Abstract
In this work, the presence of selected emerging contaminants has been investigated in two reservoirs, La Fe (LF) and Rio Grande (RG), which supply water to two drinking water treatment plants (DWTPs) of Medellin, one of the most populated cities of Colombia. An analytical method based on solid-phase extraction (SPE) of the sample followed by measurement by liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) was developed and validated for this purpose. Five monitoring campaigns were performed in each reservoir, collecting samples from 7 sites (LF) and 10 sites (RG) at 3 different depths of the water column. In addition, water samples entering in the DWTPs and treated water samples from these plans were also analysed for the selected compounds. Data from this work showed that parabens, UV filters and the pharmaceutical ibuprofen were commonly present in most of the reservoir samples. Thus, methyl paraben was detected in around 90% of the samples collected, while ibuprofen was found in around 60% of the samples. Water samples feeding the DWTPs also contained these two compounds, as well as benzophenone at low concentrations, which was in general agreement with the results from the reservoir samples. After treatment in the DWTPs, these three compounds were still present in the samples although at low concentrations (<40 ng/L), which evidenced that they were not completely removed after the conventional treatment applied. The potential effects of the presence of these compounds at the ppt levels in drinking water are still unknown. Further research is needed to evaluate the effect of chronic exposure to these compounds via consumption of drinking water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reservoirs are artificial lakes formed by the accumulation of water from rivers, creeks, streams and other natural sources, which are subject to the influence of its tributaries and associated watersheds. Storage in a reservoir increases the availability of water for various purposes, such as the generation of electric power, irrigation of crops, industrial use and supply of drinking water for human consumption, among others (Martinez-Zapata 2011). Specifically, the quality of drinking water is conditioned by the water of the reservoirs that feed the treatment plants producing drinking water. These systems are exposed to numerous natural and anthropogenic factors (Uhlmann et al. 2011). The impact of human activities on the aquatic environment has increased due to the growth in population and industrialization, which has intensified discharges from domestic, industrial and agricultural wastewater, the main sources of pollution.
Nowadays, there is a general concern on the presence of emerging contaminants in the water environment. Pharmaceuticals and personal care products (PPCPs) are of particular concern due to their wide use and their occurrence in treated wastewater and surface waters (Gracia-Lor et al. 2012a, b). These compounds are not regulated yet in water legislation and can be harmful for the aquatic ecosystem and become a hazard for human public health (Barceló and Petrovic 2007; Daughton 2004; Farré et al. 2008). These compounds are present in urban wastewater but are not efficiently removed in the wastewater treatment plants (WWTPs) that apply conventional treatments (Gracia-Lor et al. 2012b; Gros et al. 2010; Jelic et al. 2011; Lacey et al. 2008). In addition to the inefficient removal in WWTPs, some of them are also persistent and can be bioaccumulated in living organisms (Daughton and Ternes 1999; Yang et al. 2014; Zeng 2015). Within the group of PPCPs, many different families of compounds can be included, as those used in medicine, veterinary or agriculture, cosmetics, UV filters, fragrances and even additives in food (Farré et al. 2008; Martinez Bueno et al. 2016). Oppositely to most priority pollutants included in the current legislation, which have high toxicity and/or persistence in the environment, PPCPs do not commonly have these characteristics, but are continuously released in the aquatic ecosystems (via urban wastewater), where non-short-term detectable effects may occur (Muñoz et al. 2008), although long-term effects are still unknown. The fact that many PPCPs are not efficiently removed in the WWTPs means that these compounds can easily reach surface water (Dai et al. 2015; Kasprzyk-Hordern et al. 2008; Liu et al. 2015; Matamoros et al. 2012) and even drinking water (Boyd et al. 2003; Carmona et al. 2014; Kumar and Xagoraraki 2010; Sodré et al. 2010; Vulliet et al. 2011).
Colombia has many aquatic ecosystems providing water for human and industrial consumption. Specifically, Antioquia province is very rich in hydric resources, which are used for energy and drinking water production. This is the case of the reservoirs La Fe (LF) and Rio Grande (RG), which are the source of water supply of the two drinking water treatments plants (DWTPs) of Medellin: La Ayurá (LA) and Manantiales (MA). Although these reservoirs are located in low population areas, they can receive the impact of wastewater, both from urban and agricultural origins (Martinez and Peñuela 2013). Therefore, it becomes necessary to investigate the occurrence of emerging contaminants to have a better knowledge on the presence of these compounds in the reservoirs and eventually in drinking water.
The objective of this work was to investigate the presence of selected PPCPs in water samples from these two reservoirs and in samples entering in two DWTPs that use water from these reservoirs, as well as in treated drinking water samples. To this aim, an analytical method based on the use of liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS) was developed and validated for the determination of ibuprofen (IBU), diclofenac (DIC), clofibric acid (CFA), carbamazepine (CBZ), benzophenone (BZP), benzophenone-3 (BZP-3), methylparaben (MePB), ethylparaben (EtPB) and butylparaben (BuPB) in surface water. A solid-phase extraction (SPE) was applied to water samples, which allowed to obtain a pre-concentration factor of 200. Five monitoring campaigns were performed in each reservoir, collecting waters from seven sites (LF reservoir) and 10 sites (RG reservoir) at three different water column depths: sub-surface, photic zone limit and bottom. In addition, samples from the entrance and the exit of the DWTPs were also analysed for these compounds. Considering the general lack of data on PPCPs in Colombian surface waters, particularly in the Medellin area, this paper was conceived as a preliminary study to investigate the presence of some of the most consumed/used compounds in the area under study. Further studies will be required, including more PPCPs and metabolites/transformation products (TPs), to have a better knowledge of the issue treated and to suggest subsequent actions to protect the aquatic environment, if needed.
Experimental
Regents and chemicals
Reference standards of ibuprofen (99%), diclofenac (99%), clofibric acid (99.5%) and carbamazepine (99.5%) were from Dr. Ehrenstorfer (Germany); benzophenone (99.4%), methylparaben (99.5%), ethylparaben (99.5%) and butylparaben (99.5%) from Chemservice (West Chester, USA); and benzophenone-3 (100%) from Accustandard (New Haven, USA). Isotope-labelled internal standard (ILIS) of ibuprofen-d3 (IBU-d3) was from Toronto Research Chemicals TRC (Toronto–Ontario, Canada) and ethylparaben-d4 (EtPB-d4) from CDN Isotopes (Quebec, Canada). LC–MS-grade methanol was from Merck (NJ, USA) and acetonitrile from Honeywell B&J Brand (USA). HPLC-grade water was obtained by purifying demineralized water in a Milli-Q Gradient A10 (Millipore, Bedford, MA, USA). Formic acid 98–100% was from Merck (NJ, USA).
Individual stock solutions of 1000 mg/L of both reference standards and ILIS were prepared by dissolving 10 mg in 10 mL MeOH. From these individual solutions, a mix solution of 100 mg/L containing the PPCPs was prepared by dilution in MeOH. Working mix solutions of 10, 1 and 0.1 mg/L PPCPs were prepared in MeOH from the 100 mg/L mix solution. A mix solution of ILIS of 2 mg/L IBU-d3 and 10 mg/L EtPB-d4 was also prepared in MEOH from a 100 mg/L solution.
Sample extraction was made by SPE using Oasis HLB cartridges (60 mg) from Waters Corp. (Milford, MA, USA).
Liquid chromatography
An Acquity UPLC system (Waters Corp., Milford, MA, USA) was used, equipped with a quaternary solvent manager and a sample manager. Chromatographic separation was performed using an Acquity UPLC BEH C18 column, 2.1 × 50 mm, 1.7 μm (Waters), at a flow rate of 0.3 mL/min. The column was kept at 40 °C, and the sample manager was maintained at 5 °C. The mobile phase consisted of water with a 0.01% HCOOH/methanol gradient. The methanol percentage was changed linearly as follows: 0 min, 10%; 9 min, 90%; 9.5 min, 90%; 10.5 min, 100%; 10.6 min, 100%; and 11.7 min, 10%. Analysis run time was 12.7 min. The sample extract injection was 20 μL.
Mass spectrometry
An Acquity UPLC system was coupled to a triple quadrupole (TQD) mass spectrometer with an orthogonal Z-spray-electrospray (ESI) (Waters Corp., Milford, MA, USA). Both the drying gas and the nebulising gas were nitrogen generated from pressurized air in a N2 LC–MS nitrogen generator (Peak Scientific, EE. UU). The cone gas and the desolvation gas flows were set at 80 and 800 L/h, respectively. For operation in MS/MS mode, the collision gas was Argon 99.995% at 2 × 10−3 mbar in a T-Wave collision cell. Capillary voltages of −2.8 kV (negative ionization mode) and 3.0 kV (positive ionization mode) were applied. The interface temperature was set to 450 °C, and the source temperature to 150 °C. Dwell times of 0.1 s were selected. Masslynx 4.1 (Micromass, Manchester, UK) software was used to process the quantitative data.
The studied compounds were measured in ESI positive and/or ESI negative mode during the same injection acquiring two MS/MS transitions per compound. The acquisition of two transitions, of which the most sensitive was used for quantification (Q) and the other was used for confirmation (q), ensured the appropriate identification of the analytes in the samples. Only one transition was available for IBU 205 > 161 (Q) due to the poor fragmentation of this compound under the conditions applied. The transitions selected and the optimized conditions of cone voltage and collision energy are shown in Table 1.
Sample procedure
Water samples were firstly centrifuged (Boeco Centrifuge U-320R, Germany) for 10 min at 4500 rpm to separate particulate matter (filtration was discarded to avoid potential losses in the filter). Then, SPE was applied within 48 h after samples collection. When this was not possible, the centrifuged samples were stored in the freezer at −20 °C (Revco-Thermo Scientific). Conditioning of the SPE cartridges was made with 3 mL MeOH and 3 mL ultrapure water. After conditioning, 200 mL of the water sample (adding 50 μL of the mix ILIS solution (2 mg/L IBU-d3, 10 mg/L EtPB-d4)) was loaded into the SPE cartridge at a flow of 1–2 mL/min. The cartridges were air dried, and analytes were eluted with 5 mL MeOH. The eluate was evaporated under a gentle nitrogen stream and the residue was reconstituted 1 mL MeOH–H2O (10:90, v/v). Finally, 20 μL of the extract was injected into the UHPLC–MS/MS system under conditions indicated in Table 1.
Quantification was made with standards prepared in solvent, using relative responses analyte/ILIS or absolute responses, depending on whether ILIS was used for correction or not. All compounds measured in negative mode were quantified using ILIS for matrix effects correction. Thus, parabens (MePB, EtPB and BuPB) were quantified using EtPB-d4, while IBU was quantified using IBU-d3. After a previous study on the best ILIS to be used for matrix effects correction, EtPB-d4 was selected for quantification of DIC and CFA. The three compounds measured in positive mode (CBZ, BZP, BZP-3) were quantified by external calibration using standards in solvent without ILIS due to the unavailability of appropriate ILIS in our laboratory. In any case, our data did not show relevant matrix effects for these compounds in the samples tested.
Study area and sample collection
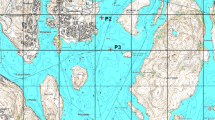
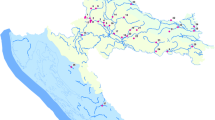
The present study was carried out in two reservoirs (RG and LF) that are used for drinking water supply to the city of Medellin after the water being treated in two DWTPs. Samples were taken from three depths: the sub-surface, photic zone limit and reservoir bottom in order to evaluate the presence of pollutants along the overall water column. Ten sampling locations were selected in reservoir RG (Fig. 1) and seven locations in reservoir LF (Fig. 2). Additionally, water samples were also collected in each tributary at locations before the entry to the each reservoir (Fig. 1, numbers 12, 15, 17 and 19; Fig. 2, numbers 8, 9, 10 and 11). The samples were collected using a Schindler bottle. An aliquot of 200 mL of each sample was transferred to amber glass bottles that were previously cleaned and heated to 450 °C for 8 h to remove any presence of organic matter. Then, they were transported to the laboratory under cooled conditions (4 °C).
Influent and effluent samples from the two DWTPs (MA fed with water from reservoir RG and LA fed with water from reservoir LF) consisted on 24-h composite samples. The effluent samples (i.e. drinking water supplied to Medellin) were amended with sodium thiosulphate immediately after collection to eliminate residual chlorine (Martinez and Peñuela 2013).
The monitoring plan for reservoirs, tributary rivers and DWTPs is summarized in Table 1SI (Supplementary Information).
Results and discussion
The selection of the compounds under study was made based on the main human activities in the area and on previous data reported in a preliminary study performed on the presence of PPCPs (Gracia-Lor et al. 2012a). The area around reservoir LF and their tributaries has experimented an increased urbanization process and recreational events, including the touristic park Los Salados. Reservoir RG is more affected by agricultural activities, and several farms (mainly cattle and pigs) are located in the nearby areas. Despite the presumably presence of pesticides, because of the agricultural activities, we focused our study on pharmaceuticals. Thus, four compounds were selected such as ibuprofen and diclofenac, which are analgesic/anti-inflammatory drugs commonly consumed around the world (Mendez-Arriaga et al. 2010; Scheurell et al. 2009) and specifically in Colombia; they are easily accessible for consumers and are included in the Colombian national strategy for Human Health. Carbamazepine is used for epilepsy treatment and is frequently found in natural and waste waters (Bade et al. 2015; Hernández et al. 2015; Sun et al. 2013) and clofibric acid, which is used to decrease cholesterol and triglyceride levels in blood (Saravanan et al. 2011).
A preliminary study made by our own group in collaboration with the University Jaume I of Castellon (Gracia-Lor et al. 2012a) revealed the presence of several personal care products in waters from these two reservoirs, specifically benzophenones, commonly used in UV filters and other products of wide use. Benzophenone and benzophenone-3 were identified in several of the samples. Parabens, used as preservatives in cosmetics and even as additives in food, were also found in the water samples (methyl paraben, ethyl paraben and butylparaben).
Altogether, nine PPCPs were selected for this study, four of them being drugs of wide consumption and/or frequent detection in waters and five being personal care products.
Analytical methodology
The method linearity was studied in the range 1–100 μg/L. Calibration curves showed satisfactory correlation coefficients (greater than 0.99), and residuals were lower than 30%. The method accuracy was evaluated in surface water spiked (n = 5) at three concentration levels (0.05, 0.25 and 0.45 μg/L). The results were satisfactory in terms of precision, expressed as relative standard deviation (RSD) (lower than 20%), and accuracy, expressed as percentage recovery (between 70 and 120%) for the analysis of PPCPs under study (Table 2).
Limits of detection (LOD) were calculated as a function of the standard deviation for a 99% confidence level (Brown and Berthouex 2002; Martinez-Zapata 2011) (n = 7) in a sample spiked at 0.25 μg/L. Limits of quantification (LOQ) were taken as 3.3 × LOD (Table 2).
Confirmation of positive findings was carried out by calculating the peak area ratios between the quantification (Q) and confirmation (q) transitions. The finding was considered as true positive when the experimental ion–ratio was within the tolerance range (Gracia-Lor et al. 2012b), and the retention time in the sample within ±2.5% when compared with a reference standard (see Table 1 for Q/q ratios empirically obtained for reference standards).
A problem occurred in the determination of BZP, which seemed to be related to a contamination of the LC–MS/MS system used for analyses. For this reason, data were available only for samples collected until October 2012. From then on, although a peak clearly differentiated from the blank was observed for several of the samples (suggesting that the sample was positive for this compound), we could not accurately quantify this analyte.
Reservoir samples
The validated method was applied to water samples collected from reservoirs and the drinking water treatment plants. In every sequence of analysis (around 20 samples), a calibration curve was injected. Quality control (QC) samples (i.e. blank samples fortified at 0.25 μg/L with the selected PPCPs) were also analysed in every sequence in order to test the robustness and applicability of the method to the samples under study. Confirmation of positive findings was based on the agreement of Q/q ratios and chromatographic retention times between samples and reference standards (see the “Analytical methodology” section).
Reservoir Rio Grande (RG)
The highest percentage of positive samples corresponded to BZP, MePB and IBU, which were detected in many of the samples analysed (Table 3). In relation to DIC, it was only found in the samples from April (11% positives) and November (3% positives), always at levels below LOQ. BZP-3 was only detected in samples from April with an average concentration of 238 ng/L, and EtPB was scarcely found, with only 4% of positives in the monitoring of April (all <LOQ). BuPB, CFA and CBZ were not detected in any of the samples from this reservoir. In general, not a clear trend was observed in the concentrations of these compounds as a function of the sampling date.
Concentrations of MePB, and mainly IBU, seemed to slightly increase along the depth of the water column in most of sampling stations (Fig. 1SI), which would imply that these compounds are sorbed onto the sediments (IBU, log K ow 3.97; MePB, log K ow 4.5) (Fatta-Kassinos et al. 2013; Vergili 2013). In addition, humic material tends to accumulate into the bottom, which might facilitate the complexation with the contaminants (Hincapie-Upegui 2014) that would be associated to the sediments and/or to the bottom of the water column.
We did not observe a clear trend in the spatial distribution of MePB in the different stations monitored in this reservoir, although a notable increase in concentration in the bottom samples was found in stations 7 and 8 in October 2012 (Fig. 1SI), a fact that could not be easily explainable. For IBU, the trend of the concentrations was more uniform in sub-surface, photic limit zone and bottom samples. In general, concentrations in station 1, which was near the tributary Rio Chico (number 12), were a bit higher and then decreased along stations 2 and 3 (see map shown in Fig. 1). However, the possible influence of the other tributaries was not so evident in those stations that might be more affected, i.e. stations 6, 4 and 10.
Additionally to samples from reservoir RG, water samples were also collected in each tributary at locations just before the entry to the reservoir (Fig. 1) in order to evaluate whether they were the main source of pollution of the reservoir. Concentration data found in tributaries 12, 15, 17 and 19, in the two monitoring campaigns, are shown in Table 4.
In the tributaries, similarly to the reservoir samples, the predominant compounds were BZP, MePB and IBU, although BZP-3 was also found at notable concentrations in samples collected in April. The presence of IBU in tributary 12 (Rio Chico) in both monitoring campaigns may explain the presence of this compound in reservoir samples of station 1, located near the entry of this tributary. From sampling station 1 onwards, IBU concentrations slightly decreased along the distance (stations 2 and 3).
BZP and MePB were present in all tributaries, which might explain the presence of these compounds in the reservoir. Similarly to the results found in the reservoir, BZP-3 was only found in the samples collected in April 2012. Although no relevant differences were observed in general between the tributaries in terms of analyte concentrations, the tributary 15 was the only one where the three parabens included in this study, BuPB, EtPB and MePB, were found, with the latter being present at relatively high concentrations (242 ng/L).
Reservoir La Fe (LF)
In general, the behaviour and concentrations found in this reservoir were similar to those found in reservoir RG. The higher concentrations were commonly observed in the first monitoring, June 2012, although no significant variations were observed along the different periods of the year.
The compounds most detected in this reservoir were again BZP, MePB and IBU, although other parabens (BuPB, EtPB) were also found in several of the samples at detectable concentrations (mostly below 60 ng/L) (Table 5).
Additionally, the most important tributaries (numbers 8, 9, 10, 11; see map in Fig. 2) were also monitored in October and December 2012 and February 2013 (Table 6). The PPCP concentrations in these tributaries were lower than tributaries from the other reservoir (see Table 4), as only IBU and MePB were found at detectable concentrations (no data available for BZP). In all positives, concentrations were below 80 ng/L. This fact illustrates that, at least for the compounds selected in this study, the tributaries seemed not be an important source of pollution in the reservoir.
No clear trends were observed in the spatial distribution of the compounds in this reservoir (Fig. 2SI) and, as stated above, the influence of the tributaries was not perceived.
The presence of PPCPs in urban wastewater has been widely reported in the scientific literature, at concentrations that are sometimes well above 1000 ng/L. High levels can be found even in effluent wastewater, as many of these compounds are not efficiently removed in WWTPs (Alidina et al. 2014; Anumol and Snyder 2015; Carmona et al. 2014; Gracia-Lor et al. 2012b; Gros et al. 2010; Jelic et al. 2011; Lacey et al. 2008; Liu and Wong 2013). This fact explains that a notable number of PPCPs are present in surface water, including the compounds studied in this work.
Specifically, MePB has been found in wide concentrations ranges, with average values near those reported in our work for reservoir samples (around 100 ng/L) (Carmona et al. 2014; Kasprzyk-Hordern et al. 2008; Peng et al. 2008). Ibuprofen is one of the compounds more frequently detected in surface water around the world (Fernández et al. 2010; Kasprzyk-Hordern et al. 2008; Lindholm-Lehto et al. 2016; Liu et al. 2015; Liu and Wong 2013; Matamoros et al. 2012; Peng et al. 2008; Tran et al. 2014; Wang et al. 2010). Concentrations reported are commonly higher than those found in reservoirs RG and LF. Thus, values up to 2700 and 1800 ng/L have been reported for IBU in Spain and Vietnam, respectively (Fernández et al. 2010; Tran et al. 2014), which are clearly higher than in our work (≤60 ng/L).
Less data exist on BZP levels in surface water, and most studies are focused on BZP-3 (Kim and Choi 2014). The presence of BZP has been reported in surface water from California, with maximum concentrations of 5.1 ng/L (Alvarez et al. 2014). Although not detected in our study, BZP-3 is commonly found in surface water, at levels up to 125 ng/L (Kim and Choi 2014).
In the present work, several compounds selected (DIC, CBZ, CFA, EtPB, BuPB) were not detected in the reservoir samples. However, their presence in surface water has been reported in several previous studies. For example, CBZ has been found at low concentrations in Finland (1.2 ng/L) (Lindholm-Lehto et al. 2016) and at much higher concentrations in Vietnam (5110 ng/L) (Tran et al. 2014). DIC is commonly reported in surface water, at concentrations from 10 ng/L, in Switzerland, to 700 or 800 ng/L, in China or Spain (Fernández et al. 2010; Wang et al. 2010). Parabens (Carmona et al. 2014; Esteban et al. 2014; Kasprzyk-Hordern et al. 2008) and CFA are usually present at low levels in fresh waters (around or below 50 ng/L) (Gros et al. 2006; Hernando et al. 2006; Kasprzyk-Hordern et al. 2008; Öllers et al. 2001; Wang et al. 2010).
Data from above show that the compounds selected in our study are commonly present in fresh water from many countries around the world. In general, the levels reported in our work for reservoir water and tributaries samples are lower than reported in the majority of studies performed in other countries. However, more data and extensive monitoring programs would be necessary to confirm this fact.
Drinking water treatment plant samples
Four monitoring campaigns were carried out in two DWTPs: Los Manantiales (MA) and La Ayurá (Table 1SI). The DWTP of MA receives water from the reservoir RG, and the catchment is located at station 4 (Fig. 1), while DWTP of LA receives water from reservoir LF, with the catchment being located at station 3 (Fig. 2).
The results obtained for analyses of influent water (water from the reservoir collected in the catchment station) and in effluent water (treated water already used for drinking water supply) are shown in Table 7. The compounds IBU, MePB and BZP were the most frequently detected, similarly to the reservoir and tributary samples. These were present in both influent and treated water of the two drinking water treatment plants. The concentrations found in the influents were, in general, consistent with those of the reservoir samples, specifically from stations 4 (RG) and 3 (LF), where the catchments are located. This supports the observation that these compounds are present in the reservoir water and enter in the DWTP.
In addition to the three major contaminants, BZP-3, DIC and BuPB were occasionally detected in the influent of MA, although at concentrations <30 ng/L. The first two compounds were also occasionally found in the RG reservoir samples in some monitoring campaigns, but BuPB was never found in the reservoir, a fact that needs further research for confirmation. A similar situation was observed in influent samples from LA, where in addition to the three major compounds, BZP-3 and DIC were occasionally detected although at low concentrations (<30 ng/L), while they were not detected in the LF reservoir samples.
The comparison of data from influent and effluent samples from DWTPs showed that PPCPs present in the catchment were not completely removed in the conventional treatment applied. For example, the removal efficiency for BZP, MePB and IBU was found to be between 30–60% (BZP), 20–60% (MePB) and 0–50% (IBU) in MA DWTP, while it was much lower in the LA DWTP (Table 7). These results are in agreement with previous data reported on drinking water treatment (de Jesus Gaffney et al. 2014; Padhye et al. 2014). Both DWTPs apply conventional treatments, based on coagulation, flocculation, sedimentation and filtration, which would be expected to efficiently remove hydrophobic compounds of high molecular weight, less soluble in water, but not necessarily emerging contaminants that are more polar in nature. For this reason, polar emerging compounds, as the majority of PPCPs, when present in water at low concentrations, would remain in the aqueous phase and would not be removed along the coagulation–flocculation process, leading to low removal efficiencies in the treatment plant (Rodríguez-Paniagua 2015).
In addition, both DWTPs also apply chlorination, a process that allows to eliminate the great majority of organic compounds, although not all of them. It has been reported that the use of oxidizing agents is appropriate for removal of emerging contaminants in drinking water treatment (Huber et al. 2005); however, the kinetics of the process can be slow and other by-products (in some occasions of unknown effects on human health) can be formed (Rodríguez-Paniagua 2015). According to our data, it seems that the low chlorine doses used in the DWTPs were not sufficient to completely remove some PPCPs. Therefore, it would be necessary to improve the treatment in order to remove trace levels of PPCPs in drinking water, for example, using higher doses of chlorine or implementing tertiary treatments, such as advanced oxidation systems, that are more efficient to this aim.
In any case, the levels found in drinking water (effluent already treated and used for drinking water supply) were always below 50 ng/L, with the only exception of MePB in the sample collected in MA in September 2012 (80 ng/L) (Table 7). Although concentrations were below 0.1 μg/L (the reference value used by default for pesticide residues in drinking water), no sufficient data exist to assess the potential harmful effects on human health. Therefore, more research is required in the near future on this issue.
Confirmation of positive findings was based on the agreement of Q/q ratios and chromatographic retention times between samples and reference standards (see the “Analytical methodology” section). Similarly to the reservoir and tributary samples, QCs (drinking water samples fortified at 0.25 μg/L with the selected PPCPs) were analysed in every sequence in order to test the robustness and applicability of the method.
As illustrative example, Fig. 3 shows the LC–MS/MS chromatograms for an effluent sample from Manantiales DWTP, where BZP and MePB were found and their identity confirmed by Rt and ion/ratio agreement with the reference standard.
Our findings are in the line of previous data reported on occurrence of PPCPs in drinking water around the world. It is not rare to find some of these compounds in drinking water at the few nanogrammes per litre level. Specifically, the compounds selected in our work have been found in drinking waters, as summarized in a recent review (Bialk-Bielinska et al. 2016), where maximal concentrations reported for diclofenac, carbamazepine and ibuprofen are given. Thus, IBU was frequently reported in DW from USA, Japan, China, Portugal and Spain, typically at concentrations between 10 and 30 ng/L. DIC was also reported in DW from Japan and China at maximal concentrations of 16 and 10 ng/L, respectively, and also in Spain, at average concentrations of 18 ng/L (Bialk-Bielinska et al. 2016; Carmona et al. 2014). However, it seems that this compound was not present in finished water, after disinfection (Cai et al. 2014). Contrarily to our work, where CBZ was not detected in DW, this compound was frequently found at maximal concentrations between 2 and 5.6 ng/L, in DW from Canada, Spain and USA, and between 14 and 35 ng/L in Portugal, Japan and China. CBZ was also found in finished water subjected to disinfection in all the five samples analysed from Beijing, China, at concentrations between 0.4 and 1.2 ng/L (Cai et al. 2014).
There are very few data on the presence of parabens in drinking water (Haman et al. 2015). In the USA, MePB was never detected in a study on drinking water sources and treated drinking water (Loraine and Pettigrove 2006). However, in Spain, MePB (17 and 40 ng/L) was quantified in tap water (Blanco et al. 2009; Casas Ferreira et al. 2011). These two studies did not observe any EtPB, PrPB or BuPB. Another recent Spanish study (Carmona et al. 2014) demonstrated the presence of MePB (12 ng/L), PrPB (9 ng/L) and BuPB (28 ng/L), but not EtPB. In our work, MePB was also found at concentrations between 21 and 80 ng/L, while EtPB was not detected, and BuPB was only once at 7 ng/L.
Although the presence of the UV filters benzophenones in effluent wastewater and surface water has been reported, there are very little studies on drinking water. BZP-4 was found in DW from Galicia, Spain, at maximum concentrations of 62 ng/L (Rodil et al. 2012), close to the levels reported in our work (around 40 ng/L). Benzophenone was also reported in an early study made in the USA in drinking water at concentrations above 100 ng/L (Loraine and Pettigrove 2006).
The regulations on PPCPs in drinking water are still insufficient around the world, and no maximum levels have been established in most countries yet. Taking as a reference the maximum level allowed for pesticides in drinking water according to the European Directive (European Union 1998) (0.1 μg/L), the concentrations found for the PPCPs under study were lower than this value. However, it is not possible to fully evaluate the potential hazard of PPCPs at these low levels for public health due to the lack of long-term exposition risk data for these compounds. However, the application of the precautionary principle seems necessary, and measurements should be taken to efficiently remove PPCPs in the DWTPs.
The research presented in this paper can be considered as a preliminary study that reveals the occurrence of selected PPCPs in reservoirs of Medellin and their potential presence in drinking water produced from these reservoirs. Subsequent actions will be needed in the near future, including ambitious monitoring programs where the number of compounds should be notably increased. In addition, selected metabolites/TPs should be also included in the monitoring, because most pharmaceuticals are excreted as metabolites more than as the parent compound and can also suffer degradation/transformation processes in the aquatic environment. Therefore, the occurrence of TPs can be more frequent than that of the parent compounds (Boix et al. 2016; Ibáñez et al. 2016).
Conclusions
In this work, several PPCPs have been monitored in two important reservoirs near Medellin, as well as in the main tributaries of these reservoirs. Our data showed that some preservatives (parabens, mainly MePB); UV filters, as BZPs and BZP-3; and the pharmaceutical IBU were present in most of the samples analysed. The presence of these compounds in the tributaries may explain their detection in the reservoir water samples, although other sources of pollution should not be discarded.
As reservoir water is used for catchment of drinking water treatment plants, these compounds (mainly MePB, IBU and BZP) were also present in the influent of the DWTPs. Analysis of treated water showed that these compounds were not completely removed after the conventional treatments applied for drink water supply (coagulation, flocculation, sedimentation, filtration and subsequent disinfection by chlorination). This reveals that conventional treatments, which had been designed to remove the great majority of organic matter, are not efficient enough to remove polar contaminants present at low concentrations in the influent water, as occurs for most of emerging contaminants.
The PPCP concentrations found in drinking water were all below 80 ng/L (e.g. below 0.1 μg/L, which is the maximum concentration allowed for pesticides—a group of contaminants subjected to strict regulation in drinking water). Insufficient information exists to assess the potential harmful effects of these low levels on human health. It seems necessary that the application of the precautionary principle until more data is available to properly evaluate the risks for the population. More research is required in the near future considering a higher number of PPCPs, including the most consumed by population, because synergic effects have to be taken into account. The presence of metabolites and transformation products is another issue of concern, as many pharmaceuticals are hardly excreted as parent compound; therefore, the presence of some parent pharmaceuticals may only be the visible part of the iceberg of this complex and of current concern issue.
References
Alidina M, Hoppe-Jones C, Yoon M, Hamadeh AF, Li D, Drewes JE (2014) The occurrence of emerging trace organic chemicals in wastewater effluents in Saudi Arabia. Sci Total Environ 478:152–162
Alvarez D a, Maruya K a, Dodder NG, Lao W, Furlong ET, Smalling KL (2014) Occurrence of contaminants of emerging concern along the California coast (2009-10) using passive sampling devices. Mar Pollut Bull 81:347–354
Anumol T, Snyder SA (2015) Rapid analysis of trace organic compounds in water by automated online solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. Talanta 132:77–86
Bade R, Rousis NI, Bijlsma L, Gracia-Lor E, Castiglioni S, Sancho JV, Hernandez F (2015) Screening of pharmaceuticals and illicit drugs in wastewater and surface waters of Spain and Italy by high resolution mass spectrometry using UHPLC-QTOF MS and LC-LTQ-Orbitrap MS. Anal Bional Chem 407:8979–8988
Barceló D, Petrovic M (2007) Pharmaceuticals and personal care products (PPCPs) in the environment. Anal Bioanal Chem 387:1141–1142
Bialk-Bielinska A, Kumirska J, Borecka M, Caban M, Paszkiewicz M, Pazdro K, Stepnowski P (2016) Selected analytical challenges in the determination of pharmaceuticals in drinking/marine waters and soil/sediment samples. J Pharm Biomed Anal 121:271–296
Blanco E, Casais MC, Mejuto MC, Cela R (2009) Combination of off-line solid-phase extraction and on-column sample stacking for sensitive determination of parabens and p-hydroxybenzoic acid in waters by non-aqueous capillary electrophoresis. Anal Chim Acta 647:104–111
Boix C, Ibáñez M, Bagnati R, Zuccato E, Sancho JV, Hernández F, Castiglioni S (2016) High resolution mass spectrometry to investigate omeprazole and venlafaxine metabolites in wastewater. J Hazard Mater 302:332–340
Boyd GR, Reemtsma H, Grimm DA, Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario. Canada Sci Total Env 311:135–149
Brown LC, Berthouex PM (2002) Statistics for environmental engineers. Limit of detection, 2nd edn. CRC Press, pp 512
Cai M-Q, Wang R, Feng L, Zhang L-Q (2014) Determination of selected pharmaceuticals in tap water and drinking water treatment plant by high-performance liquid chromatography-triple quadrupole mass spectrometer in Beijing, China. Env. Sci. Pollut Res 22:1854–1867
Carmona E, Vicente A, Picó Y (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci Total Env 484:53–63
Casas Ferreira AM, Möder M, Fernández Laespada ME (2011) GC-MS determination of parabens, triclosan and methyl triclosan in water by in situ derivatisation and stir-bar sorptive extraction. Anal Bional Chem 399:945–953
Dai G, Wang B, Huang J, Dong R, Deng S, Yu G (2015) Occurrence and source apportionment of pharmaceuticals and personal care products in the Beiyun River of Beijing, China. Chemosphere 119:1033–1039
Daughton C, Ternes T (1999) Special report: pharmaceuticals and personal care products in the environment: agents of subtle change? Env Heal Perspec 107:907–938
Daughton CG (2004) Non-regulated water contaminants: emerging research. Env Impact Asses 24:711–732
de Jesus Gaffney V, Almeida CMM, Rodrigues A, Ferreira E, Benoliel MJ, Cardoso VV (2014) Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res 2:1–10
Esteban S, Gorga M, Petrovic M, González-Alonso S, Barceló D, Valcárcel Y (2014) Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci Total Env 466–467:939–951
European Union (1998) Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Communities L330:32–54
Farré M l, Pérez S, Kantiani L, Barceló D (2008) Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC 27:991–1007
Fatta-Kassinos D, Bester K, Kümmerer K (eds) (2013) Xenobiotics in the urban water cycle, mass flows, environmental processes, mitigation and treatment strategies. Environmental Pollution 16
Fernández C, González-Doncel M, Pro J, Carbonell G, Tarazona JV (2010) Occurrence of pharmaceutically active compounds in surface waters of the Henares-Jarama-Tajo river system (Madrid, Spain) and a potential risk characterization. Sci Total Env. 408:543–551
Gracia-Lor E, Martinez M, Sancho JV, Peñuela G, Hernandez F (2012a) Multi-class determination of personal care products and pharmaceuticals in environmental and wastewater samples by ultra-high performance liquid-chromatography-tandem mass spectrometry. Talanta 99:1011–1023
Gracia-Lor E, Sancho JV, Serrano R, Hernandez F (2012b) Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 87:453–462
Gros M, Petrovic M, Barcelo D (2006) Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 70:678–690
Gros M, Petrovic M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Enviroment Int 36:15–26
Haman C, Dauchy X, Rosin C, Munoz JF (2015) Occurrence, fate and behavior of parabens in aquatic environments: a review. Water Res 68:1–11
Hernández F, Ibáñez M, Botero-Coy A-M, Bade R, Bustos-López MC, Rincón J, Moncayo A, Bijlsma L (2015) LC-QTOF MS screening of more than 1,000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogotá, Colombia. Anal Bioanal Chem 407:6405–6416
Hernando MD, Heath E, Petrovic M, Barceló D (2006) Trace-level determination of pharmaceutical residues by LC-MS/MS in natural and treated waters. A pilot-survey study. Anal Bioanal Chem 385:985–991
Hincapie-Upegui DA (2014) Caracterización espacio-temporal y a nivel molecular de la materia orgánica disuelta en los embalses abastecedores de agua potable de los embalses Riogrande II y La Fe—Antioquia. Universidad de Antioquia, Colombia
Huber MM, Korhonen S, Ternes TA, Von Gunten U (2005) Oxidation of pharmaceuticals during water treatment with chlorine dioxide. Water Res 39:3607–3617
Ibáñez M, Borova V, Boix C, Aalizadeh R, Bade R, Thomaidis NS, Hernández F (2016) UHPLC-QTOF MS screening of pharmaceuticals and their metabolites in treated wastewater samples from Athens. J Hazard Mater. doi:10.1016/j.jhazmat.2016.03.078
Jelic A, Gros M, Ginebreda A, Cespedes-Sánchez R, Ventura F, Petrovic M, Barceló D (2011) Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res 45:1165–1176
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res 42:3498–3518
Kim S, Choi K (2014) Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review. Env Int 70:143–157
Kumar A, Xagoraraki I (2010) Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: a proposed ranking system. Sci Total Environ 408:5972–5989
Lacey C, McMahon G, Bones J, Barron L, Morrissey A, Tobin JM (2008) An LC–MS method for the determination of pharmaceutical compounds in wastewater treatment plant influent and effluent samples. Talanta 75:1089–1097
Lindholm-Lehto PC, Ahkola HSJ, Knuutinen JS, Herve SH (2016) Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland. Env. Sci Pollut Res 23:7985–7997
Liu J, Lu G, Xie Z, Zhang Z, Li S, Yan Z (2015) Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci Total Env 511:54–62
Liu J-L, Wong M-H (2013) Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Env int 59:208–224
Loraine GA, Pettigrove ME (2006) Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in southern California. Env Sci Technol 40:687–695
Martinez M, Peñuela GA (2013) Analysis of triclosan and 4n-nonylphenol in Colombian reservoir water by gas chromatography-mass spectrometry. Water Env J 27:387–395
Martinez Bueno MJ, Herrera S, Munaron D, Boillot C, Fenet H, Chiron S, Gómez E (2016) POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment. Env. Sci Pollut Res 23:5019–5029
Martinez-Zapata M (2011) Estudio de la presencia y distribución de los contaminantes emergentes Triclosán y Nonilfenol en los embalses Riogrande II, La Fe, las plantas de potabilización Manantiales, La Ayurá y su degradación a Nivel de laboratorio. Universidad de Antioquia.
Matamoros V, Arias CA, Nguyen LX, Salvadó V, Brix H (2012) Occurrence and behavior of emerging contaminants in surface water and a restored wetland. Chemosphere 88:1083–1089
Mendez-Arriaga F, Esplugas S, Giménez J (2010) Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res 44:589–595
Muñoz I, Gomez JM, Molina-Díaz A, Huijbregts MAJ, Fernandez-Alba AR, García-Calvo E (2008) Ranking potential impacts of priority and emerging pollutants in urban wastewater through life cycle impact assessment. Chemosphere 74:37–44
Öllers S, Singer HP, Fässler P, Müller SR (2001) Simultaneous quantification of neutral and acidic pharmaceuticals and pesticides at the low-ng/l level in surface and waste water. J Chromatogr A 911:225–234
Padhye LP, Yao H, Kung’u FT, Huang CH (2014) Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res 51:266–276
Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta. South China Sci Total Env 397:158–166
Rodil R, Quintana JB, Concha-Graña E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D (2012) Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 86:1040–1049
Rodríguez-Paniagua E (2015) Removal of emerging contaminants in waters by means of physico-chemical procedures. Universidad de Extremadura
Saravanan M, Karthika S, Malarvizhi A, Ramesh M (2011) Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. J Hazard Mater 195:188–194
Scheurell M, Franke S, Shah RM, Hühnerfuss H (2009) Occurrence of diclofenac and its metabolites in surface water and effluent samples from Karachi, Pakistan. Chemosphere 77:870–876
Sodré F, Locatelli MA, Jardim WF (2010) Occurrence of emerging contaminants in Brazilian drinking waters: a sewage-to-tap issue. Water Air Soil Poll 206:57–67
Sun SP, Zeng X, Lemley AT (2013) Kinetics and mechanism of carbamazepine degradation by a modified Fenton-like reaction with ferric-nitrilotriacetate complexes. J Hazard Mater 252–253:155–165
Tran NH, Urase T, Ta TT (2014) A preliminary study on the occurrence of pharmaceutically active compounds in hospital wastewater and surface water in Hanoi, Vietnam. Clean - Soil, Air, Water 42:267–275
Uhlmann D, Paul L, Hupfer M, Fischer R (2011) Lakes and reservoirs. In: Wilderer P (ed) Treatise on water science. Elsevier, Oxford, pp 157–213. doi:10.1016/B978-0-444-53199-5.00034-8
Vergili I (2013) Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J Env Manag 127:177–187
Vulliet E, Cren-Olivé C, Grenier-Loustalot MF (2011) Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Env. Chem Lett 9:103–114
Wang J, Leung D, Chow W (2010) Applications of LC/ESI-MS/MS and UHPLC QqTOF MS for the determination of 148 pesticides in berries. J Agri Food Chem 58:5904–5925
Yang G, Fan M, Zhang G (2014) Emerging contaminants in surface waters in China—a short review. Env Res Lett 9:74018
Zeng E (2015) Persistent organic pollutants (POPs): analytical techniques, environmental fate and biological effects, 1st edn. Elsevier, vol. 67, p 684
Acknowledgements
This work has been developed under the financial support provided by Empresa Públicas de Medellin. The authors wish to thank Marian Martinez from the GDCON research team for their help with the analysis. Carolina Aristizábal is especially grateful with Carlos Lopez, professor of University of Antioquia, for his technical advice in the development of this work. The advices and help of Professor Felix Hernandez, from University Jaume I, is greatly appreciated.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Ester Heath
Carolina Aristizabal-Ciro and Ana María Botero-Coy contributed equally to the design, development, writing and revision of the present article.
Electronic supplementary material
ESM. 1
In this section, two figures are included, showing the distribution of IBU and MePB in reservoir RG (Figure 1SI) and in reservoir LF (Figure 2SI) water collected at three depths for different sampling stations and monitoring dates. Also three tables, 1SI: Monitoring plan for reservoirs, tributary rivers and DWTPs, 2SI: Concentrations (ng/L) found for BZP, MePB and IBU in samples from reservoir RG, 3SI: Concentrations (ng/L) found for BZP, MePB and IBU in samples from reservoir LF, are included to support the information given in the manuscript. (DOCX 1069 kb)
Rights and permissions
About this article
Cite this article
Aristizabal-Ciro, C., Botero-Coy, A.M., López, F.J. et al. Monitoring pharmaceuticals and personal care products in reservoir water used for drinking water supply. Environ Sci Pollut Res 24, 7335–7347 (2017). https://doi.org/10.1007/s11356-016-8253-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8253-1