Abstract
A range of pesticides are available in Australia for use in agricultural and domestic settings to control pests, including organophosphate and pyrethroid insecticides, herbicides, and insect repellents, such as N,N-diethyl-meta-toluamide (DEET). The aim of this study was to provide a cost-effective preliminary assessment of background exposure to a range of pesticides among a convenience sample of Australian residents. De-identified urine specimens stratified by age and sex were obtained from a community-based pathology laboratory and pooled (n = 24 pools of 100 specimens). Concentrations of urinary pesticide biomarkers were quantified using solid-phase extraction coupled with isotope dilution high-performance liquid chromatography–tandem mass spectrometry. Geometric mean biomarker concentrations ranged from <0.1 to 36.8 ng/mL for organophosphate insecticides, <0.1 to 5.5 ng/mL for pyrethroid insecticides, and <0.1 to 8.51 ng/mL for all other biomarkers with the exception of the DEET metabolite 3-diethylcarbamoyl benzoic acid (4.23 to 850 ng/mL). We observed no association between age and concentration for most biomarkers measured but noted a “U-shaped” trend for five organophosphate metabolites, with the highest concentrations observed in the youngest and oldest age strata, perhaps related to age-specific differences in behavior or physiology. The fact that concentrations of specific and non-specific metabolites of the organophosphate insecticide chlorpyrifos were higher than reported in USA and Canada may relate to differences in registered applications among countries. Additional biomonitoring programs of the general population and focusing on vulnerable populations would improve the exposure assessment and the monitoring of temporal exposure trends as usage patterns of pesticide products in Australia change over time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are used in agricultural and domestic settings to control pests. Pesticides include but are not limited to insecticides, such as pyrethroids and organophosphates, herbicides, and insect repellents. In Australia, limited information exits about use and availability of insect repellents, but a range of pyrethroid and organophosphate pesticides are commonly available (Table 1). Pyrethroids, the most prevalent insecticide class on the Australian market, are found in an array of domestic (e.g., aerosols, medical treatments, veterinary products) and agricultural pest control products (APVMA 2015). Diazinon, maldison/malathion, and chlorpyrifos, the most commonly available organophosphates for domestic applications, are found in spray products, veterinary products, head-lice treatments, and garden treatments (APVMA 2015). Chlorpyrifos and dimethoate are the most commonly found organophosphate residues in foods sold in Australia (FSANZ 2011).
Pyrethroids and organophosphates are neurotoxins, and chronic early life exposure has been associated with a range of adverse health effects in humans, including cognitive deficits (Bouchard et al. 2011; Rauh et al. 2012; Koureas et al. 2012; Shelton et al. 2014) and increased incidence of childhood cancers (Roberts and Karr 2012; Turner et al. 2010). Routes of pesticide exposure include dermal, inhalation, and dietary/non-dietary ingestion, with food residues being an important source that varies markedly by region (Becker et al. 2006; Riederer et al. 2008; Morgan 2012; Trunnelle et al. 2014). Additional exposure pathways and sources specific to young children, such as mouthing and lower breathing zone compared to adults (Tulve et al. 2002; WHO 2011), may place young children at greater risk of both acute and chronic pesticide exposures; in one survey of insecticide-related calls to an Australian poison control center, children under the age of 4 years accounted for half of all calls (English et al. 2015).
The number of pesticides, particularly insecticides and herbicides, registered for domestic and agricultural use in Australia exceeds those in other countries and regions, including the USA and the European Union (Babina et al. 2012). Annual insecticide and herbicide sales in Australia exceeded 1.8 billion Australian dollars in 2012–2013 financial year (APVMA 2014). However, as no integrated pesticide usage reporting system exists in Australia, quantitative data are scarce (Radcliffe 2002).
Biomonitoring is a tool increasingly used for exposure assessment (NRC 2006), and urinary metabolites are commonly used biomarkers of exposure for non-persistent chemicals. Biomonitoring data of pesticides exist for populations in Europe (Becker et al. 2006; Schettgen et al. 2002; Becker et al. 2006; Roca et al. 2014), North America (CDC 2015; Fortin et al. 2008), and China (Guodong et al. 2012), but there are no large-scale data currently available for the general Australian population. Here, we present a preliminary age and sex-stratified characterization of exposure to select organophosphate insecticides, pyrethroid insecticides, the insect repellent N,N-diethyl-meta-toluamide (DEET), and the herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) in a convenience sample of Australian residents using a simple and cost-effective pooled urine-sampling approach.
Materials and methods
Study population and sample collection
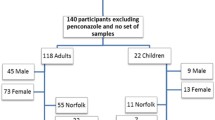
For this cross-sectional study, de-identified specimens were obtained from a community-based pathology laboratory (Sullivan Nicolaides Pathology, Taringa) from surplus-archived urine that had been collected and analyzed as part of routine testing throughout the state of Queensland, Australia, with the majority of samples collected from sub-tropical South East Queensland. Urine specimens were collected from November 2012 to November 2013 in sterile polyethylene urine specimen containers, refrigerated at 4 °C for up to 3 days, and then frozen. As this was a pre-existing, convenience population, no specific sampling protocols were employed. This work was approved by the University of Queensland ethics committee (approval number 2013000397). The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subject research.
Pooling protocol
Descriptive information about each specimen was limited to date of sample collection, sex, and date of birth of the individual. Before pooling, samples were stratified by age and sex into the following strata: 0–4, 5–14, 15–29, 30–44, 45–59, and >60 years. The mean age of each pool was calculated from the average age of the individuals making up that pool. A total of 2400 individual specimens were combined into 24 pools, with 100 individual specimens contributing to each pool; there were 2 pools for each of the 12 age-sex strata. Specimens were pooled based on volume, where each individual in the pool contributed the same volume; thus, the concentration measured in each pool is equivalent to the arithmetic mean of the concentration in each individual sample contributing to the pool (Caudill 2010). During pooling, individual urine specimens were thawed, homogenized, and aliquoted, after which the pooled sample was well mixed, divided into smaller aliquots, and frozen until analysis. A synthetic urine sample was included as a procedural blank (Calafat and Needham 2009). No measures of creatinine or specific gravity were available for individual samples.
Chemical analysis
Pooled urine samples were shipped on ice to the CDC (Atlanta, USA) for chemical analysis and analyzed for several pesticide biomarkers (Table 2), specifically six non-specific organophosphate metabolites, dimethyl phosphate (DMP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), diethyl phosphate (DEP), diethyl thiophosphate (DETP), and diethyl dithiophosphate (DEDTP); four specific organophosphate metabolites, 3,5,6-trichloro-2-pyridinol (TCPY), malathion dicarboxylic acid (MDA), 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMPY), and paranitrophenol (PNP); four pyrethroid metabolites, 3-phenoxybenzoic acid (3-PBA), 4-fluoro-3-phenoxybenzoic acid (4-F-3-PBA), cis-3-(2,2-dibromovinyl)-2,2-dimethyl cyclopropane carboxylic acid (DBCA), and trans-3-(2,2-dichlorovinyl)-2,2-dimethyl cyclopropane carboxylic acid (trans-DCCA); two phenoxy acid herbicides, 2,4-D and 2,4,5-T; and DEET and its metabolites 3-diethylcarbamoyl benzoic acid (DCBA) and N,N-diethyl-3-(hydroxymethyl) benzamide (DHMB). For analysis, we used 96-well plate-based or online solid-phase extraction and high-performance liquid chromatography–isotope dilution–tandem mass spectrometry approaches as described before (Kuklenyik et al. 2013; Davis et al. 2013; Odetokun et al. 2010). Accuracy and precision for each analytical run were monitored through the use of calibration standards, reagent blanks, and quality control materials of high and low concentrations. The limits of detection (LODs) ranged from 0.08 to 0.50 ng/mL and are listed in Table 3.
Statistical analysis
The influence of age (in years) on chemical concentration was assessed via curvilinear regression, as follows:
Concentrations below the LOD were replaced with the LOD divided by the square root of 2 (Hornung and Reed 1990). All regression analyses were conducted using Stata statistical software v12.1 (StataCorp, College Station, TX, USA). Criteria for significance were set as p < 0.05.
Results
Organophosphate insecticide metabolites were detected in >96 % of pooled samples with the exception of DMDTP (75 %), DETP (83 %), and DEDTP, which was not detected in any sample. Overall, the concentrations of these organophosphate metabolites were relatively low (geometric mean [GM] 13.6, 10.6, 0.41, 6.18, and 1.25 ng/mL for DMP, DMTP, DMDTP, DEP, and DETP, respectively) and ranged from <0.1 to 19.2 ng/mL for DAPs and from <0.1 to 3.09 ng/mL for the specific metabolites (GM 1.00, 0.38, and 1.76 ng/mL for MDA, IMPY, and PNP, respectively). The highest concentrations were for TCPY with GM 23.0 ng/mL and range 2.0–36.8 ng/mL (Table 3).
Pyrethroid metabolites DBCA, 3-PBA, and trans-DCCA were detected at GM 1.25, 1.21, and 1.89 ng/mL, respectively, with a maximum concentration of 5.51 ng/mL for trans-DCCA in females, 5–15 years (Table 3). 4-F-3-PBA was undetectable in all samples. Similarly, phenoxy acid herbicide metabolites 2,4-D and 2,4,5-T were low (maximum concentration 7.83 ng/mL and undetectable in all pools, respectively). The detection frequency of parent compound DEET was low (17 %), but metabolites DCBA and DHMB were detected in all pooled samples, with GM concentrations of 65.9 and 0.83 ng/mL, respectively (Table 4). All analytes were non-detectable in the synthetic urine sample with the exception of DCBA (0.55 ng/mL), a concentration quite close to the LOD for this analyte (0.48 ng/mL). Because the lowest DCBA concentration detected in the pools was nearly one order of magnitude greater (4.23 ng/mL; Table 3), we applied no blank correction to any DCBA results.
The association of age with urinary concentrations was examined using curvilinear modeling (see Supplementary Material 1 for regression coefficients). There was a significant association with age only for the organophosphate metabolites DMDTP, DETP, DMTP, TCPY, and PNP (Fig. 1). The percentage of variability explained by the model was 56, 42, 27, 28, and 22 %, respectively (Supplementary Material 1). The association of age with concentration was generally U-shaped, with highest concentrations at increasing extremes of age (i.e., youngest and oldest age strata), as well as a slight additional increase in concentration for the younger age groups only.
Urinary total concentration (ng/mL) versus age (years) for pesticide metabolites with significant age-concentration relationships, DMTP (a), DMDTP (b), DETP (c), TCPY (d), and DCBA (e). The triangles denote the female pools, and the squares denote the male pools. The horizontal line indicates the mean concentration of four pools in each age stratum. The curvilinear regression line (solid line) with 95th confidence intervals (dotted lines) are presented
Discussion
For the first time, we report age- and sex-stratified urinary metabolites of organophosphate and pyrethroid insecticides, an insect repellent, and select phenoxy acid herbicides in a convenience sample of the general Australian population using samples pooled by age and sex. Pyrethroid and organophosphate insecticides are widely available in Australia (Table 1), and this fact was reflected by the high detection frequency (>90 %) of all metabolites measured, with the exception of DEDTP and 4-F-3-PBA. The low detection frequency of DEDTP and 4-F-3-PBA (we did not detect these compounds in any samples) is similar to studies in other countries (CDC 2015; Roca et al. 2014; Becker et al. 2006; Guodong et al. 2012). The herbicide 2,4-D and metabolites of the insect repellent DEET, DHMB, and DCBA were also detected in all pooled samples and at relatively high concentrations.
Age has previously been demonstrated to be associated with insecticide concentrations, with higher concentrations typically found in young children (CDC 2015). However, for most of the metabolites in this study, we observed no association between concentration and age (Supplementary Material 1). Age was only significantly associated with concentrations of five organophosphate metabolites, namely, DMDTP, DMTP, DETP, TCPY, and PNP, and the association was curvilinear (Fig. 1), with higher concentrations in the younger and older (>60) age strata. With the exception of DMDTP (56 %) and DETP (42 %), the variability in metabolite concentration explained by age was low (<30 %) and the magnitude of the effect size of age on measured concentration was relatively small (Supplementary Material 1), where the greatest difference in concentration between age strata was less than one order of magnitude. This suggests that factors other than age and sex, such as specific behavioral and lifestyle factors, for example, domestic use patterns of pesticide products, also influence urinary concentrations.
The concentration differences in the youngest (<5 years) and oldest age strata (>60 years) may be related to absolute differences in external exposure compared to other age groups and/or age-related differences in behavior and physiology. Young children and older persons may have greater exposure to insecticides because of a relatively greater period of time spent in the indoor environment, where concentrations of these compounds are typically higher than outdoors (Rudel and Perovich 2009). Infants occupy different microenvironments than adults and often for prolonged periods of time. Because the floor is a critical zone for very young children (<2 years of age), consumer products that result in widespread floor contamination or are only applied to the floor, such as “trigger-spray” insecticides or aerosols, may be particularly relevant for children’s exposures. Young children experience proportionally greater chemical exposures than adults due to physiological differences, including a relatively greater surface area to volume ratio (for dermal exposure), and increased respiratory and metabolic rates (Makri et al. 2004; WHO 2011). Early life exposures are of particular concern because of the disproportionate future years of life, providing a longer time frame to manifest a disease that has a long latency period (Scheuplein et al. 2002; WHO 2011).
Older adults may also have relatively higher urinary concentrations of certain pesticide biomarkers because of differences in lifestyle factors and physiology compared with younger age groups. Glomerular filtration rate decreases with age and is associated with decreased clearance of metabolites from the blood (ABS 2013). Additionally, the age distribution of the Australian population varies geographically, with older adults (>65 years) making up a larger proportion of the population in inner regional areas than in major cities (Baxter et al. 2011). A previous study demonstrated that urinary concentrations of several insecticide metabolites were higher for participants residing in inner regional areas of South Australia compared to the major city, and this was attributed to agricultural practices (Babina et al. 2012). In the current study, no data was available regarding the geographic distribution of individuals contributing to the pooled samples or regarding the potential for occupational pesticide exposures. It is, therefore, possible that geographic differences across the age pools may also have contributed to the observed association of greater concentrations of some pesticide metabolites in urine pools from older adults. Stratification of pooled samples via sex, age, and geographic distribution would be necessary to determine the individual effect of age and geographic distribution on concentrations. Additionally, the potential contribution of occupationally exposed individuals is likely to be a very small proportion of the 2500 individual specimens, as the pathology collection center used to source the specimens is located in South East Queensland and primarily serves residential Brisbane.
Concentrations of non-specific organophosphate metabolites DMTP, DEP, and DETP measured in Australian pooled urine (GM 10.6, 6.18, and 1.84 ng/mL, respectively) were generally higher than concentrations reported in general populations in Canada (~2.03, 2.30, and not measured, respectively; Haines and Murray 2012) and the USA (2.28, <LOD, and <LOD, respectively; CDC 2015). DMTP, DEP, and DETP are non-specific organophosphate biomarkers derived from the metabolism of a wide array of commonly available organophosphates in Australia, such as chlorpyrifos (128 products), diazinon (45 products), and maldison/malathion (25 products; APVMA 2015). Urinary concentrations of TCPY, a specific metabolite of chlorpyrifos, reported in this study (GM 22.6 ng/mL [0–4 years] and 26.3 ng/mL [5–14 years]) were similar to those reported in children in South Australia (3–6 years, n = 115, arithmetic mean 21.5 μg/g creatinine; Babina et al. 2012) but substantially higher than urinary concentrations reported in children in Spain (6–11 years, n = 125, GM 3.36 ng/mL; Roca et al. 2014) and USA (6–11 years, n = 386, GM 1.12 ng/mL; CDC 2015) (Table 4). Chlorpyrifos is still available for limited domestic applications in Australia and widely available for agricultural use. By contrast, in the USA, in 2000, chlorpyrifos was restricted from homeowner applications as well as some agricultural applications (US EPA 2000), and since then, urinary concentrations of TCPY in the general USA population have been in decline (CDC 2015). PNP, a metabolite of parathion and parathion methyl, was detected in all pools in our study (GM 1.76 ng/mL, range 1.04–3.09 ng/mL). Interestingly, PNP concentrations in children 0–4 years (GM 1.90 ng/mL) were lower than those reported by Babina et al. (2012) during the period of 2003–2006, which may reflect declining exposures as use of parathion and parathion methyl was phased out in Australia in 2011, prior to the commencement of this study (Agriculture Victoria 2015).
The concentrations of pyrethroid metabolites DBCA, 3-PBA, and trans-DCCA (GM 1.25, 1.21, and 1.89 ng/mL, respectively) in this study were similar to concentrations reported in Spain (0.9, 4.76, and 2.16 ng/mL, respectively; Roca et al. 2014) and some adult populations in the USA (<0.4, 1.52, and 3.41 ng/mL, respectively; Davis et al. 2013) but higher than those reported in Canada (Fortin et al. 2008), France (Le Grand et al. 2012), Germany (Becker et al. 2006; Schettgen et al. 2002), Poland (Wielgomas and Piskunowicz 2013; Wielgomas et al. 2013), and USA National Health and Nutrition Examination Survey (NHANES) (CDC 2015) (all <0.1, <0.8, and <0.5 ng/mL, respectively; Table 4). With the exception of DBCA, which is a metabolite of deltamethrin, 3-PBA and trans-DCCA are non-specific metabolites for the commonly available pyrethroids cypermethrin, deltamethrin, and permethrin. Studies in the USA have shown that pyrethroid metabolite concentrations vary by geographical region and by pest control practices in the home, with dietary habits having less of an impact than for organophosphates (Lu et al. 2006). Higher pyrethroid metabolite concentrations in this study may therefore reflect more frequent or intense use of these household insecticides in South East Queensland.
Concentrations of primary DEET metabolite DCBA, ranging from 4.23 to 850 ng/mL (GM 65.9 ng/mL), are similar to the range reported in 2007–2008 NHANES (<0.93 to 5760 ng/mL; CDC 2016a) but not as high as in the 2009–2010 survey cycle (<0.48 to 30,400 ng/mL; CDC 2016b). These relatively high urinary concentrations (compared with other pesticide metabolites; Tables 3 and 4) may be related to low cost and high availability of DEET-containing products (Costanzo et al. 2007), particularly in Northern Queensland where DEET is used to protect against mosquito-borne diseases (Larson et al. 2000). Queensland has a hot, sub-tropical climate with a high-pest burden, which may explain relatively greater use of household insecticides. For this reason, the sampled population is unlikely to be representative of the general Australian population for all pesticide metabolites.
We have made a number of assumptions that must be considered when interpreting the results of this study: (1) pathology specimens do not introduce significant bias into the study population, (2) pooled samples provide an accurate measurement of mean concentration, and (3) spot samples provide a reasonable estimate of internal exposure over a given time frame. The study population consisted of convenience samples collected during the course of routine pathology testing. No creatinine or specific gravity data were available for the samples used in this study. However, for the interpretation of pooled measurements as representative measures of average concentration, variation in individual sample hydration status is expected to be averaged out and not introduce significant bias to the estimated average concentrations and excretion rates. For some compounds, there was a considerable difference in measured concentration for replicate pools within an age strata. For example, measured concentrations of TCPY in 0–4-year-old pools ranged from 12 to 36.8 ng/mL, while DCBA concentrations ranged from 61.6 to 769 ng/mL in 5–14-year-old pools (Table 3). As each pool contains a large number of individual samples (n = 100), the concentration of any one individual is unlikely to influence the pool mean, and thus, this variability is likely a reflection of actual variation in exposure within the given demographic stratum. One limitation of a pooled sampling approach is that it cannot provide any information on variance within a population. Ad hoc methods can be applied to estimate upper bound reference values, which may be important in a health risk context (Aylward et al. 2014).
The use of pooled specimens is advantageous as it saves significantly on analytical costs, reduces the time and resources required for recruitment, and may avoid ethical difficulties associated with reporting individual results (reviewed in Heffernan et al. 2014), and pooled pathology specimens have been successfully used previously to measure other short half-life chemicals in the Australian population (Gomez-Ramos et al. 2016; Thai et al. 2016; Heffernan et al. 2015; Van den Ede et al. 2015).
Here, we present results of the first large-scale biomonitoring study of pesticide metabolites in a convenience sample of the general Australian population. We demonstrate broad exposure to organophosphate and pyrethroid insecticides as well as to one insect repellent in Australia consistent with the availability of commercial pesticide-containing products in Australia for domestic and agricultural applications.
References
(ABS) Australian Bureau of Statistics (2013) Australian health survey: biomedical results for chronic diseases, 2011–2012 kidney disease biomarkers, Canberra.
(APVMA) Australian Pesticides and Veterinary Medicines Authority (2014) Final pesticide and veterinary medicines product sales 12–13 financial year. Commonwealth of Australia Gazette No APVMA 4.
(APVMA) Australian Pesticides and Veterinary Medicines Authority (2015) Public chemical registration information system search. Available from: https://portal.apvma.gov.au/pubcris. Accessed 30 Dec 2015
(CDC) United States Centers for Disease Control and Prevention (2015) Fourth national report on human exposure to environmental chemicals. February 2015.Updated Tables; U.S Department of Health and Human Services: Atlanta, GA, USA. Available from: www.cdc.gov/exposurereport.
(CDC) United States Centers for Disease Control and Prevention (2016a). NHANES 2007–2008 laboratory data. Available from: wwwn.cdc.gov/Nchs/Nhanes/Search/Nhanes07_08.aspx. Accessed 15 Feb 2016
(CDC) United States Centers for Disease Control and Prevention (2016b). NHANES 2009–2010 laboratory data. Available from: wwwn.cdc.gov/Nchs/Nhanes/Search/Nhanes09_10.aspx. Accessed 15 Feb 2016
(FSANZ) Food Standards Australia New Zealand (2011) The 23rd Australian total diet study.
(NRC) National Research Council (2006) Human biomonitoring for environmental chemicals. Washington, DC: The National Academies Press. doi:10.17226/11700. Available from: www.nap.edu/catalog/11700/human-biomonitoring-for-environmental-chemicals
(US EPA) Environmental Protection Ageny USA (2000) Chlorpyrifos revised risk assessment and agreement with registrants: Washington, DC: Office of Prevention, Pesticides, and Toxic Substances; Accessed 4/01/2016. Available from: http://www.ibiblio.org/london/NAFEX/message-archives/old/pdf00000.pdf.
(WHO) World Health Organisation (2011) Summary of principles for evaluating health risks in children associated with exposure to chemicals in Children’s Environmental Health. World Health Organization, Geneva, Switzerland.
Abdeen Z, Berman T, Azmi K, Abu Seir R, Agha H, Ein-Mor E, Goen T, Stein Y, Richter E, Calderon-Margalit R (2015) Urinary organophosphate metabolite levels in Palestinian pregnant women: results of the Middle East regional cooperation project. Int J Environ Health Res:1–13 [epub ahead of print]. doi:10.1080/09603123.2015.1109067
Agriculture Victoria (2015) Cancellation of products containing parathion-methyl. Accessed 30/12/2015). Available from: http://agriculture.vic.gov.au/agriculture/farm-management/chemical-use/publications/cancellation-of-products-containing-parathion-methyl
Aylward LL, Green E, Porta M, Toms LM, Den Hond E, Schulz C, Gasull M, Pumarega J, Conrad A, Kolossa-Gehring M, Schoeters G, Mueller JF (2014) Population variation in biomonitoring data for persistent organic pollutants (POPs): an examination of multiple population-based datasets for application to Australian pooled biomonitoring data. Environ Int 68:127–138
Babina K, Dollard M, Pilotto L, Edwards JW (2012) Environmental exposure to organophosphorus and pyrethroid pesticides in South Australian preschool children: a cross sectional study. Environ Int 48:109–120. doi:10.1016/j.envint.2012.07.007
Baxter J, Gray M, Hayes A (2011) Families in regional, rural and remote Australia. Australian Government Australian Institute of Family Studies. Accessed: 19/05/2016. Available from: https://aifs.gov.au/sites/default/files/publication-documents/fs201103.pdf
Becker K, Seiwert M, Angerer J, Kolossa-Gehring M, Hoppe HW, Ball M, et al. (2006) GerES IV pilot study: assessment of the exposure of German children to organophosphorus and pyrethroid pesticides. Int J Hyg Environ Health 209(3):221–233. doi:10.1016/j.ijheh.2005.12.002
Berman T, Goldsmith R, Goen T, Spungen J, Novack L, Levine H, Amitai Y, Shohat T, Grotto I (2013) Urinary concentrations of organophosphate pesticide metabolites in adults in Israel: demographic and dietary predictors. Environ Int 60:183–189. doi:10.1016/j.envint.2013.08.008
Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. (2011) Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119(8):1189–1195. doi:10.1289/ehp.1003185
Calafat AM, Needham LL (2009) What additional factors beyond state-of-the-art analytical methods are needed for optimal generation and interpretation of biomonitoring data? Environ Health Perspect 117(10):1481–1485. doi:10.1289/ehp.0901108
Caudill SP (2010) Characterizing populations of individuals using pooled samples. J Expos Sci Environ Epidemiol 20(1):29–37. doi:10.1038/jes.2008.72
Costanzo SD, Watkinson AJ, Murby EJ, Kolpin DW, Sandstrom MW (2007) ) Is there a risk associated with the insect repellent DEET (N,N-diethyl-m-toluamide) commonly found in aquatic environments? Sci Tot Environ. 384(1–3):214–220. doi:10.1016/j.scitotenv.2007.05.036
Davis MD, Wade EL, Restrepo PR, Roman-Esteva W, Bravo R, Kuklenyik P, Calafat AM (2013) Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J Chrom B 929:18–26. doi:10.1016/j.jchromb.2013.04.005
English K, Jagals P, Ware R, Wylie C, Sly PD (2015) Insecticide exposure risk by age: an analysis of Queensland Poisons Information Centre calls. Australian New Zealand Journal of Public Health (under review)
Fortin MC, Bouchard M, Carrier G, Dumas P (2008) Biological monitoring of exposure to pyrethrins and pyrethroids in a metropolitan population of the province of Quebec, Canada. Environ Res 107(3):343–350. doi:10.1016/j.envres.2008.03.002
Gomez-Ramos MJ, Heffernan AL, Toms LML, Calafat AM, Ye X, Hobson P, Broomhall S, Mueller JF (2016) Concentrations of phthalates and DINCH metabolites in pooled urine from Queensland, Australia. Environ Int 88:179–186. doi:10.1016/j.envint.2015.12.016
Guodong D, Pei W, Ying T, Jun Z, Yu G, Xiaojin W, et al. (2012) Organophosphate pesticide exposure and neurodevelopment in young Shanghai children. Environmental science & technology 46(5):2911–2917. doi:10.1021/es202583d
Haines DA, Murray J (2012) Human biomonitoring of environmental chemicals—early results of the 2007-2009 Canadian health measures survey for males and females. Int J Hyg Environ Health 215(2):133–137. doi:10.1016/j.ijheh.2011.09.008
Heffernan AL, Aylward LL, Toms LM, Sly PD, Macleod M, Mueller JF (2014) Pooled biological specimens for human biomonitoring of environmental chemicals: opportunities and limitations. J Exp Sci Environ Epidemiol 24(3):225–232. doi:10.1038/jes.2013.76
Heffernan AL, Baduel C, Toms LML, Calafat AM, Ye X, Hobson P, Broomhall S, Mueller JF (2015) Use of pooled samples to assess human exposure to parabens, benzophenone-3 and triclosan in Queensland, Australia. Environ Int 85:77–83. doi:10.1016/j.envint.2015.09.001
Hornung RW, Reed LD (1990) Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5:46–51
Koureas M, Tsakalof A, Tsatsakis A, Hadjichristodoulou C (2012) Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol Lett 210(2):155–168. doi:10.1016/j.toxlet.2011.10.007
Kuklenyik P, Baker SE, Bishop AM, Morales-A P, Calafat AM (2013) On-line solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry approach to quantify N. N-diethyl-m-toluamide and oxidative metabolites in urine Anal Chim Acta 787:267–273. doi:10.1016/j.aca.2013.05.055
Larson A, Bryan J, Howard P, McGinn D (2000) Queenslanders’ use of personal strategies to minimise risk of mosquito-borne disease. Aust N Z J Public Health 24(4):374–377
Le Grand R, Dulaurent S, Gaulier JM, Saint-Marcoux F, Moesch C, Lachatre G (2012) Simultaneous determination of five synthetic pyrethroid metabolites in urine by liquid chromatography-tandem mass spectrometry: application to 39 persons without known exposure to pyrethroids. Toxicol Lett 210(2):248–253. doi:10.1016/j.toxlet.2011.08.016
Lewis RC, Cantonwine DE, Anzalota Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, Baker SE, Alshawabkeh AN, Cordero JF, Meeker JD (2014) Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ Health 13:97. doi:10.1186/1476-069X-13-97
Lewis RC, Cantonwine DE, Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, Montesano MA, Alshawabkeh AN, Cordero JF, Meeker JD (2015) Distribution and determinants of urinary biomarkers of exposure to organophosphate insecticides in Puerto Rican pregnant women. Sci Total Environ 512-513:337–344. doi:10.1016/j.scitotenv.2015.01.059
Lu CS, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R (2006) Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environ Health Perspect 114(2):260–263
Makri A, Goveia M, Balbus J, Parkin R (2004) Children’s susceptibility to chemicals: a review by developmental stage. J Toxicol Environ Health B Crit Rev 7(6):417–435. doi:10.1080/10937400490512465
Morgan MK (2012) Children’s exposures to pyrethroid insecticides at home: a review of data collected in published exposure measurement studies conducted in the United States. Int J Environ Res Pub Health 9(8):2964–2985. doi:10.3390/ijerph9082964
Morgan MK, Sheldon LS, Croghan CW, Jones PA, Chuang JC, Wilson NK (2007) An observational study of 127 preschool children at their homes and daycare centers in Ohio: environmental pathways to cis- and trans-permethrin exposure. Environ Res 104(2):266–274. doi:10.1016/j.envres.2006.11.011
Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW (2005) Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol 15(4):297–309
Naeher LP, Tulve NS, Egeghy PP, Barr DB, Adetona O, Fortmann RC, Needham LL, Bozeman E, Hilliard A, Sheldon LS (2010) Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United States city. Sci Tot Environ 408(5):1145–1153. doi:10.1016/j.scitotenv.2009.10.022
Odetokun MS, Montesano MA, Weerasekera G, Whitehead RD Jr, Needham LL, Barr DB (2010) Quantification of dialkylphosphate metabolites of organophosphorus insecticides in human urine using 96-well plate sample preparation and high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878(27):2567–2574. doi:10.1016/j.jchromb.2010.04.027
Oglobline AN, Elimelakh H, Tattam B, Geyer R, O’Donnell GE, Holder G (2001) Negative ion chemical ionization GC/MS-MS analysis of dialkylphosphate metabolites of organophosphate pesticides in urine of non-occupationally exposed subjects. Analyst 126(7):1037–1041. doi:10.1039/B102004H
Radcliffe J (2002) Pesticide use in Australia. Australian Academy of Technological Sciences and Innovation.
Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. (2012) Brain anomalies in children exposed prenatally to a common organophosphate pesticide. PNAS 109(20):7871–7876. doi:10.1073/pnas.1203396109
Riederer AM, Bartell SM, Barr DB, Ryan PB (2008) Diet and nondiet predictors of urinary 3-phenoxybenzoic acid in NHANES 1999-2002. Environ Health Perspect 116(8):1015–1022. doi:10.1289/ehp.11082
Roberts JR, Karr CJ (2012) Pesticide exposure in children. Pediatrics 130(6):e1765–e1788. doi:10.1542/peds.2012-2758
Roca M, Miralles-Marco A, Ferre J, Perez R, Yusa V (2014) Biomonitoring exposure assessment to contemporary pesticides in a school children population of Spain. Environ Res 131:77–85. doi:10.1016/j.envres.2014.02.009
Rudel RA, Perovich LJ (2009) Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ 43(1):170–181. doi:10.1016/j.atmosenv.2008.09.025
Schettgen T, Heudorf U, Drexler H, Angerer J (2002) Pyrethroid exposure of the general population—is this due to diet. Toxicol Lett 134(1–3):141–145
Scheuplein R, Charnley G, Doursen M (2002) Differential sensitivity of children and adults to chemical toxicity: I. Biological basis. Regul Toxicol Pharmacol 35:429–447
Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. (2014) Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 122(10):1103–1109. doi:10.1289/ehp.1307044
Thai PK, Heffernan AL, Toms LML, Li Z, Calafat AM, Hobson P, Broomhall S, Mueller JF (2016) Monitoring exposure to polycyclic aromatic hydrocarbons in an Australian population using pooled urine samples. Environ Int 88:30–35. doi:10.1016/j.envint.2015.11.019
Trunnelle KJ, Bennett DH, Ahn KC, Schenker MB, Tancredi DJ, Gee SJ, et al. (2014) Concentrations of the urinary pyrethroid metabolite 3-phenoxybenzoic acid in farm worker families in the MICASA study. Environ Res 131:153–159. doi:10.1016/j.envres.2014.03.003
Tulve NS, Suggs JC, McCurdy TR, Cohen Hubal EA, Moya J (2002) Frequency of mouthing behaviour in young children. J Expo Anal Environ Epidemiol 12(4):259–264
Turner MC, Wigle DT, Krewski D (2010) Residential pesticides and childhood leukemia: a systematic review and meta-analysis. Environ Health Perspect 118(1):33–41. doi:10.1289/ehp.0900966
Van den Eede N, Heffernan AL, Hobson P, Mueller JF, Neels H, Covaci A (2015) Age-related phosphate flame retardant and plasticizer exposure in an Australian population. Environ Int 74:1–8. doi:10.1016/j.envint.2014.09.005
Wielgomas B, Piskunowicz M (2013) Biomonitoring of pyrethroid exposure among rural and urban populations in northern Poland. Chemosphere 93(10):2547–2553. doi:10.1016/j.chemosphere.2013.09.070
Wielgomas B, Nahorski W, Czarnowski W (2013) Urinary concentrations of pyrethroid metabolites in the convenience sample of an urban population of northern Poland. Int J Hyg Environ Health 216(3):295–300. doi:10.1016/j.ijheh.2012.09.001
Acknowledgments
The authors wish to thank the staff at Entox and Sullivan Nicolaides Pathology Taringa for their assistance with the sample collection and pooling. We also gratefully acknowledge Sam Baker, Mark Davis, Angela Montesano, and other CDC staff for their technical assistance in measuring the urinary concentrations of the pesticide biomarkers. ALH is funded by an NHMRC-ARC Fellowship (APP1106911), KE by an Australian Government Postgraduate Award, LMT by an ARC DECRA (DE120100161), PDS is an NHMRC Senior Principal Research Fellow (no. 1102590), and JFM is an ARC Future Fellow (FF120100546). The authors would like to thank the Australian Government Department of the Environment for their financial support. The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant. Entox is a joint venture of the University of Queensland and the Queensland Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the views of the Australian Department of the Environment.
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Supplementary Material 1
(DOC 13.6 kb)
Supplementary Material 2
(DOC 150 kb)
Supplementary Material 3
(DOC 132 kb)
Supplementary Material 4
(DOC 158 kb)
Rights and permissions
About this article
Cite this article
Heffernan, A., English, K., Toms, L. et al. Cross-sectional biomonitoring study of pesticide exposures in Queensland, Australia, using pooled urine samples. Environ Sci Pollut Res 23, 23436–23448 (2016). https://doi.org/10.1007/s11356-016-7571-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7571-7