Abstract
Mangrove ecosystems in Pattani Bay, Thailand are considered representatives for monitoring the occurrence of anthropogenic and natural pollution due to metal and radionuclide contamination. Sediments and seawater were collected from five locations to determine metal (Cd, Cr, Cu, Mn, Ni, Zn, and Pb) and radionuclide (226Ra, 232Th, and 40K) concentrations. Spatial variations in metal and radionuclide concentrations were determined among the sampling sites. A geoaccumulation index (I geo ) and enrichment factor (EF) were used to classify the impacts of metals from anthropogenic point sources. Significant values for I geo and EF were measured for Pb in site 4 (I geo 0.65; EF 28.2) and Cd in site 1 (I geo 1.48; EF 46.2). EF values in almost all sampling sites were >1 which indicates anthropogenic pollution. To assess the potential public hazard of radioactivity, the average radium equivalent activity (Raeq), the external hazard index (H ex), the internal hazard index (H in), the absorbed dose rate in air (D), and the annual effective outdoor dose rate (E) were determined. Based on these measurements, it is concluded that the probability of human health risk from radionuclides is low. However, the absorbed dose in air (D) values in sites 4 and 5 were greater than the global average value of 55 nGy h−1, indicating that sediments in these locations pose a radiological hazard. The data obtained in this study provides useful information on metal and radionuclide background levels in mangrove sediments and seawater, and can be applied toward human health risk assessment and metal and radionuclide mapping.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediment in mangrove ecosystems is a primary sink for contaminants from terrestrial sources (Mountouris et al. 2002). Contaminants become immobilized in sediments in part due to their anaerobic character combined with their enrichment in sulfides and organic matter (Peters et al. 1997). Contaminants such as lead (Pb) and cadmium (Cd) are adsorbed on ion exchange sites of fine silt or clay or are co-precipitated with other metals, i.e., manganese (Mn), aluminum (Al), and iron (Fe) (Harbison 1986). Furthermore, metals and radionuclides commonly occur in seawater in trace quantities (Yap et al. 2011; Yunus et al. 2015). However, anthropogenic and natural disturbances can increase the quantity of contaminants via redistribution from sediments to seawater. In particular, mining is considered a key source of radionuclides (Hu et al. 2014), while fertilizer runoff from agricultural lands serves as a source of 226Ra, particularly in superphosphates. Levels as high as of 571.22 Bq kg−1 have been measured (Saleh et al. 2007).
Pattani Bay is located along the southern Gulf of Thailand. Coastal zones are characterized by sand and muddy sand sediments (Swennen et al. 2001). The region consists of a diverse array of mangrove vegetation, and aquatic and terrestrial organisms. Industrial activities around Pattani Bay have, over the past few decades, improved the economic status of local communities (Cheewasedtham et al. 2003; Sowana et al. 2011). Unfortunately, however, massive quantities of wastes from these activities have been released into local ecosystems, particularly in waterways. Several facilities and sites are considered primary point sources of contaminants, which have led to bioaccumulation in food chains. Accurate and current data for metal and radionuclide concentrations in terrestrial and marine ecosystems of Pattani Bay, Thailand is essentially nonexistent (Kaewtubtim et al. 2015, 2016). Little data is available on the physicochemical status of mangrove ecosystems along Pattani Bay, as many occur in conflict zones or so-called ‘red areas’.
A number of metals and radionuclides occurring in Pattani Bay sediments are believed to be enriched from industrial, agricultural and domestic activities, and from abandoned tin mines. It is known that several metals are linked with an increased incidence of acute and chronic diseases, including those affecting the nervous system, the eyes, skin, and lungs. The rates of certain types of cancers are also elevated (Singh et al. 2011; Haddad 2012). A number of chronic diseases are known to be associated with exposure to radionuclides (Frontasyeva et al. 2001). Hence, an accurate accounting of metal and radionuclide concentrations in both sediments and seawater is necessary for proactive environmental management since harvest of marine organisms (i.e., fish, macroalgae, crab, etc.) and aquaculture are common activities in coastal ecosystems of Pattani Bay.
In this first reported investigation, the distribution of metals and radionuclides in sediment and seawater from Pattani Bay, Thailand was documented. Heavy metals including Cd, Cr, Cu, Mn, Ni, Zn, and Pb were selected for determination as they comprise key contaminants of local mangrove ecosystems. Radionuclides such as 226Ra, 232Th, and 40K are considered representative radionuclides that affect human health (Alamgir Miah et al. 2012). The Geoaccumulation Index (I geo ) and enrichment factor (EF) were used to screen the anthropogenic impacts for metals in the study sites and to determine ecological risk. This information will be used for decision making to ultimately establish an environmental management program in coastal areas of Thailand, with particular emphasis on mangrove ecosystems.
Materials and methods
Study areas and physicochemical analysis of the samples
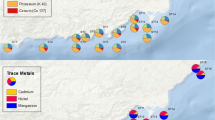
Sediment and seawater samples were collected from five sites in the coastal area of Pattani Bay (site 1: N 6° 53′ 77.6′′ E 101° 15′ 01.2′′; site 2: N 6° 53′ 68.4′′ E 101° 14′ 41.9′′; site 3: N 6° 53′ 68.4′′ E 101° 14′ 41.9′′; site 4: N 6° 54′ 85.3′′ E 101° 14′ 82.1′′; and site 5: N 6° 54′ 87.8′′ E 101° 14′ 98.6′′) (Fig. 1). The average annual temperature and precipitation in 2014 were 27.5 °C and 5.62 mm y−1, respectively. The distance between sampling sites is approximately 0.5 km. The sampling sites are located near mangrove forests, and are surrounded by residential dwellings and industrial estates.
Sediments were collected using a 2-cm diameter stainless steel hand corer, placed into plastic bags and transferred to an insulated box. All samples were subsequently transported to the laboratory. The sediments were allowed to air dry at room temperature following which they were grounded with an agate mortar and pestle, and sieved to pass a 2-mm mesh sieve.
Physicochemical properties of the sediments were determined following standard methods. One-half gram of sediment was placed into a Pyrex® test tube and digested with conc. HNO3 in a heating block (APHA, AWWA and WEF 2005). Total Zn, Pb, Mn, Cr, Cu, Ni, and Cd concentrations were determined using a flame atomic absorption spectrophotometer (FAAS; AAnalyst™ 200, PerkinElmer) or a graphite furnace atomic absorption spectrophotometer (GF-AAS; AAnalyst™ 600, PerkinElmer) after HNO3 digestion (APHA, AWWA and WEF 2005), depending on metal concentration. Extractable Zn, Pb, Mn, Mn, Cr, Cu, Ni, and Cd concentrations were determined by FAAS or GF-AAS after extraction with 0.05 M diethylene triamine pentaacetic acid (DTPA) (ICARDA 2001). Analytical accuracy and precision were determined by running standard solutions after every 20 samples. A method blank and certified reference materials (NIST SRM® 2710a Montana soil and HPS Certified Waste Water Trace Metals Lot#D532205 for sediment and seawater, respectively), were used for quality control. Percentage recovery was in the range of 100.2–118.0 and 100.2–116.0% for sediment and seawater, respectively.
Total nitrogen (N) was determined using the Kjeldahl method (Black 1965), extractable phosphorus (P) concentrations using the Bray II method (Bray and Kurtz 1945), and extractable potassium (K) concentrations using FAAS after extraction with neutral NH4OAc (Sparks 1996). Soil cation exchange capacity was determined after leaching with 1 N NH4OAc buffer (Sparks 1996). Organic matter content was measured using the Walkley–Black titration method (Walkley and Black 1934). Soil pH was measured on a 1:5 (w/v) suspension of soil deionized water using a glass electrode pH meter (Hanna instruments HI 221). Electrical conductivity (EC) was measured using an EC meter (Hanna instruments HI 993310). Soil texture was determined using the hydrometer method (Allen et al. 1974). Concentrations of 226Ra, 232Th, and 40K were determined by gamma-ray spectrometry with a high purity germanium (HPGe) detector. The energy calibration was performed using standard reference radionuclide sources 60Co and 137Cs, while the efficiency calibration was performed using reference samples 238U, 226Ra, 232Th, and 40K obtained from the Office of Atoms for Peace, Thailand.
Fifty mL of seawater was filtered through a 0.45-μm cellulose membrane filter and then acidified with 0.05 mL double-distilled HCl (Merck®) to attain pH < 2. Total metal concentrations were determined by FAAS or GF-AAS. Separate samples were analyzed for the selected radionuclides by gamma-ray spectrometry with a high purity germanium (HPGe) detector after filtering without acidified water.
The radioactivity of 226Ra, 232Th, and 40K was calculated using the following equation (IAEA 1989):
Where C i is the specific activity of the radionuclide in the plant (Bq kg−1), A is the count of the radionuclide, E is the detector efficiency of the specific gamma-ray, P is the absolute transition probability of the specific gamma-ray, T is the time (s), and W is the mass of the sample (kg).
Evaluation of sediment contamination
The Geoaccumulation Index (I geo ) is a tool for assessing possible enrichment of metals in sediments (Müller 1969). It is calculated as follows:
where C n is the measured concentration of metal ‘n’, B n is the metal concentration in ‘average shale’ (Turekian and Wedepohl 1961), and 1.5 is the background matrix correction factor due to lithogenic effects (Nowrouzi and Pourkhabbaz 2014).
I geo is divided into seven classes, where the highest value (class 6) indicates a 100-fold enrichment above background values (Ghrefat et al. 2011). The classes for I geo range from Class 0 (practically uncontaminated); I geo ≤ 0; to class 6 (extremely contaminated); I geo > 5.
The enrichment factor (EF) has been widely used for assessing the degree of metal enrichment and is useful for comparing metal enrichment from anthropogenic and natural sources in surface sediments (Karageorgis et al. 2009). For a better understanding of anthropogenic contribution of the metal, an enrichment factor was calculated for each metal by dividing its ratio to the normalizing element by the same ratio found in the chosen baseline (Taylor 1964), and is described as follows:
where C n is the concentration of metal ‘n’. The background value is the metal concentration in an ‘average shale’ (Turekian and Wedepohl 1961). Manganese was chosen as a normalizing, or reference element for determining EF values as it is one of the major components of the earth’s crust and its concentration in sediment is associated mainly with the matrix (Uduma and Awagu 2013). As proposed by Simex and Helz (1981), EF is classified into five categories, ranging from EF < 2 depletion to minimal enrichment; to ER > 40 extremely to high enrichment.
The radium equivalent activity is used to assess hazards from 226Ra, 232Th, and 40K in Bq kg−1 (Agbalagba and Onoja 2011; Tufail et al. 2011) and is calculated as follows:
Where A Ra, A Th, A K are the activity concentrations of 226Ra, 232Th, and 40K, respectively.
The external hazard index (H ex) and internal hazard index (H in) were determined. The external hazard index evaluates the hazard of natural gamma radiation, while the internal exposure to radon and its daughter products are evaluated by the internal hazard index (Agbalagba and Onoja 2011; Tufail et al. 2011):
Where A Ra, A Th, and A K are the activity concentrations of 226Ra, 232Th, and 40K, respectively.
The absorbed dose rate in air is commonly employed to assess health risk index (Jahan et al. 2016). As regard to biological effects, radiological, and clinical effects are directly associated with absorbed dose rate. Measured activity concentrations of detected radionuclides are converted into doses as follows:
Where A Ra, A Th, and A K are the activity concentrations of 226Ra, 232Th, and 40K, respectively.
Using the conversion coefficient from absorbed dose in air to effective dose of 0.7 Sv Gy−1, as recommended by UNSCEAR ( 2000), the number of hours in a year of 365 days (8760 h) and the outdoor occupancy factor of 20% (Freitas and Alencar 2004; Jibiri and Okeyode 2012), the annual effective outdoor dose rate is calculated by the equation:
Where D is the absorbed dose rate.
Statistical analysis
All data were analyzed using SPSS® version 18.0 (SPSS Inc. Chicago, IL). Analysis of variance was performed using least significant difference (LSD) post hoc comparisons, with a 95% confidence level (p ≤ 0.05).
Results and discussion
Physicochemical properties of sediments in Pattani Bay mangrove forests are shown in Table 1. Sediment pH values ranged from neutral to slightly alkaline (7.1–7.5), indicating that mobility of metallic and radionuclide contaminants should be minimal (Hu et al. 2014). Mangrove sediments in many areas worldwide tend to be slightly basic in pH due to limited buffering capacity of the sediments (Middelburg et al. 1996). Sediment EC values ranged from 0.4–8.1 mS cm−1, N from 0.02–0.18%, and extractable P from 89 to 986 mg kg−1 (Table 1). Maximum organic matter content was 2.5%, which is fairly typical for mangrove sediments of the humid tropics. The influences of organic matter accumulation in tropic sediments depend on sediment grain size, anthropogenic organic input, and decomposition rate of mangrove litterfall (Hossain et al. 2014).
The texture for all sediment samples was loam. In different mangrove forests of the tropics, the majority of sediments are reported as having high proportions of silt and clay; such colloids may trap radioisotopes on particles surfaces, resulting in elevated levels of radiation (Ibrahiem et al. 1993). Sediment texture in the study areas partly reflects parent material, but is also due to disturbance by anthropogenic activities including extensive construction as well as mining. The latter effects changed the mangrove sediment texture from a high clay proportion to loam. In addition, coastal waves affect sediment accumulation at the sampling sites which may affect sediment properties as well. Sites 1, 2, 4, and 5 possessed higher CEC and metal concentrations compared to site 3. These data demonstrate the importance of CEC for metal adsorption and/or precipitation in the sediment; however, CEC values for samples in this study may have lower levels of potentially bound metals on adsorption sites compared to those of other coastal mangrove sediments which have higher clay contents (Nematollahi and Ebrahimi 2015).
Total Cd concentrations in sediment ranged from 0.2 to 2.0 mg kg−1, from 27.4 to 65.2 mg kg−1 for Cr, from 0.4 to 23.5 mg kg−1 for Cu, from 44.4 to 213.8 mg kg−1 for Mn, from 11.4 to 41.4 mg kg−1 for Ni, from 3.9 to 30.8 mg kg−1 for Zn, and from 9.7 to 57.4 mg kg−1 for Pb (Table 1). Concentrations of Cr, Mn, Ni, and Zn were the highest in site 1, while the highest Cd and Cu concentrations were measured in site 5 and Pb in site 1. The differences in metal contents in the sediments might be influenced by mineral and organic matter content that tend to immobilize metals (Saedeleer et al. 2010).
The I geo values for sediment metals are rated as essentially unpolluted except for Cd in site 1 which is classified as moderately polluted (Table 2). This effect may be due to current circulation and sediment dynamics of large bodies of seawater. Cd can be found together with Pb and Zn via discharges from paint factories (Sekabira et al. 2010; Seshan et al. 2010). Furthermore, the high EF value for Cd in site 1 could indicate a high degree of anthropogenic impact (Table 2). EF values for Pb were >1, which indicate that anthropogenic pollution occurred in the sampling sites (Abdel Ghani 2015).
Metal concentrations of seawater samples were all significantly (p < 0.05) lower as compared to concentrations in sediments (Table 3). The lower metal concentrations may be explained by the precipitation–coprecipitation of particulates and sorbed metals during periods of limited water movement (Fonseca et al. 2011).
The activities of sediment radionuclides varied significantly with sampling site (p < 0.05). Radionuclide activities ranged from 3.1–5.8 Bq kg−1 for 226Ra, 145.9–227.2 Bq kg−1 for 40K, and 28.2–86.9 Bq kg−1 for 232Th. Highest 226Ra and 232Th concentrations were noted at site 5, whereas the highest 40K concentration was detected in site 3. Radionuclide abundance was as follows: 40K > 232Th > 226Ra. The lowest concentrations of radionuclides were from stations located farther from the river mouth (Fig. 1).
Radionuclide concentrations in seawater were generally below detection limits, except for 40K at very low concentrations (3.1 and 2.3 Bq m−3 for sites 4 and 5, respectively). However, some reports state that even low doses of radionuclides may increase the frequency of mutations in chromosomes and genes in human somatic, bone marrow, and muscle cells (Cristaldi et al. 1991; Livingston et al. 1997); therefore, radionuclide concentrations must be monitored continuously in environments where bioaccumulation may occur in the food chain. Furthermore, there is little information of natural radioactivity available in both sediment and seawater of Pattani Bay. Concentrations of 175.5, 252.6, and 58.0 Bg kg−1 for 226Ra, 40K, and 232Th, respectively, were determined in sediments of Pattani Bay in a previous investigation (Kaewtubtim et al. 2015). In that study, 226Ra concentrations were higher than those of the present study (30.1–56.4 x).
Solubility, partitioning and redox reactions are considered primary factors influencing radionuclide mobility. Furthermore, increases in radionuclide contents via water column transportation depend upon type of radionuclide, soil/sediment porosity, redox state, and types and amounts of humic materials (Payne and Edis 2012; Tchokosssa et al. 2012). The decomposition rate of organic matter must also be taken into account in mangrove sediments because radionuclides are transported in sediment via plant uptake. Sediments allow for limited distribution of radionuclides at different depths as a function of the root length (Yanagisawa et al. 2000; Uchida 2007).
In sediment samples, values for average radium equivalent activity (Raeq), external hazard index, internal hazard index, absorbed dose rate in air, and annual effective outdoor dose rate were in the ranges of 57.4–143.7 Bq kg−1, 0.2–0.4, 0.02–0.40, 26.1–62.3 nGy h−1, and 0.03–0.08 mSv y−1, respectively (Table 4). Values of Raeq were lower than standard values of 370 Bq kg−1 in sampling sites worldwide (UNSCEAR 2000). Other radiological risk assessment indices showed no potential internal or external radiation hazards for humans. The absorbed dose in air (D) values for sediments were lower than the global average value of 55 nGy h−1, except for the sediment in sites 4 and 5 (56.8 and 62.3 nGy h−1, respectively). The annual effective outdoor dose rate (E) in the sediments was within the acceptable levels provided by The International Commission for Radiological Protection of 1.0 mSv y−1. Sediment in site 5 had the highest E value, which implies that this site must be monitored for potential future environmental and public health impacts (Amekudzie et al. 2011).
Concentrations of metals in the present study varied among those of other mangrove ecosystems worldwide (Table 5). The highest values for Cu, Ni, and Mn were recorded for Vaitarna estuary (India), the highest Zn concentrations in Sungai Puloh (Malaysia), and the highest Cr in Alibag (India). Differences in sediment metal concentrations are a function of the point source involved and the quantity of metal released. As indicated in the map of the study area (Fig. 1), the influence of human activities on metal and radionuclide contaminations must be taken into account, as the affected areas are located near industrial facilities and domestic dwellings. Such activities have been cited as primary causes of metal contamination in coastal zones (Duman and Kar 2012; Sundaray et al. 2012). Furthermore, rubber, fruit, and oil palm plantations located in the Pattani watershed may be minor sources of metals via their presence in phosphate fertilizers and fungicides. In this investigation, Cd and Pb concentrations exceeded world average values for shale (Table 1), as sediments in sites 4 and 5 had Cd concentrations >0.3 mg kg−1 and Pb concentrations >20 mg kg−1 in all sampling sites except for site 2 (Turekian and Wedepohl 1961). The average Pb content from all sampling sites (47.3 mg kg−1) exceeded world average values for shale. Considering the sediment quality guidelines as proposed by Luo et al. (2010), Pb concentrations were categorized as moderately-polluted for sediment. Lead and Cd, even in low concentrations, can be toxic to both aquatic plants and animals (Galas-Gorcher 1991). The remaining metal concentrations were within world average concentrations of shale as proposed by Turekian and Wedepohl (1961).
Recent research conducted by Paiva et al. (2016) reported higher 226Ra and 40K concentrations in mangrove sediments in Chico Science (410 and 24 Bq kg−1 for 40K and 226Ra, respectively) and Rio Formoso (851 and 21 Bq kg−1 for 40K and 226Ra, respectively) in Brazil, as compared to data for this study (Table 5). Furthermore, all radionuclides had lower concentrations compared with those of global soil values of 32 Bq kg−1 for 226Ra, 420 Bq kg−1 for 40K, and 45 Bq kg−1 for 232Th (UNSCEAR 2008). However, the average 232Th activity concentration in this study was higher than that found in Wadies mouth and Safaga along the Red Sea coast of Egypt. Radionuclide activity concentrations may differ due to the effects of industrial activities; however, other point sources must be considered as minor sources, including fertilizers for palm and rubber tree plantations, clam harvesting, excavation of sediments for a deep-water port, and runoff from abandoned and active mines in nearby locations. The mangrove areas addressed in this study are considered as having low radionuclide-contaminated sediments.
Conclusion
Metal and radionuclide contamination in sediment and seawater pose major hazards for coastal biota and the coastal environment. In this study, the quantities of heavy metals and radionuclides in mangrove sediment ecosystem were relatively low; thus, there is a minimal risk of food web bioaccumulation. The geoaccumulation index (I geo ) and enrichment factor (EF) were used to assess the effect of metal pollution in surface sediments due to anthropogenic inputs. Concentrations of several metals, especially Cd and Pb should be monitored often, as they are toxic to biota even at low accumulation rates in tissue. In addition, metal concentrations found in the sampling sites, particularly Pb and Cd in sites 1 and 5, respectively, were considered to result from anthropogenic activities (presumably mining, industrial, and agricultural activities). The higher levels of radionuclides may be due to movement of fine and particulate sediments in the water column; this is particularly likely in sites 4 and 5 which are located near the river mouth. It is believed that mining, industrial, and agricultural inputs served as key point sources of radionuclide contaminants in those sites. Furthermore, natural phenomena such as river flow, intense rainfall events, and sediment resuspension may increase disturbances on suspended sediments, and dissolved materials in the water column thereby increasing radionuclide concentrations. Most of the radiological risk assessment indices had relatively low values, except for the D value in sites 4 and 5 that exceeded the standard recommended value of 55 nGy h−1. It is important, therefore, that metal and radionuclide monitoring continues in these locations.
References
Abdel Ghani SA (2015) Trace metals in seawater, sediments and some fish species from Marsa Matrouh beaches in north-western Mediterranean coast, Egypt. Egypt J Aquat Res 41:145–154
Agbalagba EO, Onoja RA (2011) Evaluation of natural radioactivity in soil, sediment and water samples of Niger Delta (Biseni) flood plain lakes, Nigeria. J Environ Radioactiv 102:667–671
Allen SE, Grimshaw HM, Parkinson HM, Quarmby JA (1974) Chemical analysis of ecological materials. Blackwell, Oxford
Alamgir Miah MM, Miah H, Kamal M, Chowdhury MI, Rahmatullah M (2012) Natural radioactivity and associated dose rates in soil samples of Malnichera tea garden, Sylhet District of Bangladesh. J Nucl Part Phys 2:147–152
Amekudzie A, Emi-Reynolds G, Faanu A, Darko EO, Awudu AR, Adukpo O, Quaye LAN, Kpordzro R, Agyemang B, Ibrahim A (2011) Natural radioactivity concentrations and dose assessment in shore sediments along the coast of greater Accra, Ghana. World Appl Sci J 13:2338–2343
APHA, AWWA & WEF (American Public Health Association, American Water Works Association and Water Environment Federation) (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Black GR (1965) Bulk density: method of soil analysis. Monograph No. 9 Part I. American Society of Agronomy Inc, Washington, DC
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soil. Soil Sci 59:39–45
Chaiyarat R, Ngoendee M, Kruatrachue M (2013) Accumulation of Cd, Cu, Pb, and Zn in water, sediments, and mangrove crabs (Sesarma mederi) in the upper gulf of Thailand. ScienceAsia 39:376–383
Cheewasedtham W, Hasamoh M, Chairattanamanokorn W, Pengpara U, Suwan-in A, Krisonpornson B, Cheewasedtham C, Tjell JC (2003) The strategy plan for control and rehabilitation of lead contamination in Pattani River. Research meeting report, Prince of Songkla University, Pattani
Cristaldi M, Ieradi L, Mascanzoni D, Mattei T (1991) Environmental impact of the Chernobyl accident: mutagenesis in bank voles from Sweden. Int J Radiat Biol 59:31–40
Cuong DT, Bayen S, Wurl O, Subramanian K, Wong KKS, Sivasothi N, Obbard JP (2005) Heavy metal contamination in mangrove habitats of Singapore. Mar Pollut Bull 50:1713–1744
Duman F, Kar M (2012) Temporal variation of metals in water, sediment and tissues of the European Chup (Squalius cephalus L). Bull Environ Contam Toxicol 89:428–433
El-Taher A, Madkour HA (2011) Distribution and environmental impacts of metals and natural radionuclides in marine sediments in-front of different wadies mouth along the Egyptian Red Sea coast. Appl Radiat Isot 69:550–558
Fonseca B, Figueiredo H, Rodrigues J, Queiroz A, Tavares T (2011) Mobility of Cr, Pb, Cd, Cu and Zn in a loamy sand soil: a comparative study. Geoderma 164:232–237
Freitas AC, Alencar AS (2004) Gamma dose rates and distribution of natural radionuclides in sand beaches—Ilha Grande, southeastern Brazil. J Environ Radioactiv 75:211–223
Frontasyeva MV, Perelygin VP, Vater P (2001) Radionuclides and heavy metals in environment. Kluwer Academic Publishers, The Netherlands
Galas-Gorcher H (1991) Dietary intake of petricide residues: cadmium, mercury and lead. Food add 8:793–780
Ghrefat HA, Abu-Rukah Y, Rosen MA (2011) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain dam, Jordan. Environ Monit Assess 178:95–109
Harbison P (1986) Mangrove muds—a sink and source for trace metals. Mar Pollut Bull 17:273–276
Haddad HH (2012) The effect of heavy metals cadmium, chromium and iron accumulation in human eyes. Am J Anal Chem 3:710–713
Hossain MB, Marshall DJ, Venkatramanan S (2014) Sediment granulometry and organic matter content in the intertidal zone of the Sungai Brunei estuarine system, northwest coast of Borneo. Carpath J Earth Environ Sci 9:231–239
Hu N, Ding D, Li G, Zheng J, Li L, Zhao W, Wang Y (2014) Vegetation composition and 226Ra uptake by native plant species at a uranium mill tailings impoundment in South China. J Environ Radioactiv 129:100–106
Jahan I, Ali ML, Haydar MA, Ali MI, Paul D, Islam SMA (2016) Distribution of natural and probable artificial radioactivity in the sediment and water samples collected from low-lying areas of Savar industrial zones, Bangladesh. J Nucl Part Phys 6:25–34
Jibiri NN, Okeyode IC (2012) Evaluation of radiological hazards in the sediments of Ogun river, South-Western Nigeria. Radiat Phys Chem 81:103–112
IAEA (International Atomic Energy Agency) (1989) Measurement of radionuclides in food and the environment. Guide Book. Technical report series no. 295. International Atomic Energy Agency, Vienna
Ibrahiem NM, Abd El Ghani AH, Shawky SM, Ashraf EM, Farouk MA (1993) Measurement of radioactivity levels in soil in the Nile Delta and Middle Egypt. J Health Phys 64:620–627
ICARDA (International Center for Agricultural Research in the Dry Areas) (2001) Soil and plant analysis laboratory manual. ICARDA and NARC, Syria
Kaewtubtim P, Meeinkuirt W, Seepom S, Pichtel J (2016) Heavy metal phytoremediation potential of plant species in a mangrove ecosystem in Pattani Bay, Thailand. Appl Ecol Env Res 14:367–382
Kaewtubtim P, Phansuke P, Yisen P (2015) Quantitative analysis of radionuclide in sediments and determination of recent sedimentation rates by 137Cs and 210Pb dating techniques in Pattani Bay. Final Report. Prince of Songkla University, Pattani. (In Thai)
Karageorgis AP, Katsanevakis S, Kaberi H (2009) Use of enrichment factors for the assessment of heavy metal contamination in the sediments of Koumoundourou Lake, Greece. Water Air Soil Pollut 204:243–258
Livingston GK, Jensen RH, Silberstein EB, Hinnefeld JD, Pratt G, Bigbee WL, Langlois RG, Grant SG, Shukla R (1997) Radiobiological evaluation of immigrants from the vicinity of Chernobyl. Int J Radiat Biol 72:703–713
Luo W, Lu Y, Wang T, Hu W, Jiao W, Naile JE, Khim JS, Giesy JP (2010) Ecological risk assessment of arsenic and metals in sediments of coastal areas of northern Bohai and Yellow Seas, China. Ambio 39:367–375
Mansour AM, Askalany MS, Madkour HA, Assran BB (2013) Assessment and comparison of heavy-metal concentrations in marine sediments in view of tourism activities in Hurghada area, northern Red Sea, Egypt. Egypt J Aquat Res 39:91–103
Middelburg JJ, Nieuwenhuize J, Slim FJ, Ohowa B (1996) Sediment biogeochemistry in an east African mangrove forest (Gazi Bay, Kenya). Biogeochem 34:133–155
Mountouris A, Voutsas E, Tassios D (2002) Bioconcentration of heavy metals in aquatic enviromnents: the importance of bioavailability. Mar Pollut Bull 442:1136–1141
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geol J 2:108–118
Nematollahi MJ, Ebrahimi M (2015) Investigation of heavy metals origin in surface sediments of Gowatr Bay, SE Iran using geostatistical analyses. Geochem J 2
Nowrouzi M, Pourkhabbaz A (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve, Iran. Chem Spec Bioavailab 26:99–105
Okuku EO, Peter HK (2012) Choose of heavy metals pollution biomonitors: a critic of the method that uses sediments total metals concentrations as the benchmark. Int J Environ Res 6:313–322
Pahalawattaarachchi V, Purushothaman CS, Vennila A (2009) Metal phytoremediation potential of Rhizophora mucronata (Lam.). Indian J Mar Sci 38:178–183
Paiva JDS, Sousa EE, Farias EEG, Carmo AM, Souza EM, Franca EJ (2016) Natural radionuclides in mangrove soils from the state of Pernambuco, Brazil. J Radioanal Nucl Chem 307:883–889
Payne TE, Edis R (2012) Mobility of radionuclides in tropical soils and groundwater. In: Twining JR (ed) Radioactivity in the environment, vol 18. Elsevier, The Netherlands
Peters EC, Gassman NJ, Firman JC, Richmond RH, Power EA (1997) Ecotoxicology of tropical marine ecosystems. Environ Toxicol Chem 16(12):40
Ratheesh Kumar CS, Joseph MM, Gireesh Kumar TR, Renjith KR, Manju MN, Chandramohanakumar N (2010) Spatial variability and contamination of heavy metals in the inter-tidal systems of a tropical environment. Int J Environ Res 4:691–700
Saedeleer V, Cappuyns V, Cooman W, Swennen R (2010) Influence of major elements on heavy metal composition of river sediments. Geol Belg 13:257–268
Saleh IH, Hafez AF, Elanany NH, Motaweh HA, Naim MA (2007) Radiological study on soils, foodstuff and fertilizers in the Alexandria region, Egypt. Turkish J Eng Env Sci 31:9–17
Sekabira K, Oryem-Origa H, Basamba TA, Mutumba G, Kakudidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Tech 7:435–446
Seshan BRR, Natesan U, Deepthi K (2010) Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. Int J Environ Sci Tech 7:291–306
Simex SA, Helz GR (1981) Regional geochemistry of trace elements in Chesapeake Bay. Environ Geol 3:315–323
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43:246–253
Souza IC, Rocha LD, Morozesk M, Bonomo MM, Arrivabene HP, Duarte ID, Furlan LM, Monferrán MV, Mazik K, Elliott M, Matsumoto ST, Milanez CRD, Wunderlin DA, Fernandes MN (2015) Changes in bioaccumulation and translocation patterns between root and leafs of Avicennia schaueriana as adaptive response to different levels of metals in mangrove ecosystem. Mar Pollut Bull 94:176–184
Sowana A, Shrestha RP, Parkpian P, Pongquan S (2011) Influence of coastal land use on soil heavy-metal contamination in Pattani Bay, Thailand. J Coastal Res 27:252–262
Sparks DL (1996) Methods of soil analysis. Part 3. Chemical methods. Book series, No.5. Soil Science Society of America, Wisconsin
Sundaray SK, Nayak BB, Kanungo TK, Bhatta D (2012) Dynamics and quantification of dissolved heavy metals in the Mahanadi river estuarine system, India. Environ Monit Assess 184:1157–1179
Swennen C, Moolenbeek RG, Ruttanadakul N, Hobbelink H, Bekker H, Hajisamae S (2001) The mollusks of the southern gulf of Thailand. Thai Studies in Biodiversity, Bangkok
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285
Tchokosssa P, Makon TB, Nemba RM (2012) Assessment of radioactivity contents and associated risks in some soil used for agriculture and building materials in Camaroon. J Environ Prot 3:1571–1578
Tufail M, Hussain MY, Akram M (2011) Short communication primordial radionuclides contamination level in fertilized farms soils of Faisalabad-Pakistan. Soil Environ 30:88–94
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Uchida S (2007) Radionuclides in tropical and subtropical ecosystems. In: Shaw G (ed) Radioactivity in the terrestrial environment. Elsevier, Amsterdam, pp. 193–209
Udechukwu BE, Ismail A, Zulgifli SZ, Omar H (2015) Distribution, mobility, and pollution assessment of Cd, Cu, Ni, Pb, Zn, and Fe in intertidal surface sediments of Sg. Puloh mangrove estuary, Malaysia. Environ Sci Pollut Res 22:4242–4255
Uduma AU, Awagu EF (2013) Manganese as a reference element for the assessment of zinc enrichment and depletion in selected farming soils of Nigeria. Res J Environ Earth Sci 5:497–504
UNSCEAR (United Nation Scientific Committee on the Effects of Atomic Radiation) (2000) Sources and effects of ionizing radiation. Report to General Assembly, with scientific annexes. United Nations, New York
UNSCEAR (United Nation Scientific Committee on the Effects of Atomic Radiation) (2008) Sources and effects of ionizing radiation. Report to General Assembly, with scientific annexes. United Nations, New York
Uosif MAM, Issa S, Zakaly HMH, Hashim M, Tamam M (2016) The status of natural radioactivity and heavy metals pollution on marine sediments Red Sea coast, at Safaga, Egypt. J Nucl Phys Mater Sci Radiat Appl 3:191–222
Volvoikar SP, Nayak GN (2014) Reading source and processes with time from mangrove sedimentary environment of Vaitarna estuary, west coast of India. Indian J Mar Sci 43:1063–1075
Yanagisawa K, Takeda H, Miyamoto K, Fuma S (2000) Transfer of technetium from paddy soil to rice seedling. J Radioanal Nucl Chem 243:403–408
Yap CK, Chee MW, Shamarina S, Edward FB, Chew W, Tan SG (2011) Assessment of surface water quality in the Malaysian coastal waters by using multivariate analyses. Sains Malays 40:1053–1064
Yunus SM, Hamzah Z, Wood AKH, Saat A (2015) Natural radionuclides and heavy metals pollution in seawater at Kuala Langat coastal area. Malaysian J Anal Sci 19:766–774
Walkley A, Black CA (1934) An examination of degradation method for determining soil organic matter: a proposed modification of the chromic acid titration method. Soil Sci 37:29–35
Wu Q, Tam NFY, Leung JYS, Zhou X, Fu J, Yao B, Huang X, Xia L (2014) Ecological risk and pollution history of heavy metals in Nansha mangrove, South China. Ecotoxicol Environ Saf 104:143–151
Acknowledgement
Financial support was provided to SAT ASEAN Scholarship (No.5604) at Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Thailand. We are also grateful for the valuable comments from reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
The original publication of this paper contains a mistake. The sentence in the caption of Table 4 “Sharp (#) indicates the difference of 40K concentration between sites 4 and 5” should be removed and retained in the caption of Table 3. The original article was corrected.
An erratum to this article is available at http://dx.doi.org/10.1007/s11356-017-8694-1.
Rights and permissions
About this article
Cite this article
Kaewtubtim, P., Meeinkuirt, W., Seepom, S. et al. Occurrence of heavy metals and radionuclides in sediments and seawater in mangrove ecosystems in Pattani Bay, Thailand. Environ Sci Pollut Res 24, 7630–7639 (2017). https://doi.org/10.1007/s11356-016-8266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8266-9