Abstract
The occurrence of five pharmaceuticals, consisting of four anti-inflammatory and one antiepileptic drug, was studied by passive sampling and grab sampling in northern Lake Päijänne and River Vantaa. The passive sampling was performed by using Chemcatcher® sampler with a SDB-RPS Empore disk as a receiving phase. In Lake Päijänne, the sampling was conducted during summer 2013 at four locations near the discharge point of a wastewater treatment plant and in the years 2013 and 2015 at four locations along River Vantaa. The samples were analyzed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) in the multiple reaction monitoring mode. The concentrations of carbamazepine, diclofenac, ibuprofen, ketoprofen, and naproxen in Lake Päijänne determined by passive sampling ranged between 1.4–2.9 ng L−1, 15–35 ng L−1, 13–31 ng L−1, 16–27 ng L−1, and 3.3–32 ng L−1, respectively. Similarly, the results in River Vantaa ranged between 1.2–40 ng L−1, 15–65 ng L−1, 13–33 ng L−1, 16–31 ng L−1, and 3.3–6.4 ng L−1. The results suggest that the Chemcatcher passive samplers are suitable for detecting pharmaceuticals in lake and river waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is an increasing need for monitoring the aquatic environment, particularly the priority pollutants listed in EU Water Framework Directive and other persistent organic pollutants (POPs) but also pseudo-persistent compounds, such as pharmaceuticals which are constantly released into the aquatic environment (EC 2000; Vrana et al. 2006). The concentrations of pollutants dissolved in water are commonly very low, usually less than 1 ng L−1. However, the low levels of contaminants in water can affect the reproduction of aquatic organisms even with very low concentration levels as they often bioconcentrate to relatively high levels in tissues (Meador and Rice 2001). For example, up to 1000 times higher levels of pharmaceuticals are found in the bile of fish compared to those in the water phase (Brozinski et al. 2013). Recently, more interest has been focused on polar organic chemicals, including pharmaceuticals and pesticides. Even though polar compounds do not have a high potential for bioaccumulation, they can pose a risk to aquatic organisms because they are often continuously present in the aquatic environment (Daughton 2002). Occurrence and distribution of chemicals in urban waters are linked to population density, consumption patterns, and water use but also to physical and chemical properties of substances which determine their fate in the environment (Dickenson et al. 2011).
Sampling of the micro pollutants in aquatic environment is often a challenging task. Water bodies are not well mixed and therefore chemicals are not evenly distributed (Pawlowski et al. 2004). Especially, small rivers are often hydrologically very dynamic (Vermeirssen et al. 2006). In case of long-term monitoring studies which aim to link exposure of chemicals to effects in organisms, grab sampling can be applied successfully only when the concentrations of analytes are fairly stable. Conventional methods for monitoring of harmful substances in surface waters are based on frequent grab sampling at fixed time intervals. Traditionally, water is collected at specific sites while the sample clean-up and enrichment is performed in the laboratory (Alvarez et al. 2005). However, the volume of water can be insufficient to meet the detection limit requirement of common analytic methods. Grab sampling is expensive, labor intensive, and gives only instantaneous concentrations which may not describe the long-term average concentrations. Additionally, it can miss periodic and occasional fluctuations in the concentrations of pollutants caused by spills or storm water runoffs (Koester et al. 2003; Guo et al. 2004; Alvarez et al. 2005).
Passive sampling technique combines sampling and enrichment steps (Vrana et al. 2005). It is based on free flow of analytes from surrounding media to the receiving phase due to the difference between chemical potentials of the compounds in these two media (Gorecki and Namiesnik 2002). Chemicals diffuse and partition until the aqueous concentrations of substances reach the equilibrium between uptake and elimination into and from the sampling device over time (Vermeirssen et al. 2009). The mass transfer of compounds from water to the sampler includes diffusion and transport across barriers, such as aqueous boundary layer, biofilm layer, the diffusion-limiting membrane (if used), and the receiving phase (Huckins et al. 1999). Under constant aqueous concentration, the concentration in the sampler increases nearly linearly over time (Vermeirssen et al. 2008). After that, the increase flattens and the concentrations in the receiving phase and in the surrounding media reach equilibrium (Mayer et al. 2003).
Integrative passive sampling is commonly used to determine the time-weighted average (TWA) concentration of a substance when the uptake into the sampler is the dominating process (Vermeirssen et al. 2009). The amount of chemical in a sampler can be divided by the sampling rate (R s) and the deployment time to calculate the TWA concentration. However, it is not straightforward to calculate TWA concentrations from passive sampling data because the diffusion and partitioning are influenced by temperature, turbulence around the sampler, salinity, and biofouling (Vrana et al. 2006; Booij et al. 2006; Togola and Budzinski 2007; Vermeirssen et al. 2008). In case of linear uptake, the amount of substance in the receiving phase can be calculated as expressed in Eq. 1 (Kingston et al. 2000; Shaw et al. 2009).
where M s (t) is the amount of substance (ng) measured in the receiving phase after the deployment time t (days), C w is the average concentration of substance (ng L−1) in water and R s the sampling rate, the extracted water volume per unit of time (L day−1) (Vrana et al. 2006).
R s is an important parameter in order to reach a proper quantification (Harman et al. 2012). R s is established for each device and compound by performing lab or field experiments (MacLeod et al. 2007). R s needs to be determined for each substance which can be time and resource-consuming in case of a large number of substances. It has been discussed if R s can be predicted based on physicochemical properties of substances (Shaw and Mueller 2009; Morin et al. 2013; Vermeirssen et al. 2013). So far, passive samplers have been calibrated in a laboratory scale by, e.g., static renewal, static depletion, and flow-through systems (Alvarez et al. 2004; Vrana et al. 2006; MacLeod et al. 2007; Harman et al. 2009; Morin et al. 2012). However, all methods give different R s and no standard method is yet available (Morin et al. 2013; Mills et al. 2014). Water matrix properties, such as temperature, pH, dissolved organic matter, and ionic strength, strongly affect the R s value, and R s calculations at laboratory conditions with nanopure or tab water do not simulate field conditions (i.e., lake or river water) very well (Harman et al. 2012). Therefore, TWA concentrations calculated with R s values based on lab experiments goes with uncertainties (Hyne and Aistrope 2008).
There are various passive sampler designs available but only two have been used to detect polar substances in water: polar organic chemical-integrated sampler (POCIS) and the polar version of Chemcatcher (Alvarez et al. 2004; MacLeod et al. 2007; Ahkola et al. 2014). Most of the published research is about POCIS and less information is available about Chemcatcher (Schäfer et al. 2008; Mills et al. 2014). For example, over 300 compounds have been shown to accumulate in POCIS, including pesticides (>100), pharmaceuticals (>90), and industrial chemicals (>30) (Harman et al. 2012). Chemcatcher passive samplers have been used for, e.g., DDT and its metabolites (de la Cal et al. 2008), polar herbicides (Stephens et al. 2005; Tran et al. 2007), and pesticides (Gunold et al. 2008) but rarely to determine the concentrations of pharmaceuticals in the aquatic environment (Vermeirssen et al. 2009). Both samplers use adsorbent material, commonly styrenedivinylbenzene-reversed phase sulfonate (SDB-RPS) for Chemcatcher and Oasis HLB powder for POCIS, and undergo reversed phase interactions with polar substances (Kingston et al. 2000; Alvarez et al. 2004). The SDB-RPS Empore disks contain a hydrophilic copolymer modified with sulfonic acids and show a high retention of polar organic compounds (Hennion et al. 1998). Among Chemcatcher phases, SDB-RPS sorbent is the most efficient for polar compounds due to the higher ratio of sorbent mass per surface area (Stephens et al. 2005).
Recently, a variety of analysis methods of pharmaceuticals was reviewed and the occurrence of selected anti-inflammatory and antiepileptic drugs was studied in lake water of northern Lake Päijänne by grab sampling from different sampling sites and depths in the summer and winter time (Lindholm et al. 2014; Lindholm-Lehto et al. 2015; Lindholm-Lehto et al. 2016). In this study, Chemcatcher passive sampling results of selected anti-inflammatory and antiepileptic drugs in Lake Päijänne and River Vantaa were analyzed as well as the results of grab sampling. Lake Päijänne is the source of raw water for the production of drinking water for the capital area of Finland, comprising about one million people and River Vantaa the backup raw water source in case water from Lake Päijänne is not available.
Experimental
Materials and chemicals
Analytical standards carbamazepine, diclofenac, ibuprofen, ketoprofen, and naproxen (purity 98 %) were purchased from Alfa Aesar (Karlsruhe, Germany). Their chemical structures, applications, and annual consumptions are listed in Table 1. HPLC grade methanol and acetonitrile were obtained from J.T Baker. The water used in the analyses was ultra high-quality (UHQ) water (Millipore, Bedford, MA, USA). The standard solutions (100 μg mL−1) were prepared by dissolving an accurate amount of pure standard in HPLC grade methanol and filtered with membrane filter 0.2-μm pore size, ME 24. Standard solutions were stored in the dark at 4 °C. The Chemcatcher sampler housing made of polycarbonate was purchased from MP-Plast Inc. (Muurame, Finland). Empore SDB-RPS disks (47 mm) were purchased from Agilent Technologies (Waghaeusel-Wiesenthal, Germany).
Passive samplers
A SDB-RPS Empore disk (diameter 47 mm, surface area 15.9 cm2) was used as a receiving phase and conditioned before use by immersing in methanol followed by immersing in UHQ water (Kingston et al. 2000). The conditioned disk was fitted in a polycarbonate Chemcatcher sampler housing which was kept in methanol overnight and rinsed with UHQ water, closed and stored in zip-lock bags at 4 °C until exposure. After the deployment time, the SDB-RPS disk was carefully removed from the sampler into Kimax tube and stored at -18 °C prior to analysis.
Calibration experiment
A calibration experiment was performed in laboratory conditions in order to determine the R s of each studied pharmaceutical. Ten passive samplers were exposed in a constant concentration system under controlled conditions of temperature, water turbulence, and analyte concentration. The experiment was performed in a dark room at 18 °C. The system consisted of a 31-L cylindrical glass tank filled with 19 L of UHQ water spiked with a 100 μL of selected pharmaceutical standards in methanol. Nominal concentration of 500 ng L−1 of each analyte was maintained throughout the experiment. The spiked UHQ water was renewed throughout the experiment every 3 or 4 days. Grab samples of 500 mL were collected from the exposure tank and the concentrations of pharmaceuticals were measured before and after each renewal.
Ten samplers were tied to an overhead stirrer with cable ties two at a time. The holder was interconnected to an overhead stirrer and it was rotated at a constant stirring speed of 90 rpm (33 cm s−1). The exposure took place for 14 days expecting a linear uptake. Duplicate samplers were removed after 3, 6, 8, 10, and 14 days and the concentrations of accumulated chemicals were determined. The R s were calculated according to Eq. 1.
Field experiment
Lake Päijänne
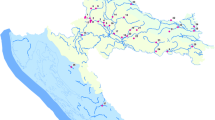
Selected pharmaceuticals (carbamazepine, diclofenac, ibuprofen, ketoprofen, and naproxen) were analyzed from water of Lake Päijänne by passive sampling and grab sampling during summer 2013. Lake Päijänne (1080 km2, average depth 16.2 m), the source of raw water for production of drinking water for the capital area of Finland, is located in central Finland (Fig. 1). The flow rate of water via Lake Päijänne is on average 240 m3 s−1 (S1). The lake receives effluent from the municipal wastewater treatment plant (WWTP) (influent 35,000 m3 day−1, at sampling site P2) of the city of Jyväskylä (population 150,000). The wastewater treatment process comprises primary clarification, removal of phosphorous with ferrosulphate and an activated sludge process.
The passive samplers were deployed at four sampling sites (P1–P4) in a depth of 1 m for 2 weeks at a time in Lake Päijänne (Fig. 1). Duplicate samplers were exposed at the sites for seven times during summer 2013. Grab water samples (500 mL) were collected with Ruttner water sampler from four locations (sites P1-P4) from the depth of 1 m. Water samples, in total eight from each location, were taken in glass bottles before and after each deployment time period of the passive samplers. Originally, the samples were taken for the method development and studies of occurrence reported by Lindholm-Lehto et al. (2015). The water samples were stored in HDPE bottles at -18 °C prior to analysis.
River Vantaa
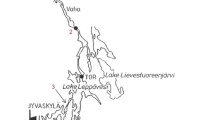
The five selected pharmaceuticals were studied from water of River Vantaa by passive sampling and grab sampling in 2013 and 2015. River Vantaa is a 101-km-long river located in southern Finland and flows into the Gulf of Finland (river basin 1685 m2, flow rate 1.4–317 m3 s−1). Flow velocities along River Vantaa increase towards the sea shore mostly due to flows from river branches (Fig. 2, S2). River Vantaa is used as a backup fresh water source for the capital city area in case of limited fresh water supply from Lake Päijänne. More than half of the river basin area comprises of forest, a third is in agricultural use, the rest being population centers or in industrial use. Altogether, 1.1 million people live along River Vantaa. The water in River Vantaa is naturally brown and turbid due to clayey soil, especially during rainy seasons.
There are several municipal wastewater treatment plants along River Vantaa which treat together 31 500 m3 day−1 of wastewater (250–12,000 m3 day−1) (S3). One of them, Riihimäki WWTP, treats municipal wastewater of 28,000 residents (12,600 m3 day−1) and industrial wastewater from a dairy product producer. It was rebuilt during 2013–2014 to enhance the pretreatment, nitrogen, and phosphorous removal and to increase its capacity (Riihimäki waterworks 2014). Its nitrogen removal is based on a biological denitrification-nitrification process and the phosphorous is precipitated mainly by ferrosulphate. In addition, a tertiary treatment stage has been built to ensure the phosphorous removal also in special cases.
Hyvinkää WWTP serves 32,900 residents and treats 10,300 m3 day−1 of municipal wastewater (Kalteva WWTP 2011). The treatment process is based on chemical and biological nitrification–denitrification with chemical precipitation of phosphorous by ferrosulphate. The Hyvinkää WWTP reaches excellent removal efficiency (BOD7 99 %, CODCr 96 %, Ptot 97 %, and Ntot 81 %).
Nurmijärvi WWTP treats municipal wastewater (2700 m3 day−1) of 5800 residents (Nurmijärvi WWTP 2011). The treatment process includes biological nitrogen removal combined with chemical precipitation. Ferrosulphate is used for the precipitation of phosphorous. The Nurmijärvi WWTP reaches good removal efficiency (BOD7 97 %, Ptot 93 %, and Ntot 51 %).
Data on pharmaceuticals were collected by passive samplers and grab samples from four locations (sites V1–V4; Fig. 2), first in August 2013 and later in May 2015. The selected sampling sites belong to the regular monitoring of harmful substances in River Vantaa (Vahtera et al. 2013). The grab samples, six from each location, were taken before and after 2 weeks of deployment time of passive samplers.
Sample preparation
Grab samples
Water samples were treated according to Lindholm-Lehto et al. (2015). In brief, the samples were concentrated with solid-phase extraction (SPE) cartridges (Varian bond Elut C18, 500 mg) which were first conditioned with 3 mL methanol followed by 3 mL UHQ water before loading the samples. The analytes were eluted with 3 mL of acetone, evaporated to dryness under a stream of nitrogen. Subsequently, they were redissolved in 300 μL of methanol and water (1:1, v/v), filtered through syringe cartridges (Cronus 0.45 μm PTFE) and analyzed immediately with liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
Passive samplers
The Empore disks were eluted first with 10 mL of acetone in an ultrasonic bath for 10 min and the solvent was collected. The procedure was repeated by using 10 mL of methanol. The solvents were combined and evaporated to a small volume by a rotary evaporator. The samples were then filtrated through (Cronus 0.45 μm PVDF) syringe cartridges and evaporated to dryness under a stream of nitrogen. Finally, they were redissolved in 300 μL of methanol and water (1:1, v/v) and analyzed with LC-MS/MS.
Instrumental analysis
The concentrations of target analytes in the extracts were quantified using LC-MS/MS as described by Lindholm-Lehto et al. (2015). The analysis was performed with 1290 Infinity series liquid chromatograph equipment coupled to a 6460 Triple Quad triple-quadrupole mass analyzer (MS/MS) with an electrospray ionization source (Agilent Technologies). The analysis method was validated by performing experiments with spiked water samples (recoveries for carbamazepine 89.6 %, naproxen 89.3 %, ketoprofen 86.3 %, ibuprofen 90.6 %, and diclofenac 87.6 %). The LOQ of water samples (ng L−1) are CBZ 0.1, DIC 25, IBU 12, KET 5.1, and NPX 9.2, and the LOQ of passive samplers (ng L−1) are CBZ 0.1, DIC 130, IBU 32, KET 22, and NPX 23.
Results and discussion
Calibration experiment
The R s determined by the laboratory calibration are presented in Table 2. The highest R s was measured for carbamazepine and the lowest for diclofenac. The R s of ibuprofen, ketoprofen, and naproxen were at the same level.
The R s values were in the similar range than those reported in the literature. For example, DiCarro et al. (2014) and Tanwar et al. (2015) studied pharmaceuticals by POCIS passive samplers and reported somewhat higher R s values of ibuprofen and ketoprofen than those of diclofenac (IBU 0.075, KET 0.066, DIC 0.058). Additionally, MacLeod et al. (2007) and Li et al. (2010) reported higher R s values of carbamazepine than ibuprofen, ketoprofen, and naproxen (CBZ 0.348–0.397, IBU 0.254, KET 0.135, NPX 0.116–0.298).
Lake Päijänne
The concentrations of selected pharmaceuticals collected both by grab and passive sampling at sites P1–P4 are shown in Table 3. The concentrations based on passive sampling were calculated by using Eq. 1 and R s determined in the laboratory calibration (Table 2).
The highest concentrations of pharmaceuticals are detected mainly at the sampling site P2 which is located at the point of effluent discharge from the WWTP (Table 3; Figs. 1 and 3). This applies to both passive and grab sampling. Generally, the concentrations were decreasing with increasing distance from the WWTP. However, all selected pharmaceuticals were also detected at site P1 even though it is located upstream from the WWTP. According to Krogerus et al. (2013), the sampling site P1 is counter current to WWTP suggesting another source of contaminants. This is supported by the different profile of pharmaceuticals in the samples from this site compared to other sites, even though differences do also occur between other sites. In grab samples, ibuprofen dominated at all sites, with the highest mean concentration at site P1 but at site P2 in passive samples (Fig. 3). At site P2, the mean concentrations of diclofenac, ibuprofen, ketoprofen, and naproxen were at a similar level in water determined with passive sampling, while at other sites ketoprofen showed the highest concentrations (Table 3; Fig. 3).
Generally, the concentrations of selected pharmaceuticals are in the similar range, tens of nanograms per liter, like the previous results of grab and passive sampling of lake and river waters (Lindqvist et al. 2005; Vieno 2007; Moschet et al. 2015). Especially, in the case of carbamazepine, the concentrations are similar both by passive sampling and grab sampling (Table 3). However, in the case of ibuprofen and diclofenac, the concentrations of grab sampling are up to ten times higher than those of passive sampling (Table 3). Additionally, others have reported higher concentrations by grab sampling compared to passive sampling. For example, Moschet et al. (2015) reported concentrations of naproxen and diclofenac ten times higher by grab sampling compared to passive sampling.
Ibuprofen is the most commonly used non-prescription drug in Finland (Table 1). Most likely, this explains the high detected concentrations, especially at site P2 near the WWTP. In the beginning of August, exceptionally high concentrations were detected both by passive and grab sampling (Fig. 4). At that time, the Neste Oil Rally took place in the Jyväskylä area. As previously reported, an organized event with thousands of visitors increases the consumption of anti-inflammatory drugs, especially ibuprofen (Daneshvar et al. 2012; Lindholm-Lehto et al. 2015).
The large range of concentrations detected by grab sampling suggests variability in concentrations over time (Table 3, Figs. 4, 5, and 6). For example, high variation in concentrations of diclofenac was detected (Fig. 5). The samples close to the WWTP (P2) showed the highest concentrations while the others decreased with distance. It is widely reported that diclofenac undergoes photochemical degradation reactions under sun light in surface waters (e.g., Packer et al. 2003; Bartels and von Tümpling Jr. 2007). Therefore, the concentrations can decrease over long distances in surface waters. It has been reported that the concentrations of diclofenac increase rapidly in deeper water layers in Lake Päijänne (Lindholm-Lehto et al. 2015). Additionally, mixing of water can lead to wide variety of concentrations by grab sampling even in the surface water.
Generally, the concentrations of carbamazepine detected by passive sampling follow the concentrations of grab sampling (Fig. 6). The values are fairly constant over the sampling period but in case of samples collected by passive sampling, they increase slightly towards fall. The solar radiation is the most intense in central Finland during summer months, especially in June and July (June 5.4 kWh m−2, July 5.4 kWh m−2, August 3.6 kWh m−2, S1). The grab samples and the passive samples were taken from the depth of 1 m which is clearly included in the photic layer of the lake enabling the UV light-induced reactions (Secchi depth 1.6–2.0 m, S1). This can explain the slight increase in concentrations in late summer (Fig. 6).
Also in this case, the highest concentrations detected by passive sampling are found at sampling site P2 at the point of effluent discharge from the WWTP decreasing with distance (Fig. 6). However, the concentrations at site P1 are also found, throughout the sampling period. The site P1 is located upstream from the WWTP indicating a release of contaminants from sediment or another unknown source (Krogerus et al. 2013). This remains as a subject for further research.
River Vantaa
The concentrations of the studied pharmaceuticals ranged from few nanograms per liter of passive samples to hundreds of nanograms per liter of grab samples (Tables 4 and 5, Figs. 7 and 8). Despite the variation, they are in the similar range as in other studies where Chemcatcher was deployed in river waters (e.g., Vieno 2007). For example, similar levels of carbamazepine and diclofenac were reported by Äystö et al. (2014) in River Vantaa. Moschet et al. (2015) detected 6–110 ng L−1 of carbamazepine, 1.4–320 ng L−1 diclofenac, and 26–87 ng L−1 naproxen in Swiss rivers by using Chemcatcher passive samplers.
Overall, the concentrations were ten times higher in August 2013 than in May 2015 (Tables 4 and 5; Figs. 7 and 8), excluding diclofenac. Most likely, the lower levels in 2015 are caused by the higher water flow in the spring time and dilution of the trace substances in the river water (April 2015, 7.8–28 m3 s−1; May 2015, 6.8–19.6 m3 s−1, S2, S3). Year 2013 was unusually dry and warm (79 % of average rainfall and 1.2–1.8 °C warmer (OIVA 2015)). The water flow has an effect on the accumulation factor of selected pharmaceuticals which can affect the results of passive sampling (Ahkola et al. 2014). Furthermore, the WWTP of Riihimäki was rebuilt in 2014 which might have improved the removal efficiency of pharmaceuticals. In addition, higher concentrations detected by grab sampling in August 2013 might only be due to occasional variations while passive samplers represent the more long-term situation.
All in all, the lowest levels of pharmaceuticals were measured at site V4 near the sea shore in 2013, excluding ketoprofen (Table 4; Figs. 7 and 8). The average flow of River Vantaa increases towards the sea shore suggesting increased dilution of trace substances (1.3–4.8 m3 s−1, S2). However, the similar levels of pharmaceuticals between sites V2 and V3 suggest several point sources of pharmaceuticals. There are several other municipal WWTPs along River Vantaa releasing their effluents to the river. The point of discharge from a local WWTP is located near all the selected sampling sites. In addition to the Riihimäki WWTP close to the site V1, there is the point of effluent discharge from the Hyvinkää WWTP (10,300 m3 day−1) near the site V2 and near the site V3 the point of effluent discharge from the Nurmijärvi WWTP (2700 m3 day−1).
Ketoprofen showed the highest concentrations of grab sampling (Table 4; Fig. 8), while Äystö et al. (2014) detected no ketoprofen in fall 2013 in River Vantaa. Carbamazepine showed the highest concentrations collected by passive samplers in 2013, while diclofenac dominated in passive samplers in 2015 (Table 4), excluding site V4.
Other factors than point sources can also contribute to the greater amount of ketoprofen towards the sea shore (Fig. 8), especially when studied by passive samplers. Ketoprofen has the ability to accumulate in the environment during winter, but in the summer, it has more labile properties (Daneshvar et al. 2010; Vystavna et al. 2013). In addition to human consumption released via WWTPs, ketoprofen is also used as a veterinary drug which might end up in the fields after consumption by farm animals and later back to the river (Vystavna et al. 2013). Along River Vantaa, there are several animal farms with horses, lambs, and cattle which may have a contribution to the load of ketoprofen. Therefore, it is possible that the concentration of ketoprofen builds up in the river water towards the sea shore (Fig. 8).
Generally, the concentrations of carbamazepine and diclofenac decreased towards the sea shore (Tables 4 and 5). The concentrations are tens of nanograms per liter when detected by passive samplers and are similar to those reported by Äystö et al. (2014). Additionally, Äystö et al. (2014) reported decreased levels of carbamazepine in the sea shore. Carbamazepine in river water most likely originated from the multiple municipal WWTPs along River Vantaa. Carbamazepine is known to undergo transformation and dissociation reactions under sun light (Andreozzi et al. 2003). However, the reactions of carbamazepine proceed relatively slowly with half-life times of hundreds of days and it is constantly released by the effluents. In addition, the water of River Vantaa is turbid and contains humic substances which are known to decrease or even reverse the UV light-induced transformation reactions (Andreozzi et al. 2003). The highest amounts of humic substances are met in the upstream of River Vantaa due to forests and their soil properties (OIVA 2015).
Concluding remarks
Results show that all selected pharmaceuticals are present both in northern Lake Päijänne and in River Vantaa. Generally, the concentrations detected by passive samplers follow those of grab samples. Therefore, the results can be considered reliable and the Chemcatcher passive samplers suitable for the monitoring of pharmaceuticals in lake and river water. Passive sampling gives results of a long-term situation while grab sampling shows occasional fluctuations of concentrations.
The highest concentrations were detected by passive sampling in Lake Päijänne at sampling site P2 at the point of effluent discharge from the WWTP. Decreasing concentrations with greater distance from the WWTP confirm that the WWTP is the main local source of pharmaceuticals. The occurrence of pharmaceuticals at the site P1 upstream from the WWTP at a similar level but with different profile suggests another source calling for further research. Although high concentrations of selected pharmaceuticals are only occasionally detected, the cocktail of different substances requires further studies, especially related to long-term toxicity to aquatic organisms.
Pharmaceuticals, such as carbamazepine and diclofenac occurred at greater concentrations in River Vantaa than in Lake Päijänne. The difference in the dominating pharmaceuticals between River Vantaa and Lake Päijänne is most likely explained by the different sources of pharmaceuticals. There are more and different types of point sources along River Vantaa than in Lake Päijänne. Additionally, the environmental conditions and the dilution of the pharmaceuticals vary due to different loads and different types of water bodies. Sampling time and environmental factors most likely explain the differences in River Vantaa water between samplings in 2013 and 2015.
In conclusion, Chemcatcher passive sampler with SDB-RPS disk is suitable for detecting pharmaceuticals in river and lake waters. It can be expected that there will be the need for more extensive monitoring of pharmaceuticals in the near future due to restricting environmental legislation. Passive sampling gives a good alternative for long-term monitoring of environmental pollutants compared to traditional grab sampling and gives an inexpensive and less labor-intensive option also for screening of pollutants in obligatory environmental monitoring.
References
Ahkola H, Herve S, Knuutinen J (2014) Study of different Chemcatcher configurations in the monitoring of nonylphenol ethoxylates and nonylphenol in aquatic environment. Environ Sci Pollut Res 21:9182–9192
Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE (2004) Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem 23:1640–1648
Alvarez DA, Stackelberg PE, Petty JD, Huckins JN, Furlong ET, Zaugg SD, Meyer MT (2005) Comparison of a novel passive sampler to standard water-column sampling for organic contaminants associated with wastewater effluents entering a New Jersey stream. Chemosphere 61:610–622
Andreozzi R, Raffaele M, Paxéus N (2003) Pharmaceuticals in STP effluents and their solar photodegradation in aquatic environment. Chemosphere 50:1319–1330
Äystö L, Mehtonen J, Kalevi K (2014) Kartoitus lääkeaineista yhdyskuntajätevedessä ja pintavedessä (In Finnish). Finnish environmental Institute/Centre for sustainable consumption and production. Final report 8.9.2014
Bartels P, von Tümpling W Jr (2007) Solar radiation on the decomposition process of diclofenac in surface waters. Sci Total Environ 374:143–155
Booij K, van Bommel R, Mets A, Dekker R (2006) Little effect of excessive biofouling on the uptake of organic contaminants by semipermeable membrane devices. Chemosphere 65:2485–2492
Brozinski J-M, Lahti M, Meierjohann A, Oikari A, Kronberg L (2013) The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant. Environ Sci Technol 47:342–348
Daneshvar A, Svanfelt J, Kronberg L, Prévost M, Weyhenmeyer GA (2010) Seasonal variations in the occurrence and fate of basic and neutral pharmaceuticals in a Swedish river–lake system. Chemosphere 80:301–309
Daneshvar A, Svanfelt J, Kronberg L, Weyhenmeyer GA (2012) Neglected sources of pharmaceuticals in river water—footprints of a Reggae festival. J Environ Monit 14:596–603
Daughton CG (2002) Environmental stewardship and drugs as pollutants. Lancet 360:1035–1036
de la Cal A, Kuster M, de Alda ML, Eljarrat E, Barceló D (2008) Evaluation of the aquatic passive sampler Chemcatcher for the monitoring of highly hydrophobic compounds in water. Talanta 76:327–332
DiCarro M, Bono L, Magi E (2014) A simple recirculating flow system for the calibration of polar organic chemical integrative samplers (POCIS): effect of flow rate on different water pollutants. Talanta 120:30–33
Dickenson ERV, Snyder SA, Sedlak DL, Drewes JE (2011) Indicator compounds for assessment of wastewater effluent contribution to flow and water quality. Water Res 45:1199–1212
EC (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. L327:1–72.
Gorecki T, Namiesnik J (2002) Passive sampling. Trends Anal Chem 21:276–291
Gunold R, Schäfer RB, Paschke A, Schüürmann G, Liess M (2008) Calibration of the Chemcatcher® passive sampler for monitoring selected polar and semi-polar pesticides in surface water. Environ Pollut 155:52–60
Guo L, Nordmark CE, Spurlock FC, Johnson BR, Li LY, Lee JM, Goh KS (2004) Characterizing dependence of pesticide load in surface water on precipitation and pesticide use for the Sacramento River watershed. Environ Sci Technol 38:3842–3852
Harman C, Bøyum O, Thomas KV, Grung M (2009) Small but different effect of fouling on the uptake rates of semipermeable membrane devices and polar organic chemical integrative samplers. Environ Toxicol Chem 28:2324–2332
Harman C, Allan IJ, Vermeirssen ELM (2012) Calibration and use of the polar organic chemical integrative sampler—a critical review. Environ Toxicol Chem 31:2724–2738
Hennion M-C, Cau-Dit-Coumes C, Pichon V (1998) Trace analysis of polar organic pollutants in aqueous samples: tools for the rapid prediction and optimisation of the solid-phase extraction parameters. J Chromatogr A 823:147–161
Huckins JN, Petty JD, Orazio CE, Lebo JA, Clark RC, Gibson VL, Gala WR, Echols KR (1999) Determination of uptake kinetics (sampling rates) by lipid-containing semipermeable membrane devices (SPMDs) for polycyclic aromatic hydrocarbons (PAHs) in water. Environ Sci Technol 33:3918–3923
Hyne RV, Aistrope M (2008) Calibration and field application of a solvent-based cellulose membrane passive sampling device for the monitoring of polar herbicides. Chemosphere 71:611–620
Kalteva WWTP (2011) The Water Protection Association of the River Vantaa and Helsinki region: collective surveillance of wastewater treatment plants, Surveillance of use and discharge of Hyvinkää Kalteva WWTP. Annual report 4, 27 p
Kingston JK, Greenwood R, Mills GA, Morrison GM, Persson LB (2000) Development of a novel passive sampling system for the time-averaged measurement of a range of organic pollutants in aquatic environments. J Environ Monitor 2:487–495
Koester CJ, Simonich SL, Esser BK (2003) Environmental analysis. Anal Chem 75:2813–2829
Krogerus K, Liukko N, Juntunen J, Kotamäki N, Nieminen P, Huttula T (2013) Purkuputken siirron vaikutukset vedenlaatuun Pohjois-Päijänteellä Coherens-malliarvio (in Finnish). Finnish Envi-ronment Institute 12/2013
Li H, Helm PA, Metcalfe CD (2010) Sampling in the Great Lakes for pharmaceuticals, personal care products, and endocrine disrupting substances using the passive polar organic chemical integrative sampler. Environ Toxicol Chem 29:751–762
Lindholm PC, Knuutinen JS, Ahkola HSJ, Herve SH (2014) Analysis of trace pharmaceuticals and related compounds in municipal wastewaters by preconcentration, chromatography, derivatization, and separation methods. BioResources 9:3688–3732
Lindholm-Lehto PC, Knuutinen JS, Ahkola HSJ, Herve SH (2015) Occurrence of pharmaceuticals in municipal wastewater, in the recipient water, and sedimented particles of northern Lake Päijänne. Environ Sci Pollut Res 22:17209–17223
Lindholm-Lehto PC, Ahkola HSJ, Knuutinen JS, Herve SH (2016) Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in central Finland. Environ Sci Pollut Res 23:7985–7997
Lindqvist N, Tuhkanen T, Kronberg L (2005) Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res 39:2219–2228
MacLeod SL, McClure EL, Wong CS (2007) Laboratory calibration and field deployment of the polar organic chemical integrative sampler for pharmaceuticals and personal care products in wastewater and surface water. Environ Toxicol Chem 26:2517–2529
Mayer P, Tolls J, Hermens JLM, Mackay D (2003) Equilibrium sampling devices. Environ Sci Technol 37:184A–191A
Meador JP, Rice CA (2001) Impaired growth in the polychaete Armandia brevis exposed to tributyltin in sediment. Mar Environ Res 51:113–129
Mills GA, Gravell A, Vrana B, Harman C, Budzinski H, Mazzella N, Ocelka T (2014) Measurement of environmental pollutants using passive sampling devices—an updated commentary on the current state of the art. Environ Sci Process Impacts 16:369–373
Morin N, Miège C, Coquery M, Randon J (2012) Chemical calibration, performance, validation and applications of the polar organic chemical integrative sampler (POCIS) in aquatic environments. Trends Anal Chem 36:144–175
Morin N, Camilleri J, Cren-Olivé C, Coquery M, Miège C (2013) Determination of uptake kinetics and sampling rates for 56 organic micropollutants using “pharmaceutical” POCIS. Talanta 109:61–73
Moschet C, Vermeirssen ELM, Singer H, Stamm C, Hollender J (2015) Evaluation of in-situ calibration of Chemcatcher passive samplers for 322 micropollutants in agricultural and urban affected rivers. Water Res 71:306–317
Nurmijärvi WWTP (2011) The Water Protection Association of the River Vantaa and Helsinki region: collective surveillance of wastewater treatment plants, Surveillance of use and discharge of Nurmijärvi WWTP. Annual report 4, 29 p
OIVA—the environmental and geographical information service for experts. Finnish Environment Institute (2015)
Packer JL, Werner JJ, Latch DE, McNeill K, Arnold WA (2003) Photochemical fate of pharmaceuticals in the environment: naproxen, diclofenac, clofibric acid, and ibuprofen. Aquat Sci 65:342–351
Pawlowski S, Ternes TA, Bonerz M, Rastall AC, Erdinger L, Braunbeck T (2004) Estrogenicity of solid phase-extracted water samples from two municipal sewage treatment plant effluents and river Rhine water using the yeast estrogen screen. Toxicol in Vitro 18:129–138
Riihimäki waterworks (2014) Publications/Annual report 2014 (In Finnish). 41 p
Schäfer RB, Paschke A, Vrana B, Mueller R, Liess M (2008) Performance of the Chemcatcher passive sampler when used to monitor 10 polar and semi-polar pesticides in 16 Central European streams, and comparison with two other sampling methods. Water Res 42:2707–2717
Shaw M, Mueller JF (2009) Time integrative passive sampling: how well do Chemcatchers integrate fluctuating pollutant concentrations? Environ Sci Technol 43:1443–1448
Shaw M, Eaglesham G, Mueller JF (2009) Uptake and release of polar compounds in SDB-RPS EmporeTM disks; implications for their use as passive samplers. Chemosphere 75:1–7
Finnish Medicines Agency Fimea and Social Insurance Institution (2013) Suomen lääketilasto/ Finnish Statistics on Medicines 2013. http://www.fimea.fi/download/27596_SLT_2013_net.pdf. Accessed 23 February 2015
Stephens BS, Kapernick A, Eaglesham G, Mueller J (2005) Aquatic passive sampling of herbicides on naked particle loaded membranes: accelerated measurement and empirical estimation of kinetic parameters. Environ Sci Technol 39:8891–8897
Tanwar S, Di Carro M, Magi E (2015) Innovative sampling and extraction methods for the determination of nonsteroidal anti-inflammatory drugs in water. J Pharm Biomed Anal 106:100–106
Togola A, Budzinski H (2007) Development of polar organic integrative samplers for analysis of pharmaceuticals in aquatic systems. Anal Chem 79:6734–6741
Tran ATK, Hyne RV, Doble P (2007) Calibration of a passive sampling device for time-integrated sampling of hydrophilic herbicides in aquatic environments. Environ Toxicol Chem 26:435–443
Vahtera H, Männynsalo J, Lahti K (2013) Vantaanjoen yhteistarkkailu (In Finnish). Publication 70/2013
Vermeirssen ELM, Suter MJ-F, Burkhardt-Holm P (2006) Estrogenicity patterns in the Swiss midland river Lützelmurg in relation to treated domestic sewage effluent discharges and hydrology. Environ Toxicol Chem 25:2413–2422
Vermeirssen ELM, Asmin J, Escher BI, Kwon J-H, Steimen I, Hollender J (2008) The role of hydrodynamics, matrix and sampling duration in passive sampling of polar compounds with Empore™ SDB-RPS disks. J Environ Monit 10:119–128
Vermeirssen ELM, Bramaz N, Hollender J, Singer H, Escher BI (2009) Passive sampling combined with ecotoxicological and chemical analysis of pharmaceuticals and biocides—evaluation of three Chemcatcher™ configurations. Water Res 43:903–914
Vermeirssen ELM, Dietschweiler C, Escher BI, van der Voet J, Hollender J (2013) Uptake and release kinetics of 22 polar organic chemicals in the Chemcatcher passive sampler. Anal Bioanal Chem 405:5225–5236
Vieno N (2007) Occurrence of pharmaceuticals in Finnish sewage treatment plants, surface waters, and their elimination in drinking water treatment processes. Dissertation, Tampere University of Technology
Vrana B, Mills GA, Allan IJ, Dominiak E, Svensson K, Knutsson J, Morrison G, Greenwood R (2005) Passive sampling techniques for monitoring pollutants in water. Trends Anal Chem 24:845–868
Vrana B, Mills GA, Dominiak E, Greenwood R (2006) Calibration of the Chemcatcher passive sampler for the monitoring of priority organic pollutants in water. Environ Pollut 142:333–343
Vystavna Y, Le Coustumer P, Huneau F (2013) Monitoring of trace metals and pharmaceuticals as anthropogenic and socio-economic indicators of urban and industrial impact on surface waters. Environ Monit Assess 185:3581–3601
Acknowledgements
Financial support from Maa- ja vesitekniikan tuki ry. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1
Table S1. Variables of Lake Päijänne and Jyväskylä region, June-August 2013d. Table S2. Average (min…max) flow velocities m3 s−1 in River Vantaa in August 2013, April and May 2015 (OIVA 2015). Table S3. Average (min…max) discharge of treated effluent m3 d−1 from the WWTPs along River Vantaa in August 2013, April and May 2015 (OIVA 2015) and the share (%) of effluent compared to the total water flow at the point of discharge. (DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lindholm-Lehto, P.C., Ahkola, H.S.J., Knuutinen, J.S. et al. Suitability of passive sampling for the monitoring of pharmaceuticals in Finnish surface waters. Environ Sci Pollut Res 23, 18043–18054 (2016). https://doi.org/10.1007/s11356-016-6778-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6778-y