0 then for pH = 3.0 or natural pH (η H2O2 = 0.49 vs 0.47 and 0.46, respectively). Moreover, pH values of real OMW usually range from 3.5 to 5.5 (Ochando-Pulido 2016), which is an important economical aspect for the possible scale-up of the process, as little to no pH adjustment may be required before the oxidation process. Therefore, pH of 5.0 was selected as a reference for the following sections.
Effect of the reaction temperature
Reaction temperature influence on Fenton’s oxidation efficiency was studied in the 20–50 °C range. Following the best results of the parametric study so far, experimental conditions for this set of experiments were [H2O2] = 2.0 g/L, [Fe2+] = 100 mg/L, initial pH = 5.0, and reaction time = 300 min. As stated previously, the temperature was controlled and kept constant through the entire reaction for each run. Figure 6 shows that near complete abatement of the phenolic character of the effluent was achieved (> 98% TPh removal) for the entire temperature range studied. As to organic matter degradation (expressed as TOC removal), maximum efficiency was achieved in the 30–40 °C range (56.6–57.5%), with η H2O2 values around 0.50. Similar results were obtained by Lucas and Peres (2009) and Nieto et al. (2011) on their studies on organic matter degradation of OMW through homogeneous Fenton oxidation.
When operating at lower temperatures (T = 20 °C), the TOC removal performance observed (49.0%) may suggest that the extent of the reaction was not complete within 300 min of reaction. In fact, analysis of the residual H2O2 detected the highest levels of unreacted oxidant in solution for T = 20 °C (ca. 11% of unreacted H2O2 after 300 min—Table 3). Increasing the temperature to T = 50 °C slightly hindered the TOC depletion efficiency (54.5%—Fig. 6), indicating that under those conditions the temperature influences more the thermal decomposition of H2O2 into molecular oxygen and water (Eq. (8)) than in the degradation of organic matter (Rivas et al. 2001; Nieto et al. 2011). This hypothesis is confirmed by the lower levels of residual H2O2 in solution, especially after 15 and 30 min of reaction, when compared to the other temperatures tested (see Table 3).
It is also worth mentioning that an additional experiment was performed at room temperature (T ~ 22 °C) without control of the reaction medium’s temperature throughout the run (Fig. 7a). Due to the exothermal nature of the process, temperature inside the reactor increased approximately 8 °C after 300 min (Fig. 7b). However, due to the time gap to achieve the optimum temperature of 30 °C, TOC degradation rate under such conditions is considerably slower, when compared to the experiment performed with temperature control throughout the reaction, although similar overall mineralization levels were achieved. Though this could imply that temperature control would not be required at an industrial level, one should also be aware of the climatic conditions of an actual olive mill installation during the harvest season, since it usually occurs in winter time (from November to February).
a % TOC degradation for constant temperature (T = 30 °C) throughout the entire reaction (multiplication sign) vs. unadjusted temperature (T0 = 22 °C—room temperature) (empty circle); b reaction’s temperature variation througout the experiments. Experimental conditions: [H2O2] = 2.0 g/L, [Fe2+] = 100 mg/L, pH0 = 5.0, reaction time = 300 min
Due to the minor difference on the TOC degradation efficiency for T = 30 vs. 40 °C (56.6 and 57.5%, respectively—Fig. 6), T = 30 °C was considered the most viable temperature for this process, as the small TOC degradation increase would not make up for the energy used to heat the system the additional 10 °C.
CSTR catalytic tests
With the industrial application of Fenton’s process in perspective, which entails a large number of advantages when compared to biological or even other oxidative processes such as ozonation or photo-Fenton (e.g., possibility of dealing with refractory or toxic effluents, operation at atmospheric pressure and low temperature, equipment and operation simplicity, low operating cost (Canizares et al. 2009; Hodaifa et al. 2013)), additional experiments were performed in a CSTR to evaluate the process’s effectiveness under continuous mode.
As seen in “Influence of H2O2 and Fe2+ doses” section, Fenton’s oxidation for the degradation of synthetic OMW consists in two phases: first, TOC and TPh removal happens quickly within the first minutes of reaction; then, in the second phase (also known as Fenton-like stage), oxidation proceeds at a much slower rate until reaching a plateau (Ramirez et al. 2009). This occurs because in the first stage of the process, Fe2+ reacts very quickly with H2O2 (rate constant ca. 78 mol−1 dm3 s−1) to produce large amounts of HO· (Eq. (1)) which rapidly mineralize the organic matter in solution; after this stage, ferric ions produced earlier can react with H2O2 to produce HO2 · and restore Fe2+ (Eq. (2)), although the reaction rate for iron regeneration is much slower (ca. 0.02 mol−1 dm3 s−1), making the rate of oxidation of this second stage much slower than the first one (Ramirez et al. 2005). This indicates that the space-time (τ) within the CSTR is an important parameter to consider. In an ideal continuous stirred-tank reactor (where the contents within the vessel are well-stirred and uniform throughout), the space-time is equal to the mean residence time and is calculated according to Eq. (9),
Before changing the space-time, three runs with an arbitrary space-time value (τ = 90 min, equivalent to a Q feed = 10.2 mL/min) were performed varying the H2O2 feed dosages ([H2O2]feed = 2.0, 3.0, and 4.0 g/L). This set of experiments was performed not only to find the time required to reach steady-state condition, but also to clearly determine the best H2O2 feed dosage for the conditions applied, as it is a crucial factor for both the efficiency and the overall costs of the Fenton’s process (and often holding back its upscale application (Hodaifa et al. 2013)). Oxidant dose below the stoichiometric one, i.e., [H2O2] = 1.0 g/L, was not selected due to the poor results achieved in the batch mode operation. The remaining best operational conditions found on the foregoing experiments were applied for the CSTR runs, i.e., [Fe2+]feed = 100 mg/L, initial pH = 5.0, and T = 30 °C. It is worth mentioning that dimensionless reaction time values are presented as θ = t/τ—each θ unit is equivalent to one space-time (τ) of that run’s experimental conditions. TOC and TPh values were then monitored for 450 min, equivalent to five space-times values.
Results in Fig. 8 indicate that steady-state was achieved after ca. 2.5–3 space-time values (225–270 min for this set of runs), both for TOC and TPh degradation. Regarding the effluent’s TOC abatement, an increase in the hydrogen peroxide dose fed lead to slightly higher removal efficiencies (steady-state values equal to 45.0, 48.4, and 48.9% for [H2O2]feed = 2.0, 3.0, and 4.0 g/L, respectively). Regarding TPh removal, values ≥ 98% were observed for all experiments in steady-state conditions. Concentration of H2O2 at the reactor outlet was also checked, and steady-state values are presented in Table S2. Residual H2O2 at the reactor’s outlet show that, for [H2O2]feed = 3.0 g/L, ~ 15% of the overall oxidant dose fed was not used. This could imply that, for the operational conditions applied in these runs, no further TOC degradation would be attained. This hypothesis was reinforced when the oxidant dose fed to the reactor was increased to 4.0 g/L, as TOC removal in steady-state maintained practically unchanged (48.9 vs. 48.4% for [H2O2]feed = 3.0 g/L) and the residual (unreacted) oxidant concentration inside the reactor increased to ~ 25% (values for θ = 5). Therefore, increasing the dose of peroxide fed above 2.0 g/L by a factor of 1.5 or 2.0 does not justify the improvement verified in the TOC degradation, which is also evidenced by the decrease in the efficiency of the oxidant use (η H2O2 = 0.41, 0.21, and 0.14 for [H2O2]feed = 2.0, 3.0, and 4.0 g/L, respectively), as previously stated in the batch tests. Thus, according to these experimental results, further experiments were performed with [H2O2]feed = 2.0 g/L and θ = 4 (which has proved to be enough to reach steady-state conditions).
Runs with five space-time values (τ = 40, 60, 90, 120, and 180 min) were performed. Figure 9 shows TOC and TPh removal values at steady-state (for transient regimen values report to Fig. S4). Mineralization of the synthetic OMW increases while increasing the space-time, with TOC and TPh removals reaching a maximum of 50.4 and 98.2%, respectively, in steady-state, when τ = 180 min. Zhang et al. (2006) have shown the same trend for TOC and COD removal profiles on the treatment of landfill leachate by Fenton’s oxidation while operating a CSTR. The higher the space-time, the more time the fluid elements/organic matter will have to react with the radicals because residence time within the reactor is increased. However, it is worth pointing out that operating at τ = 180 min requires the use of very low feed flow rates (ca. 5 mL/min), or higher reactor volume if Q is kept constant (Eq. 9). Operating at τ = 120 min provided high TOC and TPh degradation levels (48.2 and 96.9%, respectively), with the clear advantage from the upscale application point-of-view to have reduced by half the residence time of the effluent inside the reactor. It is also worth mentioning that unreacted oxidant concentration at steady-state conditions was less than 10% of the initial feed dose applied on this set of runs, which clearly suggests its efficient use (η H2O2 = 0.29, 0.36, 0.41, 0.43, and 0.44 for τ = 40, 60, 90, 120, and 180 min, respectively).
Overall performance of the batch and CSTR reactors—biodegradability and toxicity evaluation
Table 4 shows the main characteristics of the treated solutions for the best operational conditions found on both reactors. For the sake of comparison between the two configurations, Table 4 also presents a summary of the TPh and TOC removal efficiency under identical operational times, i.e., 180 min for batch (the plateau for the discontinuous operation, according to considerations in “Influence of H2O2 and Fe2+ doses” section) and τ = 180 min for the continuous reactor; performances are slightly better in the batch mode, although differences are not too significant.
High organic matter degradation was observed for both batch and CSTR operation, with COD falling from 805.9 to 164.0 and 194.1 mg O2/L, respectively, when operated in the best conditions found. Nonetheless, the synthetic effluent’s final COD fell short on accomplishing the Portuguese legal threshold of 150 mg O2/L for direct discharge of wastewaters on natural water bodies. Also, BOD5 values slightly above the legal limit of 40 mg O2/L were obtained after the Fenton’s oxidation runs in both rector configurations. This could indicate that the application of Fenton’s oxidation alone for the treatment of real OMW, which is more complex in its organic constituents (Dermeche et al. 2013) when compared to the synthetic effluent used, may be insufficient if the goal is the direct disposal on aquatic ecosystems or municipal wastewater treatment plants (El-Gohary et al. 2009; Lucas and Peres 2009; Lucas et al. 2013). Nevertheless, high reduction of lumped parameters was achieved, with > 70% of COD and BOD5 reduction on both operating modes, as well as > 96% removal of the phenolic character of the effluent.
To evaluate the potential application of a coupled chemical-biological treatment, effluent’s biodegradability and toxicity were also addressed. Biodegradability of the treated synthetic effluent was assessed by the BOD5/COD ratio; 0.4 is usually considered the cutoff point value between a biodegradable and difficult to biodegrade effluent (Scott and Ollis 1995). Improvement of this ratio (initially equal to 0.26) was achieved for the batch (0.37) and CSTR (0.31) best runs, although the cutoff point was not met for the conditions tested. Determination of the toxicity of the effluent was also performed by the acute bioluminescence inhibition assay, using the marine bacterium V. fischeri. Although similar works dealing with synthetic OMW report high toxicity of the initial (untreated) samples (Silva et al. 2007; Martins et al. 2010), the mixture used in this study was non-toxic for the referred bacterium. Nonetheless, evaluation of this parameter was performed since the effluent’s toxicity character may change after the oxidation runs, as Martins et al. (2010) reported on their study of synthetic phenolic wastewater remediation by Fenton’s oxidation. The authors stated that after 15 min of Fenton’s oxidation, the treated wastewater toxicity was even higher than the one obtained for the initial solution, which shows that this parameter depends on the compounds eliminated and generated by oxidation. For the wastewater used in this study, toxicity of the treated effluent (after 300 min for the batch run and at steady-state for the CSTR with τ = 120 min) was found to be null.
Conclusions
This work focused on a parametric study of homogeneous Fenton oxidation of a synthetic effluent comprising six phenolic acids under batch operation; then, the process was tested in a continuous reactor. First, the effect of hydrogen peroxide dose, iron load, reaction’s temperature, and initial pH was addressed for the batch operation mode. It was found that oxidant and catalyst dose are closely related and have a strong influence on the treatment efficiency. Up to some extent, increasing the Fe2+ and H2O2 doses has a positive influence on TOC and TPh removal. Increasing the H2O2 concentration from 1.0 to 4.0 g/L improved TOC degradation up to ~ 36% and the use of oxidant doses higher than the stoichiometric one lead to ≥ 99% of TPh depletion. The best initial pH value was found to be 5.0, since it decreases to ca. 3.2 a few minutes after the beginning of the reaction, falling in the range of 3.0–3.5 (well-known as the range that maximizes Fenton’s efficiency). Also, reaction temperature rise up to 40 °C had a positive effect on organic matter mineralization. Best conditions for the parameters tested were defined not only based on lumped parameters degradation (TOC, TPh, COD, and BOD5) but also on oxidant efficient use and actual olive mill conditions.
Based on the best operational parameters found for the batch runs, further experiments were performed on a continuous flow reactor. For the CSTR operation, steady-state conditions were attained after approximately three times the space-time (τ) value; enhacement of TOC and TPh removal values was achieved by increasing τ from 40 to 180 min (30.5 and 87.7% vs. 50.4 and 98.2%, respectively). High reduction of COD and BOD5 values was also verified—over 70% of the initial values both for batch and CSTR experiments; while biodegradability was slight improved (maximum BOD5/COD ratio equal to 0.37). This study shows that Fenton’s oxidation could be effectivly applied for the treatment of phenolic wastewaters, such as OMW, under descontinuous or continuous flow operation. High reduction of lumped parameters lead to a final effluent that almost complies with the legal threshold of Portuguese legislation for direct discharge into water bodies.
References
Aggoun M, Arhab R, Cornu A et al (2016) Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem 209:72–80
Ahmadi M, Vahabzadeh F, Bonakdarpour B et al (2005) Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J Hazard Mater 123:187–195
Allouche N, Fki I, Sayadi S (2004) Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J Agric Food Chem 52:267–273
Amaral-Silva N, Martins RC, Nunes P, et al (2016) From a lab test to industrial application: scale-up of Fenton process for real olive mill wastewater treatment J Chem Technol Biotechnol 92:1336-1344
APHA, AWWA, WEF (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC, USA
Bautista P, Mohedano AF, Casas JA et al (2008) An overview of the application of Fenton oxidation to industrial wastewaters treatment. J Chem Technol Biotechnol 83:1323–1338
Bigda RJ (1995) Consider Fenton’s chemistry for wastewater treatment. Chem Eng Prog 12:62–66
Canizares P, Paz R, Sáez C, Rodrigo MA (2009) Costs of the electrochemical oxidation of wastewaters: a comparison with ozonation and Fenton oxidation processes. J Environ Manag 90:410–420
Chen A, Ma X, Sun H (2008) Decolorization of KN-R catalyzed by Fe-containing Y and ZSM-5 zeolites. J Hazard Mater 156:568–575
Daâssi D, Lozano-Sánchez J, Borrás-Linares I et al (2014) Olive oil mill wastewaters: phenolic content characterization during degradation by Coriolopsis gallica. Chemosphere 113:62–70
De Heredia JB, Torregrosa J, Dominguez JR, Peres JA (2001) Kinetic model for phenolic compound oxidation by Fenton’s reagent. Chemosphere 45:85–90
De Laat J, Truong Le G, Legube B (2004) A comparative study of the effects of chloride, sulfate and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe(III)/H2O2. Chemosphere 55:715–723
Deng Y, Rosario-Muniz E, Ma X (2012) Effects of inorganic anions on Fenton oxidation of organic species in landfill leachate. Waste Manag Res 30:12–19
Dermeche S, Nadour M, Larroche C et al (2013) Olive mill wastes: biochemical characterizations and valorization strategies. Process Biochem 48:1532–1552
El-Abbassi A, Kiai H, Hafidi A (2012) Phenolic profile and antioxidant activities of olive mill wastewater. Food Chem 132:406–412
El-Gohary FA, Badawy MI, El-Khateeb MA, El-Kalliny AS (2009) Integrated treatment of olive mill wastewater (OMW) by the combination of Fenton’s reaction and anaerobic treatment. J Hazard Mater 162:1536–1541
Fajardo AS, Rodrigues RF, Martins RC et al (2015) Phenolic wastewaters treatment by electrocoagulation process using Zn anode. Chem Eng J 275:331–341
Hodaifa G, Ochando-Pulido JM, Rodriguez-Vives S, Martinez-Ferez A (2013) Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem Eng J 220:117–124
ISO 11348-3. (2007) Water quality - Determination of the inhibitory effect of water samples on the light emission of Vibrio fischeri (Luminescent bacteria test) - Part 3: Method using freeze-dried bacteria
IOOC (2017) International Olive Oil Council. http://www.internationaloliveoil.org/ Accessed 13 Feb 2017
Kallel M, Belaid C, Mechichi T et al (2009) Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem Eng J 150:391–395
Kang N, Lee D, Yoon J (2002) Kinetic modeling of Fenton oxidation of phenol and monochlorophenols. Chemosphere 47:915–924
Kapellakis IE, Tsagarakis KP, Crowther JC (2008) Olive oil history , production and by-product management. Rev Environ Sci Bio/Technol:1–26
Lucas MS, Peres JA (2009) Removal of COD from olive mill wastewater by Fenton’s reagent: kinetic study. J Hazard Mater 168:1253–1259
Lucas MS, Beltran-Heredia J, Sanchez-Martin J et al (2013) Treatment of high strength olive mill wastewater by Fenton’s reagent and aerobic biological process. J Environ Sci Health A 48:954–962
Martins RC, Quinta-Ferreira RM (2011) Remediation of phenolic wastewaters by advanced oxidation processes (AOPs) at ambient conditions: comparative studies. Chem Eng Sci 66:3243–3250
Martins RC, Rossi AF, Quinta-Ferreira RM (2010) Fenton’s oxidation process for phenolic wastewater remediation and biodegradability enhancement. J Hazard Mater 180:716–721
McNamara CJ, Anastasiou CC, O’Flaherty V, Mitchell R (2008) Bioremediation of olive mill wastewater. Int Biodeterior Biodegrad 61:127–134
Niaounakis M, Halvadakis CP (2006) Olive processing waste management—literature review and patent survey, 2nd edn. Elsevier, Amsterdam
Nieto LM, Hodaifa G, Rodriguez S et al (2011) Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem Eng J 173:503–510
Ochando-Pulido JM (2016) A review on the use of membrane technology and fouling control for olive mill wastewater treatment. Sci Total Environ 563–564:664–675
Ramirez JH, Costa CA, Madeira LM (2005) Experimental design to optimize the degradation of the synthetic dye Orange II using Fenton’s reagent. Catal Today 107–108:68–76
Ramirez JH, Duarte FM, Martins FG et al (2009) Modelling of the synthetic dye Orange II degradation using Fenton’s reagent: from batch to continuous reactor operation. Chem Eng J 148:394–404
Ratanatamskul C, Chintitanun S, Masomboon N, MC L (2010) Effect of chloride ions on nitrobenzene oxidation by the fluidized-bed Fenton process. Fresenius Environ Bull 19:2665–2671
Rivas FJ, Beltra FJ, Gimeno O, Frades J (2001) Treatment of olive oil mill wastewater by Fenton’s reagent. J Agric Food Chem:1873–1880
Sabhi S, Kiwi J (2001) Degradation of 2, 4-dichlorophenol by immobilized iron catalysts. Water Res 35:1994–2002
Scott JP, Ollis DF (1995) Integration of chemical and biological oxidation processes for water treatment: review and recommendations. Environ Prog 14:88–103
Sellers RM (1990) Spectrophotometric determination of hydrogen peroxide using potassium titanium( IV) oxalate. Analyst 105:950–954
Siedlecka EM, Wieckowska A, Stepnowski P (2007) Influence of inorganic ions on MTBE degradation by Fenton’s reagent. J Hazard Mater 147:497–502
Silva AMT, Nouli E, Xekoukoulotakis NP, Mantzavinos D (2007) Effect of key operating parameters on phenols degradation during H2O2-assisted TiO2 photocatalytic treatment of simulated and actual olive mill wastewaters. Appl Catal B Environ 73:11–22
Truong GL, De Laat J, Legube B (2004) Effects of chloride and sulfate on the rate of oxidation of ferrous ion by H2O2. Water Res 38:2383–2393
Walling C (1975) Fenton’s reagent revisited. Acc Chem Res 8:125–131
Xu LJ, Wang JL (2011) A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J Hazard Mater 186:256–264
Zazo JA, Casas JA, Mohedano AF, Rodriguez JJ (2009) Semicontinuous Fenton oxidation of phenol in aqueous solution. A kinetic study. Water Res 43:4063–4069
Zazo JA, Pliego G, García-Muñoz P et al (2016) UV-LED assisted catalytic wet peroxide oxidation with a Fe(II)-Fe(III)/activated carbon catalyst. Appl Catal B Environ 192:350–356
Zhang H, Choi H, Huang C (2006) Treatment of landfill leachate by Fenton’s reagent in a continuous stirred tank reactor. J Hazard Mater 136:618–623
Acknowledgements
The authors are thankful to WWTP of Parada for providing the aerobic biological sludge for the respirometric tests.
Funding
This work was the result of the project NORTE-01-0145-FEDER-000005—LEPABE-2- ECO-INNOVATION, supported by North Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Carmen Rodrigues is grateful to Fundação para a Ciência e Tecnologia—FCT—for the financial support through the Postdoctoral grant (SFRH/BPD/ 115879/2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
ESM 1
(DOCX 63 kb)
Rights and permissions
About this article
Cite this article
Esteves, B.M., Rodrigues, C.S.D. & Madeira, L.M. Synthetic olive mill wastewater treatment by Fenton’s process in batch and continuous reactors operation. Environ Sci Pollut Res 25, 34826–34838 (2018). https://doi.org/10.1007/s11356-017-0532-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0532-y








 ) and TPh (
) and TPh ( ) removal efficiencies and efficiency of oxidant use (η H2O2) (
) removal efficiencies and efficiency of oxidant use (η H2O2) ( ) after the batch runs, for Fe2+ concentrations equal to 25 (a), 50 (b), and 100 (c) mg/L. Experimental conditions: pH0 = 3.7, T = 30 °C, reaction time = 300 min
) after the batch runs, for Fe2+ concentrations equal to 25 (a), 50 (b), and 100 (c) mg/L. Experimental conditions: pH0 = 3.7, T = 30 °C, reaction time = 300 min



