Abstract
Causticized calcium carbonate (CCC), a solid waste derived from kraft black recovery process, can be used as an alternative for the conventional precipitated calcium carbonate (PCC). However, the application of the CCC has been limited due to its low sizing efficiency in its filled paper. In this study, the characteristics of the CCC were studied aiming to improve the alkyl ketene dimer (AKD) sizing performances of the CCC filled papers, and the results were compared with those from PCC filled papers. The results showed that the CCC had higher pore structure, higher specific surface area, and more negative charge density than the PCC, thus leading to a higher cationic AKD adsorption onto the CCC filler. The lower AKD sizing efficiency in the CCC filled paper can be explained by the combination of higher AKD adsorption and migration, both of which resulted in preferred AKD adsorption onto/into the CCC fillers, rather than the cellulose fibers. Based on the above, the prior addition of polyamide-polyamine epichlorhydrin (PAE) resin to the CCC filler system was proposed to remedy the related issues, thus improving the sizing efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the recycling and utilization of the solid waste, such as agricultural residuals and industrial wastes, have attracted increasing attention, and such a strategy fits well to the “sustainable development” strategy (Zhang et al. 2014). Causticized calcium carbonate (CCC), also known as “lime mud” or “lime sludge,” is a by-product that derives from the kraft pulping black liquor recovery system. The CCC can be used as an alternative for the conventional paper fillers such as precipitated calcium carbonate (PCC) and talc (Sohara and Ciccarelli 2008). There are many advantages to use the CCC as paper fillers, including the secondary pollution reduction (Tang 2003), cost savings, product performance improvements, etc. (Wang et al. 2012).
Much effort has been made aiming at commercializing the use of CCC fillers in the paper industry, including the fundamental understanding, processing conditions optimization, and morphology of the prepared CCC products. For example, the crystallization of calcium carbonate polymorphs and their relevant processing conditions for the CCC preparation have been studied (Cuesta et al. 2005, Kitamura et al. 2002). Wang et al. also investigated the effect of slaking temperature, slaking time, and causticizing time on the crystal morphology of the formed CCC (Wang et al. 2012). Naari et al. prepared the CCC in various forms such as rice-, spindle-, and needle-like particles; when applied as paper fillers, a high opacity and a low plastic-wire abrasion were also found (Nanri et al. 2008). Konno et al. also reported an industrial-scale process to manufacture the CCC (Konno et al. 2009).
PCC and GCC are common inorganic fillers in the paper industry. The filled papers, consisting mainly of cellulose fibers and fillers as minor components, are used for printing, writing and packaging, and decorating products. A satisfactory moisture resistance is desired for some of these products, particularly for the packaging and decorating papers. Paper sizing and coating by using some water-resistant agents have become effective approaches to improve the moisture resistance of these filled paper products. In practice, alkyl ketene dimer (AKD) has been widely used as one of the important neutral-alkaline sizing agents due to the development of papermaking process from the acid to neutral-alkaline environment (Priest 1989, Kondo and Makino 1993, Mattsson 2002, Dumas 1981).
Recently, many CCC filler production lines have been established in China and the CCC have been used commercially in the papermaking process. Unfortunately, the AKD sizing efficiency would decrease when the CCC was used as an alternative filler. Therefore, the addition of more AKD would be required in the CCC filled paper as compared to PCC filled paper when a similar water resistance was targeted. In this study, the AKD sizing performances on the CCC and PCC filled papers were compared. Furthermore, the mechanisms associated with a lower sizing efficiency for the CCC filled paper were also studied by determining the filler accessibility/morphology and charge properties. In addition, a potential technique for improving the AKD sizing efficiency on CCC filled paper was proposed.
Experimental section
Materials
Two industrial grade fillers, namely CCC and PCC, and the cationic polyacrylamide (CPAM) with a molecular weight (Mw) of 4 ~ 6 MDa were kindly provided by a paper mill in China. AKD emulsion, a neutral-alkaline sizing agent, with 1.45 eq/g of positive charge density and 15% of solids content, was purchased from Jinquan chemical additive Co., Ltd. in China. A polyamide-polyamine epichlorhydrin (PAE) resin, with 0.073 mmol/g of positive charge density and 12.5% of solids content, was also purchased from Jinquan chemical additive Co., Ltd. in China.
Characterization of the materials
The particle size of fillers was determined using a BT-9300H laser particle size analyzer (Mastersizer, UK). The pore volume and specific surface area of fillers were measured based on the Brunauer-Emmett-Teller (BET) analysis of nitrogen absorption isotherms using a Belsorp-Max volumetric gas adsorption instrument (Bel Japan, Inc., Osaka, Japan).
Determination of AKD adsorption on the fillers
It involved two steps. In the first step, using an ultraviolet spectrophotometer (PerkinElmer, Lambda 25, USA), the absorbance of different concentrations of AKD dispersion was determined at a wavelength of 238 nm. In the second step, different amount of AKD (based on filler) was added into the CCC suspension and stirred for 5 min. Then, the mixture was centrifuged for 10 min in the centrifuge at 3000 rpm. The supernatant liquor was used to determine the absorbance at the wavelength of 238 nm. The content of AKD in supernatant liquor was calculated according to the calibration curve. The AKD adsorbed by CCC was the difference between the amount of the total addition AKD and the amount of AKD in the supernatant liquor.
AKD sizing on the CCC or PCC filled papers
0.2% of AKD (based on oven-dry stock) was added to the dispersed stock, which contained 30 wt.% (based on the total weight of paper) of PCC or CCC. Then, 0.03% of CPAM was added after 30 s stirring, the stock was poured into the Rapid-Koethen sheet former, and handsheets were prepared with a basis weight of 80 g/m2. The wet paper was pressed under the pressure of 4 kg/cm2 for 3 min and dried for 5 min at 95 °C. The sheet was conditioned at 23 °C and relative humidity of 50% for 24 h, then, tested for the Cobb60 value.
Improvement of AKD sizing efficiency for the CCC filled paper
Different dosages of PAE were added into CCC slurry in advance, then, the prepared CCC slurry (30 wt.%, based on the total weight of paper) was added to the well-dispersed stock, which contained 0.2% of AKD (based on the oven-dry stock). After that, 0.03% of CPAM was added after 30 s stirring, the stock was poured into the Rapid-Koethen sheet former, and handsheets were prepared with a basis weight of 80 g/m2. The wet paper was pressed under the pressure of 4 kg/cm2 for 3 min and dried for 5 min at 95 °C. The sheet was conditioned at 23 °C and relative humidity of 50% for 24 h, then, tested for the Cobb60 value.
Acid treatment of sheet
The dried sheet was immersed into 300 mL of 0.1 mol/L HCl solution for 12 h and decomposed the calcium carbonate. Then the wet paper was dried again. The Cobb60 value was tested after conditioning the samples at 23 °C and relative humidity of 50% for 24 h.
Determination of Cobb60 for the papers
Cobb60, as an indicator of moisture (water) adsorption capacity of the sheet, was determined according to the standard method of ISO 535:2014. The Cobb60 was defined as the absorbed amount of water on an area of 1 dm2 when one side of the paper sheet was exposed to a water pressure of 1 cm height for 60 s at the temperature of 23 ± 1 °C. The Cobb60 was calculated according to Eq. (1):
where m a is the weight of the sheet exposed to water, and m b is the weight of the sheet before exposure.
Zeta potential measurement
The filler slurry was prepared with concentration approximately 10 g/L, and zeta potential instrument (SZP 06, Mütek, Germany) was used for determining the zeta potential of the filler.
Charge density measurement
The charge density of fillers was measured by using a particle charge determination (PCD-03) device. The filler was first dispersed in the deionized water to obtain a well-dispersed suspension with concentration of about 0.1 g/L. The titration of the prepared filler suspensions (10 mL) was conducted by using the PCD-03 equipped with an automated titrator system at 25 °C. The charge density was calculated according to Eq. (2):
where q is the charge density of the filler, V is the standard liquid titrating dosages of the sample, V 0 is the standard liquid titrating dosages of deionized water, C is the charge quantity of the standard liquid, and W t is the weight of oven-dried CCC in 10 mL of the sample.
Results and discussion
The AKD sizing efficiency for the two different filled papers
The results of AKD sizing efficiency in the CCC and PCC filled papers are shown in Fig. 1. Evidently, with the increase of AKD in the filled papers, the Cobb values of both CCC and PCC filled paper decreased, where the Cobb value of CCC filled paper was higher than that of PCC filled paper under a similar CCC and PCC filler loading condition. The results revealed that the AKD sizing efficiency in the CCC filled paper was much lower than that in the PCC filled paper.
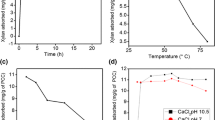
The AKD adsorption capacities onto the two different calcium-based fillers were also investigated. As shown in Fig. 2, the AKD adsorption onto the CCC filler was much higher than that onto the PCC filler as the AKD loading increased. For example, the AKD adsorption of CCC filler increased from 31.41 to 40.26 mg/g when the AKD loading increased from 5 to 10%, while for the PCC filler, it increased from 15.76 to 19.34 mg/g under the same conditions.
Proposed hypothesis for a different AKD sizing efficiency on the two filled papers
The schematic of the AKD sizing on the CCC and PCC fillers was illustrated in Fig. 3. We hypothesized that two mechanisms can be attributed to the lower AKD sizing efficiency of the industrial-grade CCC filler in comparison with the PCC filled paper:
-
1.
A higher AKD adsorption onto CCC filers, due to higher surface areas and more favorable charge density/zeta potential. As shown in Table 1, CCC filers have higher surface area (23.97 vs 11.77 m2/g); also, their charge characteristics, both the physical and electrostatic adsorption, could lead to more AKD adsorption onto the filler surfaces. In the literature, it is known that the filler morphology, e.g., specific surface area and pore structure, is strongly related to the polymer adsorption (Wang et al. 2013). Besides, the filler charge properties, e.g., surface charge or zeta potential, are also critical for the polymer adsorption (Peng et al. 2015, Yang et al. 2012).
-
2.
More AKD migration into CCC porous structure. The presence of more pores (larger pore volumes) and higher amount of AKD at the CCC filer surface would lead to more AKD migrating into the filler porous structures, in particular, during the drying process. It was reported that the amount of the adsorbed AKD and the pore structure of the filler are the two key parameters in determining the degree of AKD migration (Bartz et al. 1994).
As shown in Fig. 3, competitions of adsorbing AKD between the cellulose fibers and fillers were demonstrated in a process of AKD sizing in the filled papers. For the fillers, an initial adsorption of a number of AKD on the filler surface, followed by a melting and spreading process of these adsorbed AKD, and a final migration of a fraction of AKD into the pores structure under the drying condition would happen in both the PCC and CCC fillers (Yang et al. 2012, Yang et al. 2013). For the PCC filler (top in Fig. 3), a less amount of AKD would be adsorbed on the filler surface due to its lower accessibility and the electrostatic repulsion in comparison to the CCC filler. Moreover, a lower amount of adsorbed AKD and the filler accessibility would lead to a less migration of AKD during the following spreading and migrating processes. Therefore, more amount of AKD would be adsorbed on the cellulose fiber, thus contributing a high sizing efficiency on PCC filled paper. In a study, Barta et al. evaluated sheet drying temperature, calcium carbonate pigment size and shape, filler loading, and size addition level on alkaline sizing efficiency and reversion and found that a fraction of AKD molecules migrated into the pores of the PCC particles in drying process which led to sizing reversion (Bartz et al. 1994).
In contrast, For the CCC filler (bottom in Fig. 3), a higher AKD adsorption on its surface would be achieved because of a higher filler accessibility and the electrostatic attraction, thus leading to a more remarkable AKD migration after the spreading and migrating processes. As a consequence, more AKD would be adsorbed and migrated onto/into the fillers rather than onto the cellulose fibers, which is particularly true for the CCC filled paper, thus resulting in a low sizing efficiency.
Filler morphology and charge property
The specifications of the CCC and PCC fillers regarding morphology and charge density were showed in Table 1. For the filler morphology, it can be found that the CCC filler had a higher average particle size (4.64 μm) than the PCC filler (3.91 μm). The pore volume and specific surface area of the CCC filler were 0.045 cm3/g and 23.97 m2/g, respectively, which were consistently higher than those of the PCC filler (0.015 cm3/g and 11.77 m2/g). The above filler morphology results indicated that the CCC filler had higher filler affinity than the PCC filler, which would be partially responsible for the observed higher AKD adsorption (Fig. 3). In one study, Yang et al. found that CCC was rich in small pores with large pore area, which can exhibit significant effect on AKD sizing efficiency (Yang et al. 2013). In another study, Wang et al. reported that the higher surface areas (BET and BJH) of CCC led to a high adsorbility towards AKD (Wang et al. 2013).
For the charge property, the CCC filler had a high negative charge density (−13.98 μmol/g), with its zeta potential of −12.5 mV, while the PCC filler had a positive charge of 2.25 μmol·g−1, with zeta potential of 5.9 mV. The charge density of the AKD solution was 1.45 eq/g under the condition. Therefore, it can be expected that a strong electrostatic attraction would occur between the CCC filler and AKD, which supported the hypothesis that a stronger AKD adsorption action occurred for the CCC filler due to the electrostatic adsorption mechanism. Peng et al. investigated the effect of the pre-flocculation of CCC on AKD sizing efficiency, which revealed that the native CCC was negatively charged and assumed that AKD adsorption was affected by zeta potential of filler particles (Peng et al. 2015).
The AKD migration mechanism
The migration mechanism of the adsorbed AKD was further investigated via the determination of the Cobb60 results of the filled paper with/without acid treatment. As shown in Fig. 4, the Cobb60 of CCC filled paper remarkably decreased as a result of acid treatment, from 94.3 g/m2 of the untreated one to 23.3 g/m2 of the acid-treated one. In the acid treatment process, the calcium carbonate fillers were dissolved; thus, those AKD that were with fillers would become available to cellulose fibers, resulting an increase in the sizing degree (decrease in the Cobb60 value). In the case of CCC filled sample, the significant increase in the sizing degree was due to the fact that the entrapped/absorbed AKD (inside the CCC pores/onto the CCC surfaces) could then be re-distributed to the cellulose fibers.
Compared to a huge decrease of the Cobb60 between the treated and untreated sample for the CCC filled paper (about 71.0 g/m2), the change of Cobb60 in the PCC filled paper before and after the same acid treatment was much smaller (about 9.3 g/m2, from 29.5 to 20.2 g/m2). Therefore, it can be confirmed that a large amount of AKD had migrated and remained into the pores of the CCC filler rather than that of the PCC filler after the drying process. As a result of acid treatment, a drastic decomposition of calcium-based fillers occurred under the acid condition and allowed the release of a large amount of adsorbed and migrated AKD onto/into the CCC fillers to the cellulose fibers, thus resulting in a decreased Cobb60 or an enhanced water resistance.
Improvement of the AKD sizing efficiency for the CCC filled paper
Based on the comprehensive understanding of the adsorption and migration mechanisms mentioned above, a possible technique, i.e., the addition of PAE into the CCC slurry prior to the AKD sizing, was proposed to improve the AKD sizing performance of the CCC filled paper.
Shown in Fig. 5 are the changes of the AKD adsorption capacity and the Cobb60 values of the CCC filled papers as a function of the PAE dosage. It can be found that the adsorbed AKD initially decreased fast from about 33.3 to 16.4 mg/g as the addition of PAE increased from 0 to 0.20% and then nearly reached a plateau of 16.4 mg/g as the increase of PAE continued. It can be further found that the changes of the Cobb60 values of the modified CCC filled papers were highly in agreement with the changes of the AKD adsorption capacity. These results can be explained by the fact that due to the physical and electrostatic interactions, PAE with high cationic charge density was readily adsorbed onto the CCC filler prior to the addition of AKD, thus decreasing the AKD adsorption onto the CCC fillers, consequently the AKD migration to the inside pores of these CCC fillers via electrostatic repulsion between the already adsorbed PAE and the AKD. Interestingly, when the dosage of PAE resin was 0.2% (based on the weight of filler), the Cobb60 of the modified CCC filled paper (35.4 g/m2) was a little higher than that of the PCC filled paper (29.5 g/m2, shown in Fig. 1). Therefore, it can be concluded that the addition of PAE can be used as one of the effective solutions to improve the AKD sizing efficiency of the CCC filled paper, which may serve as a practical method to expand the application of CCC as a low-cost filler for the paper industry.
Conclusions
Causticized calcium carbonate (CCC) from the kraft pulping black liquor recovery process can be alternative fillers for fine paper production. In this paper, the performances of AKD sizing on the CCC filled papers and the related mechanisms were investigated, and the results were compared to those from the PCC filled paper. It was found that the CCC filler had higher pore structure, specific surface area, and negative charge density than the PCC filler, all of which rendered a higher filler physical adsorption and a stronger electrostatic adsorption towards the AKD emulsion. Consequently, more adsorbed AKD will migrate from the surface into the pore structure of the fillers during the drying process. The lower AKD sizing efficiency for the CCC filled paper in comparison with PCC filled paper can be attributed to (1) higher AKD adsorption and (2) more AKD migration.
Based on the above further understanding, the use of cationic PAE was studied to improve the AKD sizing of the CCC filled paper, and it was found that the addition of PAE prior to the CCC filler system can decrease the adsorption and migration of AKD onto/into the CCC fillers, thus improving the AKD sizing efficiency of the CCC filled paper.
References
Bartz WJ, Darroch ME, Kurrle FL (1994) Alkyl ketene dimer sizing efficiency and reversion in calcium carbonate filled papers. TAPPI J 77:139–148
Cuesta C, Amaré J, Cebrián S, García E, Ginestra C, Martínez M, Oliván MA, Ortigoza Y, Solórzano AOD, Pobes C (2005) Synthesis of single phase aragonite precipitated calcium carbonate in Ca(OH)2-Na2CO3-NaOH reaction system. Korean J Chem Eng 22:852–856
Dumas DH (1981) An overview of cellulose-reactive sizes. Journal of the Technical Association of the Pulp and Paper Industry 64:43–46
Kitamura M, Konno H, Yasui A, Masuoka H (2002) Controlling factors and mechanism of reactive crystallization of calcium carbonate polymorphs from calcium hydroxide suspensions. J Cryst Growth 236:323–332
Kondo N, Makino S (1993) Neutral/alkaline papermaking system by using specialized cationic polymers. Mechanism of enhancing AKD sizing.:mechanism of enhancing AKD sizing. Journal of the Japanese Technical Association of the Pulp & Paper Industry 47:234–238
Konno H, Goto H, Takahashi K, Nanri Y (2009) An innovative process to manufacture calcium carbonate by the causticizing process in a kraft pulp mill. Lab Anim Sci 43:646–649
Mattsson B (2002) AKD sizing:dispersion colloidal stability, spreading and sizing with pre-flocculated dispersion. Licentiate thesis, Luleå University of Technology
Nanri Y, Konno H, Goto H, Takahashi K (2008) A new process to produce high-quality PCC by the causticizing process in a kraft pulp mill. TAPPI J 7:19–24
Peng Y, He B, Zhao L (2015) The effect of pre-flocculation of lime mud by wet-end additives on paper properties. Nord Pulp Pap Res J 30:243–249
Priest DJ (1989) Permanence and alkaline—neutral papermaking. American Chemical Society, p 2–12
Sohara JA, Ciccarelli SA (2008) Precipitated calcium carbonate from kraft pulp lime mud for use in filled and coated paper. US, 20080053337
Tang YL (2003) An overview of comprehensive utilization of white mud. World Pulp & Paper 22:53–55
Wang J, Wei P, Liu P, Sun W (2012) Identifying appropriate conditions for producing spindle-like causticizing precipitated calcium carbonate for paper filler applications. Bioresources 7:5894–5903
Wang J, Liu L, Wang Z, Xu Y (2013) AKD sizing efficiency of paper filled with CaCO3 from the kraft causticizing process. Bioresources 9:143–149
Yang Y, Liu JG, Su YQ (2012) Adsorption behaviour of alkyl ketene dimer on lime mud calcium carbonate. China Pulp & Paper 31:13–17
Yang Y, Su YQ, Liu JG (2013) Influence of drying and curing conditions on AKD sizing of lime mud calcium carbonate filled paper. Paper & Paper Making 32:40–43
Zhang J, Zheng P, Wang Q (2014) Lime mud from papermaking process as a potential ameliorant for pollutants at ambient conditions: a review. J Clean Prod 103:828–836
Acknowledgments
This work was financially supported by the China State Key Laboratory of Pulp and Paper Engineering Fund (Grant No. 201508) and the Shaanxi University of Science and Technology Scientific Research Fund (BJ12-16).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Wang, J., Dang, M., Duan, C. et al. Further understanding on the mechanism of alkyl ketene dimer sizing on the causticized calcium carbonate filled paper and its improvements. Environ Sci Pollut Res 24, 4822–4827 (2017). https://doi.org/10.1007/s11356-016-8078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8078-y