Abstract
Cyazofamid, as a fungicide of the novel cyanoimidazole chemical class, has been widely used to control tomato late blight. Understanding of cyazofamid residues in environment and crops is an essential prerequisite for its risk assessment. In this study, field investigations in four typical tomato-producing areas were conducted to explore the dissipation kinetics and residues of cyazofamid and its primary metabolite 4-chloro-5-p-tolylimidazole-2-carbonitrile (CCIM) in soil and tomato. A robust method using QuEChERS coupled with liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed for simultaneous analysis of cyazofamid and CCIM, with limits of quantification of 0.33 and 3.8 μg/kg, respectively. Field trials showed that the half-lives of cyazofamid were 3.6–6.9 days in soil and 12.2–18.3 days in tomato. The total residues of cyazofamid and CCIM in tomato collected at three time intervals were all below 0.5 mg/kg. Moreover, the potential risks of total residues via tomato intake to ten population subgroups were evaluated. We found that the risk quotient values were all generally low (0.13–1.3%), indicating that the recommended dose of cyazofamid on tomato will not result in a consumer exposure exceeding the toxicological reference value. Here, the results of field investigation provided important information for further understanding the behavior and risk of cyazofamid in the natural environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
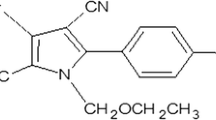
Tomato (Lycopersicon esculentum Mill.) is a critically important vegetable in China due to its various nutrition, heavy consumption, and profitability for farmers. Late blight, a major tomato disease caused by the fungus-like oomycete pathogen Phytophthora infestans, can quickly devastate tomato crops at any time during plant ontogeny (Small et al. 2015). Application of protectant fungicides is generally one of the effective practices necessary for suppression of the late blight. Among various fungicides, cyazofamid (4-chloro-2-cyano-N,N-dimethyl-5-p-tolylimidazole-1-sulfonamide) is a novel fungicide against the late blight (Lee et al. 2014).
Cyazofamid, as a fungicide of the novel cyanoimidazole chemical class, has a strong activity against a broad spectrum of oomycetes and plasmodiophoromycetes (Mitani et al. 1998). It has novel and unique mode of action to inhibit Qi site (the ubiquinone-reducing site) of complex III of the enzyme cytochrome bc1 in the mitochondrial respiratory chain (Li et al. 2014; Mitani et al. 2001a, b). Cyazofamid has been widely used to protect vegetables and fruits from various diseases, especially such as tomato late blight (caused by Phytophthora infestans), watermelon brown rot (caused by Phytophthora capsici), cucumber and melon downy mildew (caused by Psilocybe cubensis), and so on (Lozowicka 2015; Mitani et al. 2001a, b).
Cyazofamid has been observed to be toxic to cortical neuron cells, resulting in a significant decrease in cell viability both under short- and long-term exposure modes (Regueiro et al. 2015). Moreover, it could induce mitochondrial membrane depolarization (Regueiro et al. 2015). Subchronic toxicity test demonstrated that 13 weeks of dietary exposure to cyazofamid caused the microscopic kidney lesion in male rats (US EPA 2004). In addition, 4-chloro-5-p-tolylimidazole-2-carbonitrile (CCIM) is a primary metabolite of cyazofamid in crops and environment matrices (European Food Safety Authority 2016; US EPA 2004). Previous studies have already shown that CCIM appears to be more acutely toxic than cyazofamid (European Food Safety Authority 2016; US EPA 2004). Hence, cyazofamid residues in tomato would pose potential risks to human health. The Chinese government strictly regulates the residue level of cyazofamid in various food commodities through the maximum residue limit (MRL). However, the MRL of cyazofamid in tomato has not been legislated in China, and more essentially, rare fieldwork has been reported to determine the dissipation kinetics and residues of cyazofamid in tomato, which represents an uncertainty of its risk to human health. Therefore, it is of high importance to explore the dissipations and residues of cyazofamid and CCIM in tomato in agriculture production.
On the other hand, a robust, sensitive, and effective analysis method for cyazofamid and CCIM in tomato is fundamental for residue control. Methods of cyazofamid residue analysis using high-performance liquid chromatography (HPLC) have been developed with limits of quantification (LOQs) of 0.02–0.72 mg/kg (González-Álvarez et al. 2012; Lee et al. 2012; Watanabe et al. 2014). However, these methods focused on only cyazofamid and excluded the primary metabolite namely CCIM. In a recent study, Lee et al. (2014) reported a method to analyze cyazofamid and CCIM residue in vegetables like kimchi cabbage, green pepper, potato, and soybean using liquid chromatography-tandem mass spectrometry (LC-MS/MS), with LOQs of 2 and 5 μg/kg for cyazofamid and CCIM. However, its suitability to tomato matrix has remained unknown.
In this study, a rapid, robust, and sensitive method using (QuEChERS) coupled with LC-MS/MS was developed for simultaneous analysis of cyazofamid and CCIM in soil and tomato. Moreover, trial experiments both under open-field and greenhouse conditions were conducted to explore the dissipations and residues of cyazofamid and CCIM in soil and tomato. Finally, human exposure to cyazofamid residues via tomato intake was estimated, and the potential risk derived from cyazofamid residue was further evaluated. This work would be in support of the regulation of MRL for cyazofamid in tomato and of the proper and safe use of cyazofamid in agriculture.
Materials and methods
Materials and reagents
The cyazofamid standard (purity, 99.5%) was purchased from Dr. Ehrenstorfer (Augsburg, Germany). The CCIM standard (purity, 95%) was kindly donated from the manufacturer. Commercial formulation with 100 g/L of cyazofamid suspension concentrate (SC) was obtained from Ishihara Sangyo Kaisha, Ltd. (Osaka, Japan). Acetonitrile (chromatography grade) was supplied by Burdick & Jackson (Ulsan, Korea). Analytical-grade acetonitrile, formic acid, sodium chloride, and anhydrous magnesium sulfate were purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai, China). Sorbent primary-secondary amine (PSA) (particle size of 40–60 μm) and C18 (particle size of 40–60 μm) were purchased from Agela Technologies (Tianjin, China).
The properties of cyazofamid and CCIM are presented in Table S1. Standard stock solutions of cyazofamid (117 mg/L) and CCIM (95 mg/L) were prepared in acetonitrile, and then, they were diluted to obtain the standard solution of cyazofamid/CCIM mixture (11.7/9.5 mg/L). The calibration curve was prepared with cyazofamid/CCIM mixtures (5.5/4.5, 11/9.0, 22/18, 44/36, 88/72, 117/95, 176/143 μg/L). All solutions were stored in the dark at 4 °C. The compounds have shown to be stable for 3 months.
Trial experiment
The field experiments were carried out in four sites in the year of 2015. Four experiment sites were selected as typical producing areas of tomato in China, which were located in eastern, northern, and middle China. Locations and soil properties of experiment sites are depicted in Fig. 1. The weather and temperature during field experiment are shown in Tables S2, S3, S4, and S5. The site ZJ was in Cangqian, Hangzhou city, Zhejiang province, China. The site HN was in Dongan, Changsha city, Hunan province. The site BJ was located in Mulin, Beijing. The site HB was selected in Shizishan, Wuhan city, Hubei province. In every site, each plot was 15 m2 in area and experiment plots were divided by irrigation and drainage channels.
The dissipation kinetic studies were arranged in sites ZJ and HN. In site ZJ, the kinetic study was conducted under open-field condition, while it was performed under greenhouse condition in site HN. To investigate the dissipation of cyazofamid and CCIM in tomato as well as soil, cyazofamid formulation (100 g/L SC) was sprayed at 150.7 g active ingredient (a.i.)/ha (1.5 times the recommended dosage) in experiment plots each with three replicates, and the untreated plots were sprayed with water as control.
The residue investigation was carried out in all four sites. They were conducted under greenhouse condition in sites ZJ and HN and under open-field condition in sites BJ and HB. Cyazofamid formulation was sprayed at two doses, 100.4 g a.i./ha (the recommended dosage) and 150.7 g a.i./ha (1.5 times the recommended dosage). The treated plots were sprayed four or five times for each dose at an interval of 7 days. The untreated plots were sprayed with water as control.
Sampling and storage
Representative soil and tomato samples were collected from each plot at different time intervals. For the dissipation kinetic study, the samples were collected at 2 h and 1, 3, 5, 7, 10, 14, 21, 28, 35, and 42 days after cyazofamid application. To determine the residue of cyazofamid and CCIM, soil and tomato samples were collected at intervals of 1, 3, and 5 days after the last spray. Soil samples (1.0 kg) were homogenized thoroughly. Tomato samples (2.0 kg) were chopped and mashed thoroughly once after they were transported to the laboratory. All the samples were kept in a freezer at −20 °C until analysis. The samples were stored no longer than 4 months.
Sample preparation
Approximately 10 g of sample (soil or tomato) was weighed into a 50-mL polyethylene centrifuge tube. Ten milliliters of acetonitrile was added to extract cyazofamid and CCIM. The tube was capped and vortexed for 2 min. Then, the mixture was centrifuged (4000 r/min, 5 min) and the supernatant was collected. The extraction was repeated once more the same as described. The supernatants were combined, and an aliquot (5 mL) of extract was transferred into a 10-mL single-use polyethylene centrifuge tube, which contained an amount of sorbent (200 mg of C18, 40 mg of PSA, and 500 mg of MgSO4). In addition, 50 μL of formic acid was added, and then, the mixture was vortexed again for 1 min followed by centrifuging for 5 min at a speed of 10,000 r/min. The upper organic layer was filtered using a 0.22-μm nylon syringe filter and subsequently transferred to a vial for LC-MS/MS analysis.
Instrumental analysis
Cyazofamid and CCIM were measured using an ultra-fast liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS, LCMS 8050, Shimadzu, Japan). The LC was equipped with a Waters Acquity UPLC BEH C18 column (2.1 mm × 100 mm, 1.7-μm particle size, Milford, MA, USA). The mobile phase consisted of 0.1% formic acid in water (A) and acetonitrile (B), and they were passed through a 0.22-μm pore size filter before use. For gradient elution, the initial combination was 40:60 (A/B, v/v) and the B solution was increased to 90% in duration of 1.5 min and then held for 1.5 min. At 3.01 min, the solution B was changed back to 60% and held for 5 min. The flow rate was 0.20 mL/min, and the sample injection volume was 5 μL. The column was kept at 40 °C, and the temperature in the sample manager was maintained at 4 °C.
The mass system was equipped with an electrospray ionization (ESI) source. The MS/MS detection was performed in the positive ionization mode. The nebulizer and drying gas were 99.95% nitrogen, and their flow rates were 3.0 and 10.0 L/min, respectively. The heating gas was 99.95% air with a flow rate of 10.0 L/min. The collision gas was 99.99% argon with a pressure of 270 kPa. Other parameters were as follows: interface voltage 4.0 kV, interface temperature 300 °C, DL temperature 250 °C, heat block temperature 400 °C, and detector voltage 1.82 kV. The multiple reaction monitoring (MRM) mode was applied for the data acquisition. MRM transitions, Q1 pre bias, Q3 pre bias, and collision energy were optimized for the highest sensitivity and resolution (Table S1). The retention times of cyazofamid and CCIM were approximately 2.67 and 2.19 min, respectively (Fig. S1).
Method validation
Linear range, precision, accuracy, sensitivity, limit of detection (LOD), and LOQ were evaluated as parameters of the analytical methodology in this study. The recovery rate was determined using fortified blank matrices (soil and tomato) at three levels of cyazofamid and CCIM. The spiked levels were 0.01, 1.0, and 10.5 mg/kg for cyazofamid and 0.01, 0.9, and 9.5 mg/kg for CCIM. Five replicates were conducted for every spiking level. The fortified samples were allowed to equilibrate for 1 h before extraction and then treated following the process described in “Sample preparation” section. In addition, one blank sample was processed parallel.
Calculations
The SPSS 16.0 software was used for data analysis. Values below the LODs were set as zero in the calculation. The experimental data were subjected to determine the dissipation half-life. Concentrations of cyazofamid and CCIM at different intervals were fitted to Eq. (1) (Tasho and Cho 2016; Zhang et al. 2013):
where C t is the concentration (mg/kg) after time t (days), C 0 is the initial concentration (mg/kg), and k is the rate constant. Then, the half-life (T 1/2) was estimated using Eq. (2) (Tasho and Cho 2016; Zhang et al. 2013):
The total residues were the sum of cyazofamid and CCIM, calculated as the stoichiometric equivalent of cyazofamid. The total residue values were used in comparison to available MRLs in tomato and in the risk assessment. Exposure estimation and risk assessment were performed for various population subgroups in three scenarios. The three different scenarios were as follows: (i) A 5th percentile total residue level was used, representing the best scenario. (ii) A median total residue level was used, representing the mean scenario. (iii) A 95th percentile total residue level was used, representing the worst scenario. Dietary exposure calculation and risk assessment were conducted according to the following equations (Liu et al. 2014):
where ED is the daily exposure dose (mg/kg/day, bw), C is the total residue level in tomato (mg/kg), FI is the daily amount of tomato intake (kg/day), bw is the body weight (kg), ADI is the acceptable daily intake of cyazofamid (mg/kg/day, bw), and RQ is the risk quotient (%). An RQ value of more than 1 indicates that the risk of cyazofamid for human is unacceptable (Liu et al. 2014). The average body weights and the amounts of vegetable consumption were adopted from the fourth China total diet study (Wu and Li 2015). The amount of average vegetable consumption was used in this work. The ADI for cyazofamid is 0.17 mg/kg/day according to the National Food Safety Standard—MRLs for pesticides in food (Ministry of Agriculture of the People’s Republic of China 2014).
Results and discussion
Method performance
In this study, a method based on QuEChERS combined with LC-MS/MS was developed to analyze cyazofamid and CCIM in soil and tomato samples. The calibration linear was from 5.5 to 176 μg/L for cyazofamid (R 2 = 0.999) and from 4.5 to 143 μg/L for CCIM (R 2 = 0.996).
Accuracy data were provided in the recovery experiments. The recovery values and relative standard deviations (RSDs) of cyazofamid and CCIM in soil and tomato samples are shown in Table 1. The mean recoveries were in the range of 85–108% for cyazofamid in tomato and 76–90% in soil, while they ranged from 95 to 109% for CCIM in tomato and from 77 to 96% in soil. The RSDs (n = 5) of the method were varied from 0.86 to 3.3%. The recoveries and RSDs evidently indicated that the accuracy of method was sufficient for residue analysis.
The sensitivity of method was described in terms of LOD and LOQ. The LOD, a value corresponding to a signal-to-noise ratio of 3 (S/N = 3), was 0.10 μg/kg for cyazofamid in soil and tomato. It was 1.1 μg/kg for CCIM in soil and tomato. The LOQ was determined as a value corresponding to a signal-to-noise ratio of 10 (S/N = 10). It was 0.33 μg/kg for cyazofamid and 3.8 μg/kg for CCIM in both matrices. Methods for cyazofamid residue analysis in various crops have been established using high-performance liquid chromatography-photodiode array detection (HPLC-DAD) and gas chromatography-ion trap mass spectrometry (GC-ITMS) with LOQs of 6.7–720 μg/kg (González-Álvarez et al. 2012; González-Rodriguez et al. 2009; Lee et al. 2012; Watanabe et al. 2014). Obviously, our method is much more sensitive than these methods. Lee et al. (2013) developed a multiresidue analysis method using HPLC-MS/MS with a LOQ of 0.13 μg/kg for cyazofamid, which is comparable in sensitivity to our method. Even though the residue analysis of cyazofamid has been developed, methods for analyzing CCIM residues are rare. Recently, Lee et al. (2014) reported a method to analyze cyazofamid and CCIM residue in vegetables like kimchi cabbage and potato using LC-MS/MS, and the LOQ was 5 μg/kg for CCIM. It is evidently comparable in the LOQ value of CCIM to our method.
Dissipation kinetics
Dissipation of cyazofamid and CCIM in tomato
The dissipation studies were carried out in sites ZJ and HN. In site ZJ, the initial concentration of cyazofamid in tomato was 0.153 mg/kg, while 0.048 mg/kg was measured in tomato in site HN. The significant differences in the initial cyazofamid level of tomato may be due to different tomato species (Schulin et al. 1993; Singh et al. 2007). Cyazofamid dissipated slowly in tomato within 5 days after application. The half-lives of cyazofamid dissipation in tomato in sites ZJ and HN were 12.2 and 18.3 days, respectively (Table 2), which is evidently much longer than that in grape (Zhu et al. 2015).
In site ZJ, the concentration of CCIM in tomato was 0.00696 mg/kg at 2 h, increased to 0.00752 mg/kg on the first day after application, and then decreased gradually. More importantly, CCIM was not detected in tomato samples after 28 days of cyazofamid application. The half-life of CCIM in tomato in site ZJ was 12.3 days (Table 2). A similar tendency was observed in site HN; the concentration of CCIM was 0.00287 mg/kg initially and increased to 0.00545 mg/kg on the first day after application. Afterwards, CCIM was not detected in tomato after 3 days of cyazofamid application, which led to that the half-life of CCIM in tomato in site HN could not be estimated. According to previous studies, CCIM is formed in the first step of cyazofamid degradation and then converted to 4-(4-chloro-2-cyanoimidazole-5-yl) benzoic acid (CCBA) or 4-chloro-5-p-tolylimidazole-2-carboxylic acid (CTCA) through different pathways (Lee et al. 2016; US EPA 2015). We proposed that the rate of CCIM formation may be higher than that of CCIM degradation during the first day after application, which gave rise to that the concentration of CCIM peaked on the first day.
Dissipation of cyazofamid and CCIM in soil
In site ZJ, the initial concentration of cyazofamid in soil was 0.10 mg/kg, whereas it was 0.68 mg/kg in site HN. It has been reported that cyazofamid is highly affected by photolysis followed by aerobic soil degradation (Singh and Tandon 2015; US EPA 2004). In this work, the dissipation study was conducted under open-field condition in site ZJ, while it was under greenhouse condition in site HN. The dissipation of cyazofamid was probably dominated by photolysis during the first 2 h. Thus, it was enhanced when soils were exposed to sunlight directly in site ZJ, resulting in the low initial concentration of cyazofamid.
The half-life of cyazofamid in soil was 6.9 days in site ZJ, being longer than that in site HN, where the half-life was 3.6 days (Table 2). The dissipation rate of cyazofamid in site HN was greater than that in site ZJ (Fig. 2). We postulated that the different half-lives of cyazofamid were due to diverse activities of biotic degradation affected by soil texture, temperature, and pH (Lee et al. 2014; Singh and Tandon 2015). It has been shown that the fate of cyazofamid in soil is mainly controlled by biotic degradation (Singh and Tandon 2015). Zhu et al. (2015) reported that the dissipation half-life of cyazofamid in soil was 11 days under field condition. However, much shorter half-lives of cyazofamid in soil have also been observed in previous studies conducted under laboratory conditions; Singh and Tandon (2015) presented that the half-lives of cyazofamid in soils were in the range of 4.30–4.98 days, and Doshi et al. (2011) reported that the half-lives of cyazofamid varied from 3.02 to 6.1 days. Clearly, the T 1/2 of cyazofamid in this study is in comparable to the findings of Doshi et al. (2011) and Singh and Tandon (2015), but shorter than the finding of Zhu et al. (2015). As regard to CCIM, the initial concentration and dissipation half-life in site ZJ were 0.0292 mg/kg and 5.2 days respectively (Table 2). Moreover, the dissipation of CCIM in site HN was from 0.0671 mg/kg and characterized by the half-life of 11.6 days (Table 2), which was longer than that in site ZJ.
Residues
Residues of cyazofamid and CCIM in tomato and soil
The residues of cyazofamid and CCIM in tomato are presented in Table 3. When cyazofamid formulation was applied at the low dosage (recommended dosage), the residues of cyazofamid were 0.037–0.13, 0.044–0.075, 0.021–0.062, and 0.042–0.14 mg/kg in ZJ, HB, BJ, and HN, respectively. When compared to cyazofamid concentrations in tomato at the high application dosage, it indicated that the residues of cyazofamid declined with the decreasing application dosage. This phenomenon was also observed in the residues of CCIM in tomato. CCIM was sporadically detected with concentrations above the LOQ in tomato samples collected in trials with the low application dosage, while concentrations of CCIM in tomato samples were mostly higher than LOQ, with the values of 0.0026–0.020 mg/kg when cyazofamid formulation was applied at the high dosage.
The sampling interval was also a factor determining the residues of cyazofamid and CCIM. As shown in Table 3, the residues of cyazofamid generally decrease with increasing sampling intervals from 1 to 5 days. For instance, when cyazofamid formulation was sprayed at the dosage of 100.4 g a.i./ha for four times, the cyazofamid concentration was 0.13 mg/kg at sampling interval of 1 days and then deceased over 0.047 mg/kg at 3 days to 0.037 mg/kg at 5 days in site ZJ.
The residues of cyazofamid and CCIM in soil are presented in Table 4. When cyazofamid formulation was applied at the low dosage, the residues of cyazofamid were 0.045–0.11, 0.0046–0.018, 0.016–0.057, and 0.024–0.13 mg/kg in ZJ, HB, BJ, and HN, respectively, and the concentrations of CCIM were 0.0095–0.016, 0.0034–0.023, 0.0083–0.018, and 0.014–0.033 mg/kg in ZJ, HB, BJ, and HN, respectively. Clearly, there were negligible differences in cyazofamid and CCIM concentrations in soil among the four sites. It was observed that cyazofamid and CCIM concentrations were declined with the decreasing application dose and increasing sampling interval. This tendency is comparable with that observed in tomato.
Total residues of cyazofamid and CCIM
The sum of cyazofamid and CCIM, namely total residue, was calculated as the stoichiometric equivalent of cyazofamid (Tables 3 and 4). The total residues in tomato were 0.042–0.17, 0.047–0.26, 0.024–0.14, and 0.046–0.18 mg/kg in sites ZJ, HB, BJ, and HN, respectively. Also, the total residues in soil were 0.060–0.26, 0.0097–0.13, 0.028–0.13, and 0.045–0.27 mg/kg in sites ZJ, HB, BJ, and HN, respectively. The total residues in tomato and soil were dependent on weather conditions (sunlight, temperature and rain, etc.), soil characterization (pH and organic matter, etc.), crop species, planting conditions (irrigation and interplanting, etc.), and the history of pesticide application (Singh and Tandon 2015; Suciu et al. 2011; Tandon et al. 2012; Tiryaki and Temur 2010). It is of critical complication in field trials to elucidate the priorities of these factors in controlling the total residues. However, the purpose of the field tests was to evaluate the total residue originated from the application of cyazofamid formulation in typical agriculture production. In this work, the total residue was evaluated by comparing the total residue to available MRLs in tomato. As listed in Table S6, the MRL of cyazofamid in tomato has not been regulated in China, whereas it is available in countries of Korea, EU, Japan, and USA, with values ranging from 0.5 to 2 mg/kg. The total residue levels in tomato 1 day after the last application varied from 0.042 to 0.21 mg/kg at both application dosages when sprayed four times and from 0.024 to 0.26 mg/kg when sprayed five times. These residue values were all below 0.5 mg/kg, the lowest MRL of cyazofamid in tomato among available MRLs (Table S6).
Human exposure to cyazofamid via tomato intake
Intakes of cyazofamid by ten population subgroups were estimated, and the risk derived from cyazofamid via tomato intake was assessed. In agricultural practice, formulation of 100 g/L cyazofamid SC is recommended to apply on tomato at a dose of 79.5–100.4 g a.i./ha at seven to ten intervals for maximally four times, and the preharvest interval (PHI) is 1 day. Therefore, the total residues in tomato from trials of four sites corresponding to a sampling interval of 1 day were pooled and the 5th percentile, 95th percentile, and median values were calculated as 0.0375, 0.22, and 0.13 mg/kg, respectively. These values were used in the estimation of cyazofamid intakes and risk assessment for three scenarios.
The intakes of cyazofamid and RQ values of three scenarios for ten population subgroups are presented in Table 5. The intakes of cyazofamid ranged from 0.00021 to 0.00039, 0.00074 to 0.0013, and 0.0013 to 0.0023 mg/kg/day for scenarios I, II, and III, respectively. The intake of cyazofamid in scenario III was an order of magnitude higher than that in scenario I. In addition, it was highlighted that under the worst scenario, high intakes have to be expected for toddlers and children. The RQ values were all generally low (0.13–1.3%), indicating that the human risk derived from cyazofamid via tomato intake was acceptable. It is revealed that cyazofamid posed more risks on female than male within the same ages, since RQ values of females were higher than those of males (Table 5). Cyazofamid would also pose more risks on children and toddlers than adults. Based on the risk assessment, it was concluded that the recommended use of cyazofamid on tomato will not result in a consumer exposure exceeding the toxicological reference value and therefore is unlikely to pose a consumer health risk.
Conclusions
A rapid, robust, and sensitive method was established to determine cyazofamid and CCIM in soil and tomato samples, and the dissipation kinetics and residues of cyazofamid and CCIM were explored through field trials at four typical tomato-producing sites. The results showed that the dissipation half-lives of cyazofamid were 3.6–6.9 days in soil and 12.2–18.3 days in tomato. The half-lives of CCIM were 5.2–11.6 days in soil and 12.3 days in tomato. The total residues ranged from 0.024 to 0.26 mg/kg in tomato and from 0.0097 to 0.27 mg/kg in soil. Then, the risk assessment on human exposure to cyazofamid via tomato intake was performed, showing that RQ values were all generally low (0.13–1.3%). Only under the worst scenario, high intakes have to be expected for toddlers and children. Based on the risk assessment results, it was concluded that the recommended use of cyazofamid on tomato will not result in a consumer exposure exceeding the toxicological reference value and therefore it is unlikely to pose a consumer health risk. Our findings provide valuable information for risk assessment of cyazofamid. Future studies should be paid attention to cyazofamid use on all other crops and human exposure to cyazofamid via diet, including vegetables, fruits, and drinks.
References
Doshi V, Gupta VP, Dheer M (2011) Persistence studies of cyazofamid in potato plant soil and water. Academia Arena 3:25–30
Authority EFS (2016) Peer review of the pesticide risk assessment of the active substance cyazofamid. EFSA J 14. doi:10.2903/j.efsa.2016.4503
González-Álvarez M, González-Barreiro C, Cancho-Grande B, Simal-Gándara J (2012) Impact of phytosanitary treatments with fungicides (cyazofamid, famoxadone, mandipropamid and valifenalate) on aroma compounds of Godello white wines. Food Chem 131:826–836
González-Rodriguez RM, Cancho-Grande B, Simal-Gandar J (2009) Multiresidue determination of 11 new fungicides in grapes and wines by liquid-liquid extraction/clean-up and programmable temperature vaporization injection with analyte protectants/gas chromatography/ion trap mass spectrometry. J Chromatogra A 1216:6033–6042
Lee H, Kim E, Lee J, Sung J, Choi H, Kim J (2014) Analysis of cyazofamid and its metabolite in the environmental and crop samples using LC-MS/MS. B Environ Contam Tox 93:586–590
Lee H, Kim E, Moon J, Zhu Y, Do J, Oh J, Kwon K, Lee YD, Kim J (2012) Establishment of analytical method for cyazofamid residue in apple, mandarin, Korean cabbage, green pepper, potato and soybean. J Korean Soc Appl Biol Chem 55:241–247
Lee H, Kim E, Shin Y, Lee J, Hur H, Kim J (2016) Identification and formation pattern of metabolites of cyazofamid by soil fungus Cunninghamella elegans. Appl Biol Chem 59:9–14
Lee H, Park S, Lim MS, Lee H, Park H, Hwang HS, Park SY, Cho DH (2013) Multiresidue analysis of pesticides in agricultural products by a liquid chromatography/tandem mass spectrometry based method. Food Sci Biotechnol 22:1205–1216
Li H, Zhu X, Yang W, Yang G (2014) Comparative kinetics of Qi site inhibitors of cytochrome bc1 complex: picomolar antimycin and micromolar cyazofamid. Chem Biol Drug Des 83:71–80
Liu C, Lu D, Wang Y, Huang J, Wan K, Wang F (2014) Residue and risk assessment of pyridaben in cabbage. Food Chem 149:233–236
Lozowicka B (2015) Health risk for children and adults consuming apples with pesticide residue. Sci Total Environ 502:184–198
Ministry of Agriculture of the People’s Republic of China (2014) National Food Safety Standard—MRLs for pesticides in food (in Chinese).
Mitani S, Araki S, Matsuo N, Camblin P (1998) IKF-916—a novel systemic fungicide for the control of oomycete plant diseases. Brighton Crop Prot Conf Pests Dis 2:351
Mitani S, Araki S, Yamaguchi T, Takii Y, Ohshima T, Matsuo N (2001a) Antifungal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro. Pestic Biochem Phys 70:92–99
Mitani S, Araki S, Takii Y, Ohshima T, Matsuo N, Miyoshi H (2001b) The biochemical mode of action of the novel selective fungicide cyazofamid: specific inhibition of mitochondrial complex III in Phythium spinosum. Pestic Biochem Phys 71:107–115
Regueiro J, Olguín N, Simal-Gãndara J, Sunol C (2015) Toxicity evaluation of new agricultural fungicides in primary cultured cortical neurons. Environ Res 140:37–44
Schulin R, Desaules A, Webster R, von Steiger B (1993) Soil monitoring: early detection and surveying of soil contamination and degradation. Springer Basel AG.
Singh BK, Munro S, Potts JM, Millard P (2007) Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl Soil Ecol 36:147–155
Singh N, Tandon S (2015) Dissipation kinetics and leaching of cyazofamid fungicide in texturally different agricultural soils. Int J Environ Sci Technol 12:2475–2484
Small IM, Joseph L, Fry WE (2015) Development and implementation of the BlightPro decision support system for potato and tomato late blight management. Comput Electron Agr 115:57–65
Suciu NA, Ferrari T, Ferrari F, Trevisan M, Capri E (2011) Pesticide removal from waste spray-tank water by organoclay adsorption after field application to vineyards. Environ Sci Pollut Res 18:1374–1383
Tasho RP, Cho JY (2016) Veterinary antibiotics in animal waste: its distribution in soil and uptake by plants: a review. Sci Total Environ 563-564:366–376
Tandon S, Pujari A, Sand NK (2012) Dissipation studies of fentrazamide (YRC-2388) under anaerobic condition. J Environ Monit 14:2321–2526
Tiryaki O, Temur C (2010) The fate of pesticide in the environment. J Biol Environ Sci 4:29–38
US EPA (2015) Estimated drinking water concentrations of cyazofamid and its degradates of concern CCIM, CCIM-AM and CTCA, for use in human health risk assessment: petitions for the use on herb (subgroup 19A), greenhouse tomato, greenhouse pepper, and bulb vegetables (group 03–07) (PC code 085651; DP barcode D426776).
US EPA (2004) Pesticide Fact Sheet US EPA, Washington DC.
Watanabe E, Kobara Y, Baba K, Euu H (2014) Aqueous acetonitrile extraction for pesticide residue analysis in agricultural products with HPLC-DAD. Food Chem 154:7–12
Wu Y, Li X (2015) The fourth China total diet study, 1st edn Beijing
Zhang J, Chang V, Giannis A, Wang J (2013) Removal of cytostatic drugs from aquatic environment: a review. Sci Total Environ 445-446:281–298
Zhu G, Zheng Z, Jian Q, Nie S, Liang J, Fu Q (2015) Residue dynamics of cyazofamid and its metabolite in grape and soil. Argochemicals 54:438–441 (In Chinese)
Acknowledgements
This work was supported by Zhejiang Province of Natural Science Foundation (grant numbers LQ16B070003, 2015C32039), the National Natural Science Foundation of China (grant numbers 31401772, 31501685, 31501668), and Young Scientist Training Program of Zhejiang Academy of Agricultural Sciences. The authors are very grateful to anonymous reviewers for helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
ESM 1
(DOCX 324 kb)
Rights and permissions
About this article
Cite this article
Xu, Z., Zhang, C., Yu, J. et al. Field investigations of dissipations and residues of cyazofamid in soil and tomato: risk assessment of human exposure to cyazofamid via tomato intake. Environ Sci Pollut Res 24, 3483–3492 (2017). https://doi.org/10.1007/s11356-016-8106-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8106-y