Abstract
During anaerobic digestion, low-organic-content sludge sometimes is used as feedstock, resulting in deteriorated digestion performance. The operational experience of conventional anaerobic digestion cannot be applied to this situation. To investigate the feature of low-organic-content sludge digestion and explain its intrinsic mechanism, batch experiments were conducted using designed feedstock having volatile solids (VS) contents that were 30–64% of total solids (TS). The results showed that the accumulative biogas yield declined proportionally from 173.7 to 64.8 ml/g VS added and organic removal rate decreased from 34.8 to 11.8% with decreasing VS/TS in the substrate. The oligotrophic environment resulting from low-organic-content substrates led to decreased microbial activity and a switch from butyric fermentation to propionic fermentation. A first-order model described the biogas production from the batch experiments very well, and the degradation coefficient decreased from 0.159 to 0.069 day−1, exhibiting a positive relation with organic content in substrate. The results observed here corroborated with data from published literature on anaerobic digestion of low-organic-content sludge and showed that it may not be feasible to recover energy from sludge with an organic content lower than 50% through mono digestion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion is a widely used biological method that can convert organic wastes into methane and consequently leads to the stabilization and reduction of these wastes (Kafle and Kim 2013). The number and treatment capability of municipal wastewater treatment plants (WWTPs) in China and other developing countries have been increasing in recent years. As a consequence, sewage sludge discharged from WWTPs has also been increasing (Poeschl et al. 2010). Sewage sludge contains a large quantity of organic matter and nutrients, and it is an attractive feedstock for anaerobic digestion.
However, the application of conventional anaerobic digestion processes, which commonly treat the sludge having total solids (TS) content 2–6%, is not always economically feasible in every WWTP. The reasons include low benefit-cost ratios in small-capacity WWTPs (Pantaleo et al. 2013), relatively high energy requirement for heating feed sludge and digesters in regions of cold climate (Liao and Li 2015), or lack of capacity for biogas utilization in some areas that have an energy surplus. Besides these reasons, the organic content (represented by volatile solids, VS) of feedstock is also a key issue determining the performance and benefits of anaerobic digestion (Liao and Li 2015). It is well known that different types of sludge usually result in different organic degradation rates and biogas production during anaerobic digestion. Nevertheless, the performance of conventional anaerobic digestion is readily predictable because the variation of sludge organic content is commonly limited within a small range. In developed areas, sludge VS/TS ratios usually fluctuate in the range of 60–80%. Correspondingly, organic removal rates are ranged from 56 to 65%, and specific biogas production is easily estimated according to the empirical values of 0.75–1.12 m3/kg VS removed or 0.5–0.75 m3/kg VS added (Appels et al. 2008).
However, these features are not always applicable to all anaerobic digesters, especially when the VS/TS ratios in feed sludge are lower than 60% (Liao and Li 2015). In some cities of developing countries, inadequate sewage pipeline networks collect not only domestic wastewater but also rainfall runoff, industry wastewater, or some wastewater from construction sites. Because of the special effluent, inefficient grit removal, and long sludge age, the sludge discharged from these wastewater treatment systems commonly has low organic content. Furthermore, these problems cannot be solved immediately by adjusting the operation of WWTPs alone (Dai et al. 2014). Since organic degradation rates have an approximate linear relation with the VS/TS ratios in feed sludge during anaerobic digestion (Liao and Li 2015), the corresponding biogas potential of low-organic-content sludge should be much lower than the common values (Appels et al. 2008). One study reported that the organic removal rate decreased to 30% and the biogas production declined to lower than 0.4 m3/kg VS added when using this kind of sludge as the feedstock of anaerobic digestion (Duan et al. 2012). Therefore, the traditional operational experience is not applicable to those anaerobic digestion processes treating low-organic-content sludge.
Considering the huge quantity of low-organic-content sludge being produced, it is still an attractive option to reduce sludge mass and recover bioenergy through anaerobic digestion. Thus, a key question is whether this kind of sludge is suitable for anaerobic digestion, or alternatively, how much bioenergy can be recovered, because bioenergy recovery is the core advantage of anaerobic digestion (Van Stappen et al. 2016). This question is vital for selecting proper strategies of sludge treatment and disposal. In general, the lower the organic content of feedstock, the poorer the performance of anaerobic digestion. Although this rule is well known by scientific communities and practitioners, the critical threshold for organic content in feed sludge (i.e., values below which energy recovery would be infeasible) is still ambiguous. For example, a VS/TS ratio of 60% is related to “poor” sludge in developed countries, while this kind of sludge could be considered a “good” feedstock in the south of China. Despite biochemical methane potential experiments or pilot trials that could provide detailed information, it is sometimes important to preliminarily estimate the potential value of anaerobic digestion projects approximately and rapidly. Some researchers have evaluated the biogas potential from sludge anaerobic digestion but they made the assumption that sludge with different organic contents would have the same organic removal rates (Cao and Paw Owski 2012). Some researchers used low-organic-content sludge as feedstock (Duan et al. 2012; Liao et al. 2014; Liao and Li 2015), and they only recorded digestion performance (Duan et al. 2012; Liao et al. 2014). The existing information does not meet the practical demand, especially in areas suffering from a large quantity of sludge with different low organic contents.
Thus, in this work, a series of sludge substrates with gradient VS/TS ratios were prepared by adjusting inorganic content of sludge samples. They were applied to batch anaerobic digestion experiments. The results were used to characterize anaerobic digestion using low-organic-content sludge as feedstock, investigate the quantitative relation between biogas potential and sludge organic content, and analyze the intrinsic mechanism determining the performance. The results from other references were analyzed, and the effect of organic composition was discussed. Finally, criteria on sludge organic content were developed and some suggestions were provided for decision makers concerning anaerobic digestion projects using low-organic-content sludge.
Materials and methods
Sludge samples
Sewage sludge was obtained from a WWTP in Kunming City, China, and used in subsequent batch experiments of anaerobic digestion (Table 1). In this WWTP, the influent wastewater was collected from the municipal area, and a small amount of rainfall and industrial wastewater maybe also entered into the pipe network. An anaerobic-anoxic-oxidation ditch process was adopted, and primary sludge and secondary sludge were mixed and mechanically dewatered with the assistance of polyacrylamide. The collected sludge was kept at 4 °C, which would be diluted and then used as the raw substrate in the experiments. Digested sludge sourced from a laboratory-scale mesophilic anaerobic digestion reactor was used as the inoculum (Table 1). The reactor had an effective volume of 10 l, and it had been operating stably for 6 months by feeding with the diluted dewatered sludge with TS 5% at a sludge retention time (SRT) of 25–30 days. Its specific biogas production reached 370–420 ml/g VS added, indicating a good microbial activity of approximately 15 ml/(g day).
Considering that sludge organic content and composition both determine sludge digestibility, a series of sludge samples (substrate) with identical organic composition but gradient organic contents were designed. Thus, by excluding the interference of sludge organic composition, only the effect of sludge organic content was exhibited during sludge anaerobic digestion. Some of the raw dewatered sludge was dried at 105 °C in an air dry oven (DHG-9053A, Jinghong Ltd., Shanghai, China) and then burned at 550 °C in a muffle furnace (SXZ-4-10, Xinnuo Ltd., Shanghai, China). The residual ash (Table 1) was mixed with a certain quantity of the raw dewatered sludge and deionized water to generate the samples with gradient organic contents and designed TS contents. These samples were used as the substrate for the batch experiments of anaerobic digestion. A recent investigation on over 80 WWTPs in South China showed that sludge organic content ranged from 30 to 60%, the organic contents of the substrate was set at 30, 40, 50, 60, and 64%, respectively, to simulate the real-world situations. The inoculum and the substrate were mixed according to specific designs (Table 2); however, the ratios of inoculum and substrate (RI/S) were the same for all the trials. This ratio was set at 1:1 on the basis of VS, which is commonly recommended for maximum biogas production (Kameswari et al. 2012). Moreover, the organic degradation coefficient has been found to be constant when RI/S is 0.7–1.5 (Raposo et al. 2009; Liu et al. 2009).
Experimental procedures
The experiments were divided into five groups (W1–W5, excluding the control W0) according to the organic content in the substrate (Table 2), and each group except the control included 14 tests using the same mixture. Two blank tests (W0) containing only the inoculum were also conducted, so as to modify the recorded biogas productions of W1–W5. Thus, there were 14 trials in each group of five different mixtures, and in total, 72 trials were carried out simultaneously, so as to generate duplicated results.
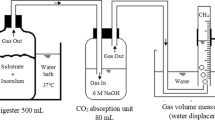
For each trial, 100 g of the mixture was added into a glass narrow-mouth bottle. The air in the headspace was removed by purging with pure N2, and then the bottle was sealed using a rubber plug. All the bottles were placed in a water bath (DKZ, YiHeng Instrument, Shanghai, China) to keep a constant temperature of 35 ± 2 °C. In addition, the water bath shook at a frequency of 60 min−1. The biogas was transported from each bottle through a polyvinyl chloride pipe and then measured by displacement of a saturated sodium chloride solution.
To monitor the variation in digestate, two bottles (as parallel samples) were taken out from the water bath every 3 or 4 days in the first 10 days and then every 5 days during the remaining experimental period. The recovered bottles were opened for collecting digestate. The actual biogas yield was calculated by deducting the biogas yield of the inoculum (W0) from the total biogas yield of each group (W1–W5). The yield was normalized to standard conditions (0 °C and 101.3 kPa). The methane content in biogas was detected using a packed column Poapak N (2 m × 4 mm) and the thermal conductivity detector in a gas chromatograph (GC-2014, Shimadzu, Kyoto, Japan). Helium was used as the carrier gas at 25 ml/min. The temperatures of the injector, column, and detector were 90, 35, and 100 °C, respectively.
Other analytical procedures
The parameters including TS, VS, total alkalinity (ALK), total nitrogen (TN), total ammonia nitrogen (TAN), and chemical oxygen demand (COD) were measured using standard methods (Ministry Of Environmental Protection 2002). For soluble parameters, the digestate collected from the bottles was centrifuged at 4390g for 10 min, and then the supernatant was filtered using 0.45-μm-mesh membranes. The filtrate was further analyzed. A pH meter (PHS-3C, Leici, Shanghai, China) was used to test the pH of the digestate. A gas chromatograph (GC-2014, Shimadzu) was used to analyze volatile fatty acids (VFAs), as previous reference (Zhang et al. 2015).
Free ammonia nitrogen (FAN) can be calculated based on TAN and pH (Anthonisen et al. 1976):
where pKa is the dissociation constant, and the value is 8.95 at 35 °C.
The carbon-to-nitrogen ratio was measured using an elemental analyzer with a thermal conductivity detector (vario EL cube, Elementar, Hanau, Germany). The temperatures of the reduction tube with copper wires as the reductant and the combustion tube with tungsten trioxide as the catalyst were 850 and 1150 °C, respectively. The temperature of the detector was 60 °C, and helium was used as the carrier gas at 220 ml/min and 120–125 kPa.
To detect the organic composition of the feed sludge, polysaccharide, protein, and lipid contents were measured using a phenol-sulfuric acid colorimetric method (Dubois et al. 1956), the Lowry method (Lowry et al. 1951), and the Soxhlet method (Rice et al. 2012), respectively. The inorganic composition of the sludge ash was detected through X-ray photoelectron spectrometry (XPS, UltraDLD, Shimadzu) and an energy-dispersive X-ray spectrometer (EDX, Quanta200FEG, FEI, Hillsboro, USA).
Results and discussion
Biogas production from low-organic-content sludge
During the batch anaerobic digestion experiments, biogas production was measured (Fig. 1) and the final methane content in the collected biogas ranged from 60 to 62%. According to Fig. 1, lower organic content in the substrate evidently resulted in lower organic degradation efficiency and less biogas yield in a 30-day digestion time. For the trials feeding the substrate with VS/TS of 64 and 60%, the biogas production ended approximately at the 19th and 25th day, respectively. For the tests using the substrates with VS/TS lower than 50%, the biogas production extended to 30 days. Furthermore, the deterioration was most drastic when the VS/TS ratio in the substrate decreased from 40 to 30%. During conventional anaerobic digestion processes, SRT is commonly less than 30 days, and the typical values are 15–25 days (Appels et al. 2008), which is consistent with the performance of the groups W4 and W5. Hence, for a normal anaerobic digestion process, the lower threshold of sludge organic content could be 60%. Correspondingly, low organic contents (< 60%) imply that the sludge may require much longer digestion time.
On the other hand, the different organic contents in the substrates also changed the conversion rate of organic substrate to biogas, despite that fact that all substrates had an identical organic composition. The organic removal rates decreased from 36.8 to 11.8% when the VS/TS ratio in substrate decrease from 60 to 30% (Fig. 2). In the period of 30 days, the groups W4 and W5 both finished their biogas production, and the group W3 almost finished. However, in the groups W1 and W2, the low-organic-content substrate caused a poor conversion rate of substrate to biogas; that is, some initial organic substances seemed to have been transformed into final products or some intermediates without further conversion to biogas. This speculation is supported by the variation of ammonia. Notably, the specific biogas production, even in the groups W4 and W5, was lower than the common range. This indicated that the raw sludge had a relatively low proportion of biodegradable substances in the total organic content, which was due to the long sludge age used in the WWTP where the sludge was obtained for these experiments.
The variations of ammonia, pH, ALK, soluble COD, and VFAs in the supernatant of digestate are shown in Fig. 3. The soluble ammonia was mainly derived from the degradation of protein, which is the major component in sludge organic substances. During the batch anaerobic digestion experiments, TAN concentration increased gradually, and the soluble ammonia strengthened total alkalinity in all the groups. Notably, ammonia concentration was nearly proportional to the VS/TS ratios in substrate. In the groups W1 and W4, TAN increased by 179 and 366 mg/l, respectively, after 30-days digestion. Assuming all the soluble ammonia was generated from the protein in substrate, the degradation rates of protein (assuming its average nitrogen content is 16%) should be 22% in group W1 and 30% in group W4. Therefore, compared to the other groups, the group W1 had the lowest organic conversion rates owing to the low-organic-content substrate.
Variations of a total ammonia nitrogen (TAN); b free ammonia nitrogen (FAN); c pH; d alkalinity (ALK); e soluble chemical oxygen demand (SCOD); and f total volatile fatty acids (VFAs) during batch anaerobic digestion experiments using different ratios of volatile solids to total solids in the substrate
The concentration of FAN decreased at the beginning of the experiments and then recovered to a steady level. This behavior is consistent with the variation of pH. The concentration of FAN remained at a low level, and the value of pH fluctuated in the range of 7.0–7.5. These parameters indicated that all the batch anaerobic digestion experiments operated steadily without any inhibition from ammonia. Thus, the deteriorated performance should be attributed to factors other than ammonia. For example, aliphatic acid is a common intermediate product possibly inhibiting anaerobic digestion (Palatsi et al. 2009). However, in all the groups, the concentration of VFAs remained less than 40 mg/l, which is far below the inhibition threshold. The concentrations of soluble COD and VFAs both decreased to a steady level gradually, indicating no accumulation of any soluble intermediate. At the initial stage, soluble organic matter was converted to biogas quickly, although VFAs were produced simultaneously and relatively slowly. Thus, the quick consumption of soluble organic matter, corresponding to the quick generation of biogas, resulted in the decrease of SCOD and VFAs at the initial stage.
Because the substrates in these trials had an identical organic composition, the deteriorated digestibility of low-organic-content sludge should be attributed to the lack of available organic substrate other than different substrate compositions. As a consequence, the oligotrophic condition decreased the activity of functional microorganisms and seemed to change the metabolic pathways in different degrees. The digestion efficiencies exhibited in these batch experiments reflected the variation of microbial activity along with organic content in substrate. To support the speculation on metabolic pathways, the composition of VFAs was further analyzed.
Intrinsic variations derived from low-organic-content feed sludge
During the batch experiments, the composition and evolution of VFAs was recorded along with digestion time (Fig. 4). The quantity of VFAs was expressed on the basis of VS because the mass or molar concentration was not convenient for the comparison between the groups using different quantities of organic substrate. On the other hand, histograms can reflect the ratios of different VFAs more clearly than line charts. In Fig. 4, butyric acid includes n-butyric acid and isobutyric acid, and valeric acid includes n-valeric acid and isovaleric acid.
Acetic acid was the major type among VFAs in all the groups, but its relative concentration was the highest in group W1. Moreover, the relative concentration of valeric acid was also higher than that in other groups. The high concentration of VFAs remaining in the digestate indicated their low conversion rate to biogas, which was consistent with the low biogas yield in the group W1. Besides acetic acid, the other VFAs were also significantly different, especially the ratio of propionic acid to butyric acid. For the groups with low-organic-content substrates (W1, W2, and W3), the concentration of propionic acid was higher than that of butyric acid; but for the other groups (W4 and W5), the reverse was true. Furthermore, along with the consumption of organic substrate in W4 and W5, propionic acid gradually replaced butyric acid as the second-most dominant type among VFAs. In general, the quantitative order of VFAs was acetic acid > propionic acid > valeric acid > butyric acid in the groups W1, W2, and W3. However, in the groups W4 and W5, the order became acetic acid > butyric acid > valeric acid > propionic acid. The switch of VFA types indicated that propionic fermentation was greater under the oligotrophic condition, while butyric fermentation was applied to the substrate with relatively high organic contents.
In general, VFAs containing branched alkyls can be biodegraded more rapidly than straight-chain VFAs and the rate order should be butyric acid > acetic acid > valeric acid > propionic acid (Wang et al. 1999). In the groups W1, W2, and W3, propionic fermentation retarded biochemical chain reactions. From W1 to W5, the concentration of propionic acid was reduced with increasing VS/TS ratios, and this is consistent with the digestion performance of these groups.
Relationship between sludge organic content and anaerobic digestion performance
Notably, the fluctuation of acetic acid concentration was limited within a small range in all the groups, indicating that acetate was converted into biogas rapidly without any accumulation. Hydrolysis is commonly recognized as the rate-limiting step of sludge anaerobic digestion (Koch et al. 2015; Liu et al. 2016). Thus, a first-order model should be the simplest representation for interpreting hydrolysis kinetics and describing batch anaerobic digestion (Luo et al. 2012), as expressed by Eq. (2):
where k is the rate constant (1/day), t is the digestion time (d) and C is the concentration of organic substrate. Integrating Eq. (2) from time 0 to t gives Eq. (3):
where C0 represents the initial concentration of organic substrate (mg/l) and Ct is the concentration at time t (mg/l). Because there was a proportional relationship between organic removal rate and biogas yield, the biogas production was expressed using Eq. (4):
where yt is the biogas yield at time t (ml/g VS added) and ym is the maximum biogas yield (ml/g VS added). The biogas yield over time can be expressed as Eq. (5).
In this study, ym and k were calculated based on the biogas production over time, using nonlinear fitting and the least squares method. The results are shown in Fig. 1, and the parameters are shown in Table 3. The R2 (> 0.994) and root-mean-square error (RMSE) values (< 0.1) indicated that the model can describe the biogas production of all the groups very well, and shows that the digestion efficiency increased with the increasing organic content in substrate. Moreover, the trend was more significant when sludge VS/TS ratio exceeded 50%. In other words, feed sludge with a VS/TS ratio less than 50% implied quite low digestion efficiency.
Besides organic content in substrate, digestion performance is also influenced by organic composition in substrate. Notably, the calculated values obtained using the relationships shown in Fig. 4 were lower than the values in some other reports. For example, a k value 0.17 day−1 was reported for the batch anaerobic mono digestion of sludge having VS/TS of 56.7% (Dai et al. 2013), while from Table 3, the evaluation value should be 0.12 day−1. Likewise, a k value was reported to be 0.19 day−1 for sludge with VS/TS of 50.7% (Liao et al. 2016), but according to Table 3, the value should be 0.09 day−1. And, in another study, the k value declined to 0.084 day−1 during anaerobic digestion of fish waste and bread waste having a VS/TS of 32.8% (Kafle et al. 2013), but Table 3 indicates this value should be 0.07 day−1. The reason for these differences could be that the sludge samples used in the present study had low biodegradability because they originated from the WWTP operating with a long sludge age. Consequently, the amount of resistant organics (such as humic substances) in the total organic content of sludge used in the present study was possibly large, which would have been reflected in a low degradation rate constant.
Based on the values of k, the digestion time needed to reach 95 (T95) or 80% (T80) of the maximum biogas yield can be evaluated using Eq. (3) and is shown in Fig. 5. According to these relationships, for the low-organic-content sludge with k values higher than 0.05, most of sludge biodegradable substances could be digested within 30 days. This digestion time is acceptable for batch or continuous anaerobic digestion of sewage sludge; however, the low VS content in substrate and the corresponding low biogas yield possibly decrease the potential benefits of anaerobic digestion.
Energetic potential from low-organic-content sludge
Bioenergy recovery is the core advantage of anaerobic digestion. For continuous anaerobic digestion systems, the energy recovered from biogas commonly is used first for heating feed sludge and maintaining digesters at approximately 35 °C. Only when biogas supply exceeds these heat requirements can sludge anaerobic digestion export surplus energy. To examine the possibility of energy export, a hypothetical digester treating 100 t/day sludge having TS content of 5% was evaluated. For a completely stirred digester having a SRT of 30 days, the working volume should be 3000 m3. The total volume of the digester was set at 3600 m3 (incorporating a headspace and working volume) and the surface area was approximately 1300 m3 (assuming a cylindrical digester with a 1:1 ratio of height to diameter). The heat capacity of feed sludge was assumed to be 4.2 kJ/(kg K) and the heat transfer coefficient of the digester wall was taken as 2.5 kJ/(m2 h K). Thus, the heat consumption for heating feed sludge from 10 to 35 °C and maintaining the digester at 35 °C was calculated to be 10.5 and 2.0 GJ/day, respectively.
To maintain an energy balance, the biogas generated from the digester should reach 680 m3/day or more if the heating value of biogas is 23 MJ/m3 and if 80% of the heat can be transferred effectively to heat the sludge. Based on this deduction, the critical specific biogas yields for maintaining an energy balance should be 227, 272, 340, and 453 ml/g VS added for feed sludge with VS/TS ratios of 60, 50, 40, and 30%, respectively. Thus, energy recovery from sludge anaerobic digestion is feasible only when the specific biogas yield of feed sludge is higher than these critical values. As is obvious from the experimental results in Table 3, the sludge used in this study cannot meet this standard. However, as stated previously, sludge sourced from different WWTPs may have the same organic content (VS/TS) but different organic compositions, and these compositions also determine the biogas potential of sludge. Furthermore, the digestibility reflected in batch experiments is commonly somewhat lower than that observed in the actual performance of digesters during continuous anaerobic digestion processes (Jensen et al. 2011; Zhang et al. 2013).
Therefore, published literature was investigated to obtain more evidence on the potential energy recovery from low-organic-content sludge. Although reports of research on relevant practices are rare, some useful examples were found. A full-scale sludge treatment plant located at Qingdao, China, used 12 digesters and had a designed treatment capability of 600 t/day dewatered sludge. In this plant, the diluted sludge with TS 5% and VS/TS 60% produced biogas of 380 ml/g VS added. A similar result (373 ml/g VS added) in laboratory-scale experiments was reported for sludge with a VS/TS of 60% (Duan et al. 2012). Compared with the critical value for biogas yield (227 ml/g VS added), these results indicate that sludge with a VS/TS of 60% can generate surplus energy. Other research demonstrated that even when the organic content of feed sludge was 50%, biogas yield of 340 ml/g VS added (Liao and Li 2015) or 333 ml/g VS added (Liao et al. 2016) could be obtained, both of which are higher than the required critical value (272 ml/g VS added). Because no reports were found on anaerobic digestion of sludge with a VS/TS ratio of 40%, it was reasonably assumed that such sludge would have a biogas yield lower than 340 ml/g VS, which is the critical value for sludge of this organic content and implies that this sludge cannot be used for bioenergy recovery. In fact, feed sludge with a VS/TS ratio of 50% can only barely maintain the energy balance, considering other energy requirements or losses (for example, energy consumption during biogas purification and condensation, heat loss during sludge transfer and recycle). Therefore, it may be not feasible to recover energy from sludge with an organic content lower than 50% through conventional anaerobic digestion. As an alternative option, low-organic-content sludge can be co-digested with other high-organic-content wastes (such as food waste) to improve biogas production significantly (Zhen et al. 2015; Di Maria et al. 2016).
Conclusions
Characteristics of low-organic-content sludge digestion and their intrinsic variations were investigated using batch experiments with specially designed feedstock. The accumulative biogas yield declined proportionally from 173.7 to 64.8 ml/g VS added and the organic removal rate decreased from 36.8 to 11.8%, as the VS/TS ratios in substrate decreased from 64 to 30%. The oligotrophic environment with a reduced bioavailable substrate resulted in decreased microbial activity and altered metabolic pathways. In the batch experiments using low-organic-content substrate, propionic fermentation competed over butyric fermentation. A first-order model described the biogas production of low-organic-content sludge very well, and the degradation coefficient k exhibited a positive relation with organic content in the substrate.
References
Anthonisen AC, Loehr RC, Prakasam T, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. JWater Pollut Control Fed 48:835–852
Appels L, Baeyens J, Degrève J, Dewil R (2008) Principles and potential of the anaerobic digestion of waste-activated sludge. Prog Energ Combust 34:755–781
Cao Y, Paw Owski A (2012) Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: brief overview and energy efficiency assessment. Renew Sust Energ Rev 16:1657–1665
Dai X, Duan N, Dong B, Dai L (2013) High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: stability and performance. Waste Manag 33:308–316
Dai X, Zhao Y, Sha C, Dai L, Dong B (2014) Investigation on the status and causes of sludge sand content of wastewater treatment plants in China. Water Wastewater Eng75-78, 79
Di Maria F, Micale C, Contini S (2016) Energetic and environmental sustainability of the co-digestion of sludge with bio-waste in a life cycle perspective. Appl Energ 171:67–76
Duan N, Dong B, Wu B, Dai X (2012) High-solid anaerobic digestion of sewage sludge under mesophilic conditions: feasibility study. Bioresour Technol 104:150–156
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Jensen PD, Ge H, Batstone DJ (2011) Assessing the role of biochemical methane potential tests in determining anaerobic degradability rate and extent. Water Sci Technol 64:880–886
Kafle GK, Kim SH (2013) Anaerobic treatment of apple waste with swine manure for biogas production: batch and continuous operation. Appl Energ 103:61–72
Kafle GK, Kim SH, Sung KI (2013) Ensiling of fish industry waste for biogas production: a lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour Technol 127:326–336
Kameswari KSB, Kalyanaraman C, Porselvam S, Thanasekaran K (2012) Optimization of inoculum to substrate ratio for bio-energy generation in co-digestion of tannery solid wastes. Clean Technol Envir 14:241–250
Koch K, Helmreich B, Drewes JRE (2015) Co-digestion of food waste in municipal wastewater treatment plants: effect of different mixtures on methane yield and hydrolysis rate constant. Appl Energ 137:250–255
Liao X, Li H (2015) Biogas production from low-organic-content sludge using a high-solids anaerobic digester with improved agitation. Appl Energ 148:252–259
Liao X, Li H, Cheng Y, Chen N, Li C, Yang Y (2014) Process performance of high-solids batch anaerobic digestion of sewage sludge. Environ Technol 35:2652–2659
Liao X, Li H, Zhang Y, Liu C, Chen Q (2016) Accelerated high-solids anaerobic digestion of sewage sludge using low-temperature thermal pretreatment. Int Biodeter Biodegr 106:141–149
Liu C, Li H, Zhang Y, Chen Q (2016) Characterization of methanogenic activity during high-solids anaerobic digestion of sewage sludge. Biochem Eng J 109:96–100
Liu G, Zhang R, El-Mashad HM, Dong R (2009) Effect of feed to inoculum ratios on biogas yields of food and green wastes. Bioresour Technol 100:5103–5108
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Luo K, Yang Q, Li X, Yang G, Liu Y, Wang D, Zheng W, Zeng G (2012) Hydrolysis kinetics in anaerobic digestion of waste activated sludge enhanced by α-amylase. Biochem Eng J 62:17–21
Ministry of Environmental Protection, China. Standard methods for the examination of water and wastewater. Beijing: China Environmental Science Press, 2002
Palatsi J, Laureni M, Andrés MV, Flotats X, Nielsen HB, Angelidaki I (2009) Strategies for recovering inhibition caused by long chain fatty acids on anaerobic thermophilic biogas reactors. Bioresour Technol 100:4588–4596
Pantaleo A, Gennaro BD, Shah N (2013) Assessment of optimal size of anaerobic co-digestion plants: an application to cattle farms in the province of Bari (Italy). Renew Sust Energ Rev 20:57–70
Poeschl M, Ward S, Owende P (2010) Evaluation of energy efficiency of various biogas production and utilization pathways. Appl Energ 87:3305–3321
Raposo F, Borja R, Martín MA, Martín A, de la Rubia MA, Rincón B (2009) Influence of inoculum–substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: process stability and kinetic evaluation. Chem Eng J 149:70–77
Rice EW, Baird RB, Eaton AD, Clesceri LS (2012) Standard methods for examination of water and wastewater. APHA AWWA WEF, Washington, DC
Van Stappen F, Mathot ML, Decruyenaere V, Loriers A, Delcour A, Planchon V, Goffart J, Stilmant D (2016) Consequential environmental life cycle assessment of a farm-scale biogas plant. J Environ Manag 175:20–32
Wang Q, Kuninobu M, Ogawa HI, Kato Y (1999) Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 16:407–416
Zhang C, Su H, Tan T (2013) Batch and semi-continuous anaerobic digestion of food waste in a dual solid–liquid system. Bioresour Technol 145:10–16
Zhang Y, Li H, Cheng Y (2015) Influencing mechanism of high solid concentration on anaerobic mono-digestion of sewage sludge without agitation. Front Env Sci Eng 9:1108–1116
Zhen G, Lu X, Kobayashi T, Li Y, Xu K, Zhao Y (2015) Mesophilic anaerobic co-digestion of waste activated sludge and Egeria densa: performance assessment and kinetic analysis. Appl Energ 148:78–86
Funding
This work was supported by the China Major Science and Technology Program for Water Pollution Control and Treatment (grant number 2011ZX07302); the Natural Science Foundation of China (grant number 51478239); and the Shenzhen Science and Technology Project (grant number JCYJ20170307152224920).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Bingcai Pan
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, H. Energy recovery from wastewater treatment plants through sludge anaerobic digestion: effect of low-organic-content sludge. Environ Sci Pollut Res 26, 30544–30553 (2019). https://doi.org/10.1007/s11356-017-0184-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0184-y