Abstract
Effects of laser irradiation on photosystem II (PS II) photochemical efficiencies, growth, and other physiological responses of Microcystis aeruginosa were investigated in this study. Results indicate that laser irradiation (wavelengths 405, 450, 532, and 650 nm) could effectively inhibit maximal PS II quantum yield (Fv/Fm) and maximal electron transport rates (ETRmax) of M. aeruginosa, while saturating light levels (Ek) of M. aeruginosa did not change significantly. Among the four tested wavelengths, 650 nm laser (red light) showed the highest inhibitory efficiency. Following 650 nm laser irradiation, the growth of M. aeruginosa was significantly suppressed, and contents of cellular photosynthetic pigments (chlorophyll a, carotenoid, phycocyanin, and allophycocyanin) decreased as irradiation dose increased. Meanwhile, laser irradiation enhanced the enzyme activities of superoxide dismutase (SOD) and peroxidase (POD) in M. aeruginosa cells. Lower irradiation doses did not change the intracellular microcystin contents, but higher dose irradiation (>1284 J cm−2) caused the release of microcystin into the culture medium. Transmission electron microscope examination showed that the ultrastructure of M. aeruginosa cells was destructed progressively following laser irradiation. Effects of laser irradiation on M. aeruginosa may be a combination of photochemical, electromagnetic, and thermal effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Overloading of nutrients leads to the frequent occurrence of algal blooms in lakes and reservoirs worldwide (Dodds et al. 2009; Paerl et al. 2011), which reduces water transparency, deteriorates water quality, affects other aquatic organisms, and decreases biodiversity (Hudnell 2010). The wide spread of algal blooms limits the use of lakes and reservoirs for drinking water sources and recreational activities (Weirich and Miller 2014). Toxins produced by cyanobacteria can pose serious risks to aquatic organisms, even human beings, and may cause fatal consequences (Carvalho et al. 2013; Steffen et al. 2014). Therefore, the prevention and control of algal blooms are of great importance for the health of humans and animals.
Controlling methods of algal blooms are usually divided into physical, chemical, and biological ones. Physical methods such as salvage, filtration, and flotation are commonly used when algal bloom occurs. Sonication is a more recently developed approach (Wu et al. 2012), and other physical methods such as ultraviolet radiation (Holzinger and Lutz 2006) and gamma-ray irradiation (Zheng et al. 2012) have also been considered as feasible methods to control algal blooms. But they are not widely applied probably because of their high cost and potential risks to other aquatic organisms. Chemical methods are economic and efficient in controlling algal blooms. Chemicals such as ozone (Li et al. 2011), chlorine dioxide (Zhou et al. 2014a), hydrogen peroxide (Matthijs et al. 2012), potassium ferrate (Zhou et al. 2014b), and copper sulfate (Song et al. 2011) were previously studied. Flocculation using clays, soils, and sediments modified by chitosan was reported to be an effective way to remove harmful algae (Zou et al. 2006). Pinho et al. (2015) reported that a solar-driven TiO2 photocatalytic process can efficiently destroy the cyanobacteria Microcystis aeruginosa. However, chemical methods have been applied less frequently recently, primarily due to the environmental safety concerns (Jia et al. 2013). Biological methods using macrophytes, fish, zooplankton, and filter-feeding bivalves were also successfully applied in controlling the growth of algae (He et al. 2014; Hong et al. 2008; Lu et al. 2006; Wang et al. 2012). Most biological methods have a good effect on algae control in small scales. However, applying these methods in large natural water bodies can be difficult and may have limited effectiveness due to the complicated ecological processes involved and a considerably long time to take effect.
Laser is a single-color light with very high energy density and directivity. Presently, laser has been widely used in various fields such as welding, cutting, lighting, communication, phototypesetting, and breeding. Biostimulation by low-intensity laser irradiation on agricultural seeds has been studied previously. Hernández et al. (2015) reported that laser at a 650 nm wavelength induced temperature changes in maize seeds, and the thermal effect of laser was believed to associate with biostimulation. Laser irradiation was found to have considerable biological effects on the metabolism of wheat during germination and later vegetative growth (Jamil et al. 2013). Suitable doses of laser irradiation can enhance the physiological attributes such as photosynthetic rate, chlorophyll content, transpiration rate, and water use efficiency and increase production of biochemicals including proteins, carotenoids, enzyme activities, essential oil, and abscisic acid (El-Kereti et al. 2013; Gao et al. 2015; Perveen et al. 2011). Some researchers have pointed out that laser irradiation could increase the biomass of hydrophytes, accelerated heavy metal accumulation, and phytoremediation of phosphorus and nitrogen compounds from wastewater and contaminated soil (Dobrowolski et al. 2012). In the meanwhile, some researchers have reported that laser irradiation could inhibit the marine plankton growth like diatoms and dinoflagellate, preventing biofouling for marine environmental sensor (Nandakumar et al. 2003, 2009; Delauney et al. 2010). The effect of laser irradiation on organisms can be related to the exposure dosage. The possible mechanisms of biostimulation from laser irradiation include heat, light, and electromagnetic effects. Lower dose of laser irradiation usually stimulates organisms, but higher dose of laser irradiation can have inhibitory or even lethal effects. Therefore, we hypothesize that higher laser irradiation intensity may induce physiological damages to algae.
Information on the effects of high-intensity laser irradiation on growth, physiological attributes, and ultrastructural changes of phytoplankton remains unclear to date. Here, we propose that laser irradiation could be an effective way to control the growth of phytoplankton and the effectiveness of laser irradiation on a bloom-forming cyanobacterium M. aeruginosa was investigated. Therefore, the objectives of this study were to investigate the inhibitory effects of laser irradiation on M. aeruginosa and to elucidate the possible mechanisms involved in the processes.
Materials and methods
Materials
M. aeruginosa FACHB-915 was obtained from the Freshwater Algal Culture Collection of Institute of Hydrobiology, Chinese Academy of Sciences. The cell culture was conducted in 500 mL sterilized glass flasks containing 200 mL of BG11 medium, which were placed under fluorescent lamps at 2000 lx with a 12-h/12-h light dark/cycle, at 25 ± 1 °C. Four low-intensity lasers (50 mW power, beam diameter 3 mm, light intensity 0.71 W cm−2) at visible wavelength (405, 450, 532, and 650 nm) and a high-power laser (1 W power, beam diameter 6 mm, light intensity 3.57 W cm−2) at wavelength of 650 nm were used in the experiment. All lasers were purchased from Xi’an MingHui Optoelectronic Technology Co., Ltd, China.
Irradiation experiment
A mixed algal suspension with a density of 7.5 × 106 cells mL−1, which was at the exponential growth phase, was prepared. Laser irradiation experiments were performed as illustrated in Fig. 1. Laser was held perpendicularly on an iron stand. Firstly, 5 mL of the prepared algal suspension was added into a 10 mL centrifuge tube wrapped with aluminum foil. Then, the centrifuge tube was placed 2 cm beneath the laser in a tube rack. After the laser was turned on, the algal suspension was irradiated for different durations (50 mW laser irradiated for 30 min, 1 W laser irradiated for 3, 6, 9, 12, and 15 min). The irradiation dosage was calculated by multiplying light intensity by exposure time, which has a unit of Joules per square centimeter. After exposure, the algal suspension was mixed and harvested for the analysis. An aliquot of 1 mL algal mixture was prepared for cell ultrastructure imaging while 5 mL was used for the measurement of other parameters. All experiments were performed in triplicate. The low-intensity lasers (50 mW) were used to select the laser wavelength, and the high-power laser (1 W) was used subsequently to study the effects of laser irradiation on M. aeruginosa.
Cell growth analysis
After being irradiated by a 650 nm laser for 642, 1284, 1926, 2568, and 3210 J cm−2 respectively, 5 mL algal suspension of M. aeruginosa was inoculated in 250 mL Erlenmeyer flasks containing 100 mL of BG11 previously sterilized at 120 °C for 15 min in an autoclave. Then, the samples were cultured under fluorescent lamps at 2000 lx with a 12 h/12 h light/dark cycle, at 25 ± 1 °C. Cell density of M. aeruginosa was determined using a flow cytometer (Accuri C6, BD, USA). The sampling volume was 0.5 mL, and the flow cytometer was operated at a flow rate of 10 μL min−1. The cell density analysis was performed every 2 or 3 days.
Fluorescence measurements
Chlorophyll fluorescence measurements of M. aeruginosa were conducted using a Water-PAM fluorometer (Walz, Germany). After a 30 min irradiation (50 mW lasers) at four wavelengths (dosage 1278 J cm−2), 5 mL algal suspension was diluted to 50 mL and incubated in the dark for 5 min. Then, the maximum photosystem II (PS II) optical quantum yield (Fv/Fm), maximal relative electron transport rates through PS II (ETRmax), and saturating light levels (Ek) were determined in a cuvette containing 3 mL of algal suspension.
Photosynthetic pigment measurement
Chlorophyll a (Chl. a) and carotenoid were determined spectrophotometrically using an Agilent Cary 60 UV-Vis spectrophotometer after algal cells were irradiated by a 650 nm laser for 642, 1284, 1926, 2568, and 3210 J cm−2, respectively. Firstly, samples were filtered by Whatman 1.2-μm GF/C glass fiber filters and then extracted with 5 mL of 95 % ethanol overnight at 4 °C. After that, the samples were centrifuged at 4000 rpm for 10 min and the absorbance of the supernatant was determined at 665, 649, and 470 nm. The cellular pigment contents were calculated using Eqs. (1) and (2) as described by Xiao et al. (2010):
Phycocyanin (PC) and allophycocyanin (APC) were determined as described by Bennett and Bogorad (1973). Samples were resuspended in 0.1 M phosphate-buffered saline (PBS) and homogenized by an ultrasonic cell pulverizer (JY92-IIN, Xinzhi Co., China, 200 W, ultrasonic time 5 s; rest time 4 s; cycle 60) with ice-bath. Then, the homogenate was centrifuged at 10,000 rpm for 10 min, and the absorbance of the extraction was determined at 615 and 652 nm. Contents of PC and APC were calculated using Eqs. (3) and (4):
Ratios of carotenoid/Chl. a, PC/Chl. a, and APC/Chl. a were also calculated. Carotenoid/Chl. a is correlated with the capacity of light-protecting mechanisms (Kuster et al. 2004). PC/Chl. a and APC/Chl. a reflect the regulation of phycobilisome content according to light intensity and quality (Jiang and Qiu 2005).
Enzyme activity assay
After being irradiated by a 650-nm laser for 642, 1284, 1926, 2568, and 3210 J cm−2, algal cells were harvested by centrifugation at 4000 rpm for 10 min at 4 °C, and then the supernatant was discarded. After that, the pellets were resuspended in 2.5 mL PBS. Then, the cells were homogenized with ice-bath using the ultrasonic cell pulverizer (200 W, ultrasonic time 3 s; rest time 5 s; cycle 60). The homogenate was centrifuged at 11,000 rpm for 15 min at 4 °C. The supernatant, cell-free enzyme extract, was used to measure the total protein contents and activities of superoxide dismutase (SOD) and peroxidase (POD). The total extracted protein was determined by binding of Coomassie Brilliant Blue G-250 to protein according to Bradford (1976), and the enzyme activities were determined using commercial kits from Nanjing Jiancheng Biology Engineering Institute. SOD and POD activities were expressed as enzyme-activity unit per milligram of protein (U mg protein−1).
Microcystin analysis
Samples were centrifuged at 4000 rpm for 10 min after being irradiated by a 650 nm laser for 642, 1284, 1926, 2568, and 3210 J cm−2. Then, the supernatant was filtrated through a 0.45 μm glass fiber filter and concentrated for extracellular (in culture medium) microcystin (MC) analysis according to Cong et al. (2006). The residue was freeze-dried, then 1 mL 75 % methanol was added into the centrifuge tubes. Thereafter, the cells were disrupted by ultrasonication (300 W, 1 min) and extracted in the dark for three times. Finally, the homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C and the supernatant was filtered using a 0.45 μm PTFE syringe filter for intracellular MC analysis.
Concentration of MC was determined using a high-performance liquid chromatography system (Waters 2695, USA) coupled with a photodiode array detector (Waters 2996, USA) operated at 238 nm according to the method of Wiedner et al. (2003).
Cell ultrastructure imaging
The samples of the M. aeruginosa cells were treated by different laser irradiation doses (1284, 2568, and 3210 J cm−2) and then fixed with 2.5 % glutaral and prepared by ultrathin sections. Prepared samples were observed using a HT-7700 transmission electron microscope (TEM) (Hitachi, Japan).
Temperature measurement
In order to investigate the heat effect of laser irradiation on M. aeruginosa, temperature of the algal suspension irradiated by 1 W 650 nm laser was measured every minute using a thermometer (KangSheng Pharmaceutical Technology Co., Ltd, China). An aliquot of 5 mL algal suspension with cell density of 7.5 × 106 cells mL−1 was added into a 10 mL centrifuge tube, and the irradiation experiment was conducted as described in Fig. 1.
Statistics
All data in this study were expressed as the means ± standard deviation of three replicates. Differences between treatments and control were tested using one-way analysis of variance (ANOVA), and differences between treatments were tested by least significant difference (LSD) test using PASW Statistics 18 (SPSS Inc.). The significance level was set at 0.05.
Results
Effect of laser irradiation on chlorophyll a fluorescence parameters
Changes of Fv/Fm, ETRmax, and Ek of M. aeruginosa cells treated with lasers of four wavelengths for 30 min were presented in Fig. 2. Irradiation with lasers of four different wavelengths led to a decrease in Fv/Fm values compared to the control (p < 0.05). ETRmax of M. aeruginosa declined significantly under laser irradiation at 405, 450, and 650 nm wavelengths (p < 0.05), but it did not change obviously after being treated with a laser at 532 nm (p = 0.677). In contrast, Ek of M. aeruginosa irradiated with all the lasers had no significant change compared to the control (p > 0.057). Results of Fv/Fm and ETRmax indicated that the 650 nm laser had the highest inhibitory effect on photosynthetic efficiency followed by 405, 450, and 532 nm lasers according to LSD test. Therefore, subsequent experiments were performed using the 650 nm laser.
Effect of laser irradiation on cell growth
Results of the growth inhibition experiment showed that the 650 nm laser can effectively inhibit the growth of M. aeruginosa, and the inhibitory effects enhanced with the increase of exposure doses (Fig. 3). The cell density in control increased from 3.6 × 105 to 1.1 × 107 cells mL−1 after incubation for 20 days. Cell densities in two treatments that received 642 and 1284 J cm−2 laser irradiation increased to 7.9 × 106 and 3.0 × 106 cells mL−1, respectively, whereas cells that received 1926, 2568, and 3210 J cm−2 laser irradiation showed no growth within 20 days, suggesting the apoptosis of M. aeruginosa cells.
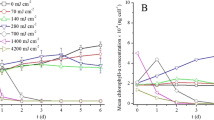
Effects of laser irradiation on pigment contents
Chl. a in solution showed no significant difference from that of control after being treated with 642 and 1284 J cm−2 irradiation (p = 0.794, p = 0.577) (Fig. 4a), but decreased at higher laser irradiation doses (p < 0.01). When irradiation dose reached 3210 J cm−2, Chl. a decreased by 57.5 % to 1.65 mg L−1. Different from the trend of Chl. a, the carotenoid content of M. aeruginosa decreased after exposure to 642 J cm−2 laser irradiation. After 3210 J cm−2 irradiation, carotenoid content decreased by 76.4 % to 0.205 mg L−1. Meanwhile, PC and APC show trends similar to Chl. a (Fig. 4b). Their concentrations did not change significantly (p > 0.532) after cells were treated with 642 J cm−2 irradiation and then decreased at higher doses (more than 1926 J cm−2, p < 0.01). After exposure to 3210 J cm−2 irradiation, PC and APC concentrations dropped to 26.1 and 13.5 % of the control, respectively. In addition, carotenoid/Chl. a ratio showed a significant decrease (p < 0.01) with increasing irradiation dosage (Fig. 4c). In contrast, the PC/Chl. a ratio decreased significantly (p < 0.05) after receiving 2568 J cm−2 laser irradiation, while APC/Chl. a shows a significant decrease (p < 0.01) compared to the control after receiving 1284 J cm−2 irradiation (Fig. 4d).
Changes of Chl. a and carotenoid (a), PC and APC (b), ratio of carotenoid to Chl. a ratio (c), and ratios of PC and APC to Chl. a (d) in M. aeruginosa cell culture receiving different laser irradiation doses. Error bar represents standard deviation of the three replicates. *(p < 0.05) and **(p < 0.01)
Effects of laser irradiation on SOD and POD activities
Changes of total protein content and SOD and POD activities during exposure were presented in Fig. 5. The results showed that the protein content decreased when receiving 1926 J cm−2 or higher doses and decreased by 80 % compared to the control with 3210 J cm−2 irradiation. Both SOD and POD activities showed no significant change receiving 1284 J cm−2 irradiation (p = 0.106, p = 0.641), but increased significantly (p < 0.05) at higher exposure doses compared to the control (Fig. 5b, c). When the irradiation dose reached 3210 J cm−2, the SOD and POD activities were 1.6 and 6.7 times higher than the control.
Effects of laser irradiation on MC content
Only microcystin-LR (MC-LR) was detected. Intracellular and extracellular MC-LR contents showed no significant difference from the control (p > 0.296) after receiving 642 and 1284 J cm−2 irradiation (Fig. 6). In contrast, intracellular MC started to decrease at higher doses, and a 42.9 % decrease was observed when receiving 3210 J cm−2 irradiation. Meanwhile, extracellular MC content increased when receiving 1926 J cm−2 or higher irradiation doses (p < 0.01). The extracellular MC was 3.5 times the control when receiving 3210 J cm−2 irradiation, whereas the total MC contents (intracellular and extracellular MC) showed no significant difference (p > 0.05) in the system.
Effects of laser on ultrastructure of M. aeruginosa
The ultrastructure was compared between control cells and those treated with 1284, 2568, and 3210 J cm−2 laser irradiation (Fig. 7). Control cells showed a normal M. aeruginosa cell ultrastructure with gelatinous layer, cell wall, cell membrane, cytoplasm, and nucleoid, and the organelles (carboxysome, thylakoid, cyanophycin granules, polyphosphate bodies, and gas vesicles) presented clearly in the nucleoplasmic area (Fig. 7a). When treated with 1284 J cm−2 laser irradiation, thylakoids were damaged, polyphosphate bodies and cyanophycin granules can still be observed in cells, and the nucleoid began to diffuse all around (Fig. 7b). With 2568 J cm−2 irradiation, cell wall deformation occurred, the shapes of thylakoids became fuzzy while the cyanophycin granules still existed, the nucleoid dispersed considerably, and larger vacuoles appeared in the nucleoplasm (Fig. 7c). With 3210 J cm−2 irradiation, plasmolysis was observed and thylakoid dissolved entirely, while the numbers of polyphosphate bodies, cyanophycin granules, and gas vesicles decreased drastically (Fig. 7d).
Transmission electron microscopic images of M. aeruginosa cells receiving different laser irradiation doses: a control; b 1284 J cm−2; c 2568 J cm−2; d 3210 J cm−2. gl gelatinous layer; cw cell wall; cm cell membrane; th thylakoid; cg cyanophycin granules; cb carboxysome; pb polyphosphate bodies; gv gas vesicles
Temperature changes
Temperature of the algal suspension irradiated by red light laser increased with the increase of irradiation doses (Fig. 8). The increasing rate went up during the first 7 min and then declined in the last 8 min. Subsequently, the temperature of algal suspension irradiated by laser increased from 26.2 to 42.1 °C while the temperature of the control did not change significantly (p > 0.05).
Discussion
The influence of biostimulation processes on organisms is linked to laser power density and wavelength. The inhibitory effect of laser irradiation on M. aeruginosa cells was wavelength dependent, and red light (650 nm) laser had the highest inhibitory effect. This, on the one hand, may be related to a better absorption of red light by M. aeruginosa cells, which has a light green color. On the other hand, the phytochromes of M. aeruginosa are sensitive to red light and infrared radiation (Chen et al. 2005b). So red light laser could induce photo-damage to M. aeruginosa. Dobrowolski et al. (2012) also found that plants irradiated with red light laser (660 nm, 20 mW power) showed the biggest increase in biomass production and better adaptation to unfavorable environmental conditions.
Fv/Fm is often used to evaluate PS II damage caused by light, pollutants, and other environmental stresses, and a low Fv/Fm value means lower photosynthetic efficiency (Yang et al. 2015). The decrease of Fv/Fm and ETRmax might indicate that an important portion of the PS II reaction center was damaged and the electron transport chain might be affected. Ek is used as an index of the photo-acclimation state of phytoplankton. High Ek values are related to growth at high irradiance and indicate a relatively greater capacity for light-saturated photosynthesis (Xing et al. 2007). Our results showed that all lasers irradiated at the 1278 J cm−2 dose did not change the photo-acclimation capacity of M. aeruginosa.
Photosynthetic pigments of M. aeruginosa play an important role in photosynthesis. Chl. a is the principal pigment that is involved in light absorption and photochemistry (Eullaffroy and Vernet 2003). Carotenoid is considered as an accessory pigment harvesting light and protecting molecules against photo-oxidative damage (Gotz et al. 1999). Our results indicated that laser irradiation could induce a decrease of both Chl. a and carotenoid, which may affect the photosynthesis of M. aeruginosa. Jiang and Qiu (2005) found that carotenoid/Chl. a of M. aeruginosa increased with enhanced UV-B irradiation probably as a result of resisting oxidative damage and accelerating the repair of damaged photosynthetic apparatus, while the carotenoid/Chl. a ratio decreased with increasing laser irradiation dose, suggesting that the damage caused by laser could not be mediated by regulating photosynthetic pigments, likely due to the high energy density of laser. Besides, a decrease of the ratios indicates that carotenoid is more sensitive than Chl. a. In addition, carotenoids are antioxidants for the removal of toxic oxygen species, and a decrease in carotenoids could lead to the impairment of the antioxidant defense system in cells.
PC and APC are antenna pigments assembled in phycobilisomes and are attached to the surface of thylakoids for photosynthesis, which play important roles in funneling light energy to the underlying PS II reaction centers (Glazer 1988). Zhou et al. (2006) reported that degradation of phycobiliproteins is a crucial acclimation response of M. aeruginosa under exogenous stress conditions. Our results revealed that both PC and APC contents were reduced, and PC/Chl. a and APC/Chl. a ratios decreased considerably following laser irradiation. The damage of phycobilisomes caused by laser irradiation might lead to less energy being transferred to the PS II reaction center, resulting in photosynthetic damage and growth inhibition. The changes of PC/Chl. a and APC/Chl. a ratios also indicated that APC was more sensitive than PC to laser irradiation at 650 nm, which might be because the maximum absorption of APC was close to 650 nm (652 nm), while the maximum absorption of PC was 615 nm (Bennett and Bogorad 1973).
To counteract the toxicity of reactive oxygen species (ROS), cells have developed a set of cellular defense system via the enzymatic and nonenzymatic antioxidants (Hong et al. 2008). SOD and POD are two important enzymatic antioxidants which can scavenge ROS. SOD is considered as a catalyst which converts superoxide anion O2 − to H2O2 and O2, which is the first line of defense in organisms to remove ROS (Hassan and Scandalios, 1990). H2O2 generated during the conversion of O2 − can be further eliminated by POD and other antioxidants (Apel and Hirt 2004). It has been reported that laser irradiation could enhance enzyme activities of seeds and plants (Chen et al. 2005a; Jamil et al. 2013; Podlesny et al. 2012). Therefore, increasing SOD and POD activities under laser irradiation could be a strategy to protect cells from oxidative damage.
MCs are cyclic heptapeptides produced by cyanobacteria, including M. aeruginosa, through nonribosomal peptide synthases. MCs are hepatotoxic and can pose a major threat to drinking water safety. Kaebernick et al. (2000) reported that the damage of the photosynthetic system of Microcystis might negatively impact intracellular MC production. Factors including light, simulated microgravity, and organic pollutants can also significantly affect MC production and release (Wang et al. 2007; Wiedner et al. 2003; Xiao et al. 2010). In our study, the detection of extracellular MC in the control group can be attributed to the high culture density (Wiedner et al. 2003). Lower dose irradiation (642 and 1284 J cm−2) did not change intracellular and extracellular MC contents, suggesting that no release of MC from M. aeruginosa cells and no degradation of MC occurred. With 1926 J cm−2 or higher doses, the intracellular MC content decreased while extracellular MC content increased, and the total MC content in the system showed no significant difference from the control, indicating that MC was released from the cell into the culture medium and no degradation of MC occurred during laser exposure. The release of MC can be attributed to the damage of the M. aeruginosa cell wall, as observed in TEM (Fig. 7). Therefore, laser irradiation may have a risk of increasing MC release, but the risk can be minimized by optimizing the exposure time.
The ultrastructural changes of M. aeruginosa cells during exposure showed that laser irradiation affect thylakoids first. Chl. a and carotenoids are attached to membrane-bound proteins of the thylakoids while phycobilins are attached to the cytosol face of the thylakoids and extend into the cytosol for photosynthesis. Therefore, the function of photosynthetic and respiratory electron transport chain was broken following laser irradiation. Then, other organelles including cyanophycin granules, polyphosphate bodies, and gas vesicles were injured by laser. Thereafter, the cell wall was affected and the permeability of the cell membrane was altered, resulting in the release of MCs. Finally, the nucleoid, cytosol, and inclusions were severely disrupted, and cellular defense and resistance disappeared (Fig. 7d). As a result, the activities of SOD and POD dropped below detection and pigment contents were further deteriorated when receiving 3210 J cm−2 irradiation.
The biostimulation effects of laser irradiation on organisms are mainly photochemical, electromagnetic, and thermal effects. Phytoplankton, especially cyanobacteria, is very sensitive to light environment (Chen et al. 2005b). Laser is a specific light of high energy intensity that can be effectively absorbed by phytochromes and cause certain photochemical reactions (Popve et al. 2007). Phytochrome is a component of photoreceptor system in plant cells, and it is located in the sub-membrane such as plasmalemma chloroplast membrane (Jamil et al. 2013). After being irradiated by laser, light absorption and photochemical reaction might directly influence the photosynthesis of M. aeruginosa. The energy of the excited molecules is transformed into chemical energy for their subsequent physiological activity (Hernández et al. 2015). The activities of related enzymes, which are modulated by phytochrome, could be enhanced. Besides, laser irradiation could induce free radicals like hydroxyl radical, which can react with other molecules (Podlesany et al. 2012). Electromagnetic fields can damage the molecular structure of protein, enzymes, and DNA (Simko 2007). Therefore, the electromagnetic effect of high-intensity laser irradiation could lead to oxidative damage and destroy the molecular structure of cellular pigments, protein, and DNA in the protoplast of the M. aeruginosa cell. As a result, the activities of SOD and POD enhanced; the cell wall, cell membrane, thylakoids, and organelles in M. aeruginosa cell were destroyed; and the intracellular MC was released after M. aeruginosa irradiated by laser.
Additionally, our results showed that temperature of algal suspension irradiated by laser increased significantly, indicating a process of heating the cells. In this way, laser irradiation could cause enhancement of enzyme activities. Some researchers found that laser irradiation could change the thermodynamic parameters of seed and the kinetic equilibrium of seed germination was broken (Hernández et al. 2015; Chen et al. 2005b). Therefore, it is inferred that the effect of laser irradiation on algal suspension may be a combination of photochemical, electromagnetic, and thermal effects.
Conclusions
The present study elucidated the effectiveness and possible mechanisms of laser irradiation on M. aeruginosa. Results indicated that laser irradiation can effectively inhibit the growth of M. aeruginosa. Assays of photosynthetic activities, pigment contents, enzymatic antioxidant activities, and MC contents combined with TEM imaging demonstrated the progressive damage of physiological functions and destruction of ultrastructure of M. aeruginosa cells, which contributed to the inhibition of growth. When exposed to lower-dose laser irradiation, M. aeruginosa can take adaptive strategies to cope with the stress while higher doses can destroy the protecting mechanisms and lead to the apoptosis of cells. Effects of laser irradiation on M. aeruginosa cells may be due to a combination of photochemical, electromagnetic, and thermal effects. The effectiveness of laser irradiation on M. aeruginosa suggested that techniques based on laser may be used for algal bloom control. In the future, further experiments will be performed to study the effectiveness, feasibility, and economical efficiency of using laser-based technology for algal bloom control in eutrophicated waters.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:19–435
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1284:248–254
Carvalho L, McDonald C, de Hoyos C, Mischke U, Phillips G, Borics G, Poikane S, Skjelbred B, Solheim AL, Van Wichelen J, Cardoso AC, Cadotte M (2013) Sustaining recreational quality of European lakes: minimizing the health risks from algal blooms through phosphorus control. J Appl Ecol 50:315–323
Chen YP, Liu YJ, Wang XL, Ren ZY, Yue M (2005a) Effect of microwave and He-Ne laser on enzyme activity and biophoton emission of Isatis indigotica fort. J Integr Plant Biol 47:849–855
Chen YP, Yue M, Wang XL (2005b) Influence of He-Ne laser irradiation on seeds thermodynamic parameters and seedlings growth of Isatis indogotica. Plant Sci 168:601–606
Cong L, Huang B, Chen Q, Lu B, Zhang J, Ren Y (2006) Determination of trace amount of microcystins in water samples using liquid chromatography coupled with triple quadrupole mass spectrometry. Anal Chim Acta 569:157–116
Delauney L, Compere C, Lehaitre M (2010) Biofouling protection for marine environmental sensors. Ocean Sci 6:503–511
Dobrowolski JW, Sliwka M, Mazur R (2012) Laser biotechnology for more efficient bioremediation, protection of aquatic ecosystems and reclamation of contaminated areas. J Chem Technol Biotechnol 87:1354–1359
Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, Schloesser JT, Thornbrugh DJ (2009) Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environ Sci Technol 43:12–19
El-Kereti MA, El-feky SA, Khater MS, Osman YA, El-sherbini EA (2013) ZnO nanofertilizer and He Ne laser irradiation for promoting growth and yield of sweet basil plant. Recent Pat Food Nutr Agric 5:169–181
Eullaffroy P, Vernet G (2003) The F684/F735 chlorophyll fluorescence ratio: a potential tool for rapid detection and determination of herbicide phytotoxicity in algae. Water Res 37:1983–1990
Gao LM, Li YF, Han R (2015) He-Ne laser preillumination improves the resistance of tall fescue (Festuca arundinacea Schreb.) seedlings to high saline conditions. Protoplasma 252:1135–1148
Glazer AN (1988) Phycobilisomes. Methods Enzymol 167:304–312
Gotz T, Windhovel U, Boger P, Sandmann G (1999) Protection of photosynthesis against ultraviolet-B radiation by carotenoids in transformants of the cyanobacterium Synechococcus PCC7942. Plant Physiol 120:599–604
Hassan HM, Scandalios JM (1990) Superoxide dismutases in aerobic organisms. In: Alscher RG, Cumming JR (eds) Stress responses in plants: adaptation and acclimatation mechanisms. Wiley, New York, pp. 175–199
He H, Liu X, Liu X, Yu J, Li K, Guan B, Jeppesen E, Liu Z (2014) Effects of cyanobacterial blooms on submerged macrophytes alleviated by the native Chinese bivalve Hyriopsis cumingii: a mesocosm experiment study. Ecol Eng 71:6423–6427
Hernández AC, Domínguez PA, Cruz OA, Tsonchev RI (2015) Thermal effects of laser irradiation on maize seeds. Int Agrophys 29:147–156
Holzinger A, Lutz C (2006) Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37:190–207
Hong Y, Hu HY, Li FM (2008) Physiological and biochemical effects of allelochemical ethyl 2-methyl acetoacetate (EMA) on cyanobacterium Microcystis aeruginosa. Ecotoxicol Environ Saf 71:527–534
Hudnell HK (2010) The state of U.S. freshwater harmful algal blooms assessments, policy and legislation. Toxicon 55:1024–1034
Jamil Y, Perveen R, Ashraf M, Ali Q, Iqbal M, Ahmad MR (2013) He-Ne laser-induced changes in germination, thermodynamic parameters, internal energy, enzyme activities and physiological attributes of wheat during germination and early growth. Laser Phys Lett 10:045606–045614
Jia Y, Yang Z, Su W, Johnson D, Kong F (2013) Controlling of cyanobacteria bloom during bottleneck stages of algal cycling in shallow Lake Taihu (China). J Freshw Ecol 29:129–140
Jiang H, Qiu B (2005) Photosynthetic adaptation of a bloom-forming cyanobacterium Microcystis aeruginosa (Cyanophyceae) to prolonged UV-B exposure. J Phycol 41:983–992
Kaebernick M, Neilan BA, Borner T, Dittmann E (2000) Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl Environ Microbiol 66:3387–3392
Kuster A, Schaible R, Schubert H (2004) Light acclimation of photosynthesis in three charophyte species. Aquat Bot 79:111–124
Li H, Yao C, Dong X, Dong W, Fan Z (2011) Effect of pH on inactivation of Microcystis aeruginosa by ozonation air in sequencing batch reactor. J Chem Technol Biotechnol 86:468–471
Lu K, Jin C, Dong S, Gu B, Bowen SH (2006) Feeding and control of blue-green algal blooms by tilapia (Oreochromis niloticus). Hydrobiologia 568:111–120
Matthijs HC, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, Talens R, Huisman J (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res 46:1460–1472
Nandakumar K, Obika H, Shinozaki T, Ooie T, Utsumi A, Yano T (2003) Pulsed laser irradiation impact on two marine diatoms Skeletonema costatum and Chaetoceros gracilis. Water Res 37:2311–2316
Nandakumar K, Obika H, Sreekumari K, Utsumi A, Ooie T, Yano T (2009) Laser damage to marine plankton and its application to checking biofouling and invasion by aquatic species: a laboratory study. Biofouling 25:95–98
Paerl HW, Hall NS, Calandrino ES (2011) Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci Total Environ 409:1739–1745
Perveen R, Jamil Y, Ashraf M, Ali Q, Iqbal M, Ahmad MR (2011) He-Ne laser-induced improvement in biochemical, physiological, growth and yield characteristics in sunflower (Helianthus annuus L.). Photochem Photobiol 87:1453–1463
Pinho LX, Azevedo J, Brito A, Santos A, Tamagnini P, Vilar VJP, Vasconcelos VM, Boaventura RAR (2015) Effect of TiO2 photocatalysis on the destruction of Microcystis aeruginosa cells and degradation of cyanotoxins microcystin-LR and cylindrospermopsin. Chem Eng J 268:144–152
Podlesny J, Stochmal A, Podlesna A, Misiak LE (2012) Effect of laser light treatment on some biochemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Regul 67:227–233
Popov AY, Popova NA, Tyurin AV (2007) A physical model of the action of low-intensity laser radiation on biological objects. Opt Spectrosc 103:671–677
Simko M (2007) Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr Med Chem 14:1141–1152
Song L, Marsh TL, Voice TC, Long DT (2011) Loss of seasonal variability in a lake resulting from copper sulfate algaecide treatment. Phys Chem Earth 642:430–435
Steffen MM, Belisle BS, Watson SB, Boyer GL, Wilhelm SW (2014) Status, causes and controls of cyanobacterial blooms in Lake Erie. J Great Lakes Res 40:215–225
Wang H, Zhong G, Yan H, Liu H, Wang Y, Zhang C (2012) Growth control of cyanobacteria by three submerged macrophytes. Environ Eng Sci 29:420–425
Wang J, Xie P, Guo N (2007) Effects of nonylphenol on the growth and microcystin production of Microcystis strains. Environ Res 103:70–78
Weirich CA, Miller TR (2014) Freshwater harmful algal blooms: toxins and children’s health. Curr Probl Pediatr Adolesc Health Care 44:2–24
Wiedner C, Visser PM, Fastner J, Metcalf JS, Codd GA, Mur LR (2003) Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl Environ Microbiol 69:1475–1481
Wu X, Joyce EM, Mason TJ (2012) Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res 46:2851–2858
Xiao Y, Liu YD, Wang GH, Hao ZJ, YJ A (2010) Simulated microgravity alters growth and microcystin production in Microcystis aeruginosa (cyanophyta). Toxicon 56:1–7
Xing W, Huang WM, Li DH, Liu YD (2007) Effects of iron on growth, pigment content, photosystem II efficiency, and siderophores production of Microcystis aeruginosa and Microcystis wesenbergii. Curr Microbiol 55:94–98
Yang Z, Kong F, Shi X, Yu Y, Zhang M (2015) Effects of UV-B radiation on microcystin production of a toxic strain of Microcystis aeruginosa and its competitiveness against a non-toxic strain. J Hazard Mater 283:447–453
Zheng B, Zheng Z, Zhang J, Luo X, Liu Q, Wang J, Zhao Y (2012) The removal of Microcystis aeruginosa in water by gamma-ray irradiation. Sep Purif Technol 85:165–170
Zhou S, Shao Y, Gao N, Li L, Deng J, Zhu M, Zhu S (2014a) Effect of chlorine dioxide on cyanobacterial cell integrity, toxin degradation and disinfection by-product formation. Sci Total Environ 482–483:208–213
Zhou S, Shao Y, Gao N, Zhu S, Li L, Deng J, Zhu M (2014b) Removal of Microcystis aeruginosa by potassium ferrate (VI): impacts on cells integrity, intracellular organic matter release and disinfection by-products formation. Chem Eng J 251:304–309
Zhou W, Juneau P, Qiu B (2006) Growth and photosynthetic responses of the bloom-forming cyanobacterium Microcystis aeruginosa to elevated levels of cadmium. Chemosphere 65:1738–1746
Zou H, Pan G, Chen H, Yuan X (2006) Removal of cyanobacterial blooms in Taihu Lake using local soils. II. Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan. Environ Pollut 141:201–205
Acknowledgments
The authors would like to thank the National Major Science and Technology Projects for Pollution Control and Management (2012ZX07104-002-005 and 2012ZX07101-007-002) and the State Key Laboratory of Freshwater Ecology and Biotechnology (2014FBZ03 and 2015FB16) for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, T., Bi, Y., Liu, J. et al. Effects of laser irradiation on a bloom forming cyanobacterium Microcystis aeruginosa . Environ Sci Pollut Res 23, 20297–20306 (2016). https://doi.org/10.1007/s11356-016-7235-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7235-7