Abstract
Vinyl chloride (VC) is a frequent groundwater contaminant and a known human carcinogen. Bioremediation is a potential cleanup strategy for contaminated sites; however, little is known about the bacteria responsible for aerobic VC degradation in mixed microbial communities. In attempts to address this knowledge gap, the microorganisms able to assimilate labeled carbon (13C) from VC within a mixed culture capable of rapid VC degradation (120 μmol in 7 days) were identified using stable isotope probing (SIP). For this, at two time points during VC degradation (days 3 and 7), DNA was extracted from replicate cultures initially supplied with labeled or unlabeled VC. The extracted DNA was ultracentrifuged, fractioned, and the fractions of greater buoyant density (heavy fractions, 1.758 to 1.780 g mL−1) were subject to high-throughput sequencing. Following this, specific primers were designed for the most abundant phylotypes in the heavy fractions. Then, quantitative PCR (qPCR) was used across the buoyant density gradient to confirm label uptake by these phylotypes. From qPCR and/or sequencing data, five phylotypes were found to be dominant in the heavy fractions, including Nocardioides (∼40 %), Sediminibacterium (∼25 %), Aquabacterium (∼17 %), Variovorax (∼6 %), and Pseudomonas (∼1 %). The abundance of two functional genes (etnC and etnE) associated with VC degradation was also investigated in the SIP fractions. Peak shifts of etnC and etnE gene abundance toward heavier fractions were observed, indicating uptake of 13C into the microorganisms harboring these genes. Analysis of the total microbial community indicated a significant dominance of Nocardioides over the other label-enriched phylotypes. Overall, the data indicate Nocardioides is primarily responsible for VC degradation in this mixed culture, with the other putative VC degraders generating a small growth benefit from VC degradation. The specific primers designed toward the putative VC degraders may be of use for investigating VC degradation potential at contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vinyl chloride (VC) is a common contaminant in groundwater, threatening both environmental and human health. This chemical is classified as a human carcinogen, with a maximum contamination level (MCL) of 0.002 mg L−1 and an MCL goal of 0 mg L−1. VC in groundwater originates primarily from the transformation of tetrachloroethene (PCE) and trichloroethene (TCE). These chlorinated ethenes are common pollutants due to their widespread use and previous careless disposal. In groundwater, natural biotic and abiotic processes degrade these solvents, leading to the accumulation of the metabolites, cis-dichloroethene, and VC (Bradley 2003; He et al. 2003). Notably, VC is the most carcinogenic of the chlorinated ethenes and has the lowest limit in drinking water.
The use of microorganisms in groundwater bioremediation occurs through monitored natural attenuation, biostimulation, or bioaugmentation. The biodegradation of VC can occur by either anaerobic or aerobic pathways and by co-metabolism or direct VC assimilation (Mattes et al. 2010). Anaerobic transformation of VC by Dehalococcoides spp. can occur either by co-metabolism or through energy generating VC reduction (Cupples et al. 2003; He et al. 2003; Loffler et al. 2013). For the aerobic metabolism of VC, several bacteria belonging to the phyla Proteobacteria and Actinobacteria have been isolated (Coleman et al. 2002a; Danko et al. 2004; Elango et al. 2006; Fathepure et al. 2005; Fullerton et al. 2014; Hartmans and Debont 1992; Hartmans et al. 1985; Jin et al. 2010; Jin and Mattes 2008; Verce et al. 2000). While these microorganisms have contributed to the current state of knowledge on VC metabolism, limitations associated with culture-based methods have likely resulted in an incomplete understanding of VC degrading microorganisms in mixed microbial communities or in groundwater at contaminated sites.
To address the limitations surrounding culture-based approaches, various molecular tools have been adopted. For instance, stable isotope probing (SIP) has the advantage of linking function (e.g., carbon uptake) to microorganism identity in mixed microbial communities (Cupples 2011). This method was first introduced for investigating methanol-utilizing microorganisms in soil (Radajewski et al. 2000) but, since then, has been used to identify microorganisms that are able to metabolize a variety of carbon and nitrogen sources. SIP involves sample or culture exposure to a labeled compound (in this work, 13C-VC) and DNA extraction with time. The DNA is then subjected to ultracentrifugation, fractionation (separates label incorporated DNA from the unlabeled DNA), and DNA sequencing. The phylotypes that are present in the heaviest fractions are considered responsible for incorporating the labeled carbon from the target chemical.
Another molecular tool important for understanding the potential for contaminant biodegradation in mixed communities and in groundwater is quantitative PCR (qPCR). In the case of VC biodegradation, qPCR assays have been developed and applied to target etnC and etnE (Jin and Mattes 2010; Patterson et al. 2013). The gene etnC encodes the alkene monooxygenase (AkMO) alpha subunit, which is involved in the initial attack on VC, to transform it to VC epoxide. The gene etnE encodes the epoxyalkane coenzyme M transferase (EaCoMT), which conjugates the VC epoxide to CoM and introduces the metabolite to the central metabolic pathway. However, qPCR on functional genes alone cannot link activity to microorganism identity.
In a previous study, we investigated the microorganisms able to uptake carbon from VC in a mixed culture capable of slower VC degradation (∼120 μmol in 45 days) using SIP and also examined etnE abundance in the heavy SIP fractions (Paes et al. 2015). In the present study, the overall objective was to determine the microorganisms associated with carbon uptake from VC in an enrichment culture (derived from different groundwater than the previous study) capable of more rapid VC degradation (∼120 μmol in 7 days). Further, unlike previous research, qPCR primers were developed toward the identified enriched phylotypes to provide more robust evidence of carbon uptake. In addition, the presence of two functional genes associated with VC degradation (etnE and etnC) in the heavy SIP fractions was investigated. Furthermore, changes in the total microbial community during VC degradation were examined. The research identifies several novel phylotypes as being linked to carbon uptake from VC.
Methods
Experimental design for SIP
The VC enrichment culture was derived from groundwater from well 63-I (collected September 2009) at a VC contaminated site in Carver, MA. At the time of sampling, the groundwater was slightly anoxic (DO 0.61 mg/L). The culture was developed using a previously described approach (Findlay et al. 2016), involving transferring 1 mL of well 63-I groundwater to 100 mL of mineral salts media (MSM) in 160 mL serum bottles with air headspace and maintaining O2 by injecting pure O2 as needed after the onset of biodegradation. The culture was repeatedly fed VC for 1 year and then subcultured at 1:100 once, followed by subculturing at 1:1000 three times.
Microcosms for the SIP experiment involved sterile serum bottles (160 mL), VC enrichment culture (as described above), MSM, and VC (approx. 47 mg L−1). These microcosms were prepared as previously described (Coleman et al. 2002b). Two abiotic control microcosms (obtained using autoclaving) and six live microcosms were amended with unlabeled VC (99 %, Specialty Gases of America, Toledo, OH). In addition, six live microcosms were amended with labeled VC (13C2 VC, 99 %, Cambridge Isotope Laboratories, Xenia, OH). The microcosms were shielded from light (21–23 °C) and were placed on a shaker (200–300 rpm). VC concentrations were determined for 7 days and DNA was extracted from labeled VC-amended and unlabeled VC-amended microcosms at days 3 and 7.
Analytical methods
Gas chromatography was used to analyze headspace samples (100 μL) (Perkin Elmer, Waltham, MA) with flame ionization detection and a capillary column (DB-624, diameter 0.53 mm; J&W Scientific, Santa Clara, CA). Aqueous phase VC concentrations were calculated using an external standard and a previously reported Henry’s law constant (Gossett 1987).
DNA extraction, ultracentrifugation, and fractionation
The UltraClean Microbial DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA) was used for DNA extraction according to the manufacturer’s recommended procedure. The cultures were centrifuged to concentrate the cells, and then the entire pellet was used for DNA extraction. The final volume of DNA extract was 150 μL. Following this, the DNA extracts (∼10 μg) were added to Quick-Seal polyallomer tubes (13 by 51 mm, 5.1 mL; Beckman Coulter, Indianapolis, IN) along with a Tris-EDTA (pH 8.0)-CsCl solution for ultracentrifugation. Before sealing (cordless Quick-Seal tube topper; Beckman), the buoyant density (BD) was quantified with a model AR200 digital refractometer (Leica Microsystems Inc., Buffalo Grove, IL) and adjusted to a final BD of 1.73 g mL−1 by the addition of CsCl solution or Tris-EDTA buffer. Tubes were ultracentrifuged, and the fractions were generated following previously described procedures (Paes et al. 2015). The BD of each fraction was measured, and glycogen-assisted ethanol precipitation was used to remove CsCl. DNA concentrations were determined using Qubit™ (Invitrogen, Carlsbad, CA) to ascertain which fractions were enriched in labeled carbon.
MiSEQ Illumina sequencing and fraction analysis
The four heaviest fractions from each time (days 3 and 7) from the labeled VC-amended microcosms were analyzed to ascertain which microorganisms were enriched in these fractions and were responsible for carbon uptake from VC. The fractions were selected based on the DNA concentration in each fraction from the labeled VC amended microcosms compared to the unlabeled VC amended microcosms. In all, 48 samples were subjected high-throughput sequencing (MiSEQ Illumina) at the Research Technology Support Facility (RTSF) at Michigan State University. These samples included six replicates per fraction, four fractions per time point, and two time points.
A previously described protocol was used to perform PCR and Illumina sequencing at the RTSF (Caporaso et al. 2011). For this, the V4 region of the 16S ribosomal RNA (rRNA) gene was amplified using a set of multiplex indexed primers. The following steps involved quantification with the Picogreen assay, the generation of a pool of equimolar amounts of each, and purification using Ampure XP beads. In addition, to ensure the elimination of non-specific products, a gel final purification step was performed. The combined library was examined using Illumina MiSEQ with a paired end (2 × 250 bp) flow cell and reagent cartridge.
Mothur (Schloss et al. 2009) was used to analyze the data generated by MiSEQ Illumina using a SOP developed by Schloss (http://www.mothur.org/wiki/MiSeq_SOP). This involved the construction of contigs, error, and chimera removal, followed by sequence alignment for OTU assignment based on the SILVA database (Pruesse et al. 2007). Final data matrices were exported to Excel 2013 SR-1 (Microsoft Corporation, Redmond, WA). To identify which OTUs were responsible for the label uptake in each fraction at each time point, the relative abundance (%) of each OTU was calculated.
Primer design, 16S rRNA gene clone libraries, and qPCR
Specific primers (sedF and sedR; aquaF and aquaR; and varF and varR) were designed to target the phylotypes responsible for the VC assimilation (identified above) using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The new primers were tested for specificity, using 16S rRNA gene clone libraries and Sanger sequencing. Following this, the primers were used in the qPCR assays across the BD gradient to confirm label uptake.
Three 16S rRNA clone libraries were generated using total DNA extracted from the labeled microcosms from day 3. DNA was PCR-amplified using 3 primer sets: sedF (5′-CGG GCA GTT AAG TCA GTG GT-3′) and sedR (5′-TGC CTT CGC AAT AGG TGT TCT-3′); aquaF (5′-CGT AGG GTG CGA GCG TTA AT-3′) and aquaR (5′-CCA TCC CCC TCT ACC GTA CT-3′); and varF (5′-TCT GTG ACT GCA TTG CTG GA-3′) and varR (5′-CGG TGT TCC TCC GCA TAT CT-3′) (Integrated DNA Technologies (IDT), Coralville, IA). The PCR program consisted of an initial denaturation (95 °C, 5 min), 29 cycles of amplification (95 °C, 30 s; 58 °C, 30 s; 72 °C, 1:30 min), and a terminal extension step (72 °C, 30 min). Agarose gel electrophoresis and the subsequent staining of the gels with ethidium bromide confirmed the presence of PCR products. The PCR amplicons were purified with QIAquick PCR purification kit (Qiagen Inc., Alameda, CA) and were then cloned into Escherichia coli TOP10 vector (TOPO TA cloning kit, Invitrogen). Clones of E. coli were grown on Luria-Bertani medium, containing 15 g agar L−1 and 50 μg ampicillin L−1 for 16 h at 37 °C. Colonies with inserts were verified by PCR with primers M13F (5′-TGT AAA ACG ACG GCC AGT-3′) and M13R (5′-AAC AGC TAT GA CAT G-3′); plasmids were extracted from the positive clones (QIAprep miniprep system, Qiagen, Inc.); and the insertions were sequenced at the RTSF. The clone sequences for each library were aligned against the Illumina sequences using MEGA 6 to determine the specificity of each primer set.
Amplification and qPCR measurements were performed in a Chromo 4 real-time PCR cycler (Bio-Rad, Philadelphia, PA), using QuantiTect SYBR Green PCR Kit (Qiagen Inc.) and the primer sets sedF-sedR, aquaF-aquaR, and varF-varR. DNA from both time points from the density gradient fractions from both the labeled VC and the unlabeled VC amended cultures was subject to qPCR in triplicate. Each 25-μL PCR mixture contained 12.5-μL QuantiTect SYBR Green PCR Master Mix solution, 1.25 μL of each 10 μM primer, 9-μL DNA-free water, and 1-μL DNA template. The PCR protocol involved of an initial denaturation (95 °C, 15 min), 40 cycles (95 °C, 15 s; 58 °C, 20 s; 72 °C, 20 s), and a terminal extension (72 °C, 2 min). Melting curves were obtained from 55 to 95 °C and read every 0.6 °C for 2 s. Cloned plasmid DNA was used as a standard, and the numbers of gene copies were determined as described previously (Ritalahti et al. 2006) (plasmid size was 3931 bp, in addition to inserts of 148 bp by sedF-sedR, 139 bp by aquaF-aquaR, and 86 bp by varF-varR).
Functional gene (etnC and etnE) qPCR
Amplification and qPCR measurements (ABI 7000 Sequence Detection System, Applied Biosystems) involved the Power SYBR Green PCR Master Mix (Applied Biosystems). Primer sets for amplifying etnC genes were RTC_F (5′-ACC CTG GTC GGT GTK STY TC-3′) and RTC_R (5′-TCA TGT AMG AGC CGA CGA AGT C-3′) and for etnE genes were RTE_F (5′-CAG AAY GGC TGY GAC ATY ATC CA-3′) and RTE_R (5′-CSG GYG TRC CCG AGT AGT TWC C-3′) (Jin and Mattes 2010). DNA from both time points from the density gradient fractions from both the labeled VC and the unlabeled VC amended microcosms was subject to qPCR in duplicate. Each 25-μL reaction mixture contained 12.5 μL of Power SYBR Green PCR Master Mix solution, 750 nM of each qPCR primers, and 1 μL of DNA template. A previously developed thermal protocol was used (Jin and Mattes 2010).
PCR products were used as standards in the qPCR assays and involved 1138 bp etnC and 890 bp etnE from Nocardioides sp. strain JS614. Primer sets to produce the qPCR standards for etnC were JS614 EtnCF (5′-GCG ATG GAG AAT GAG AAG GA-3′) and JS614 EtnCR (5′-TCC AGT CAC AAC CCT CAC TG-3′) (Jin and Mattes 2010) and CoMF1L (5′-AAC TAC CCS AAY CCS CGC TGG TAC GAC-3′) and CoMR2E (5′-GTC GGC AGT TTC GGT GAT CGT GCT CTT GAC-3′) for etnE (Coleman and Spain 2003). The 25-μL reactions contained 12.5 μL of Qiagen PCR Master Mix, 0.2 μM of each primer, and 2 ng of JS614 genomic DNA. The number of genes per microliter was determined as described previously (Jin and Mattes 2010). Real time PCR fluorescence data was generated using the ABI 7000 System SDS software (Applied Biosystems), using the auto baseline function.
Microbial community analysis during VC degradation
All of the extracted DNA from the SIP study was fractioned, and therefore, it was not possible to examine the relative abundance of the identified VC assimilators in the total microbial community. Thus, an additional experiment was performed to determine the relative abundance of these microorganisms during VC degradation.
For this, because of the lack of available liquid culture at room temperature, frozen aliquots of the above culture were utilized. The frozen aliquots were generated by centrifuging the culture and then mixing with an equal volume of glycerol (40 % final glycerol). The cell-glycerol mixture was maintained at room temperature for 1 h to allow the cells to incorporate glycerol prior to freezing. To ensure the microbial communities were similar, the frozen aliquots were incubated for 30 days in a sterile serum bottle (160 mL) MSM (as before) and VC (∼20 mg L−1). Following VC degradation, 5 mL of fresh culture was added to sterile serum bottles (160 mL) containing MSM and VC (∼47 mg L−1). These microcosms were created as previously described (Coleman et al. 2002b) and included one abiotic control microcosm (obtained via autoclaving) and three live microcosms. The microcosms were shielded from light (21–23 °C) and were placed on a shaker (200–300 rpm). VC concentrations were examined for 18 days. DNA was extracted as described above from six time points and was analyzed using MiSEQ Illumina and mothur (as described above). The percent relative abundance of the identified phylotypes was determined at each time point.
Results and discussion
VC degradation and the identification of phylotypes responsible for label uptake
Direct aerobic VC oxidation was first reported in groundwater samples (Davis and Carpenter 1990). Since then, aerobic VC degradation and the isolation of VC-degrading microorganisms have been reported in many studies (Coleman et al. 2002a; Danko et al. 2004; Fathepure et al. 2005; Hartmans et al. 1985; Jin et al. 2010; Jin and Mattes 2008; Taylor et al. 2007; Verce et al. 2000). These authors observed degradation over a wide range of time period from as little as 2–20 days (Coleman et al. 2002a; Danko et al. 2004; Elango et al. 2006; Fathepure et al. 2005) to as much as 55–476 days (Coleman et al. 2002a; Jin and Mattes 2008; Verce et al. 2000). However, as previously discussed, limited research has identified the microorganisms responsible for VC degradation within mixed communities.
In the current research, VC was degraded by the mixed enrichment culture in the SIP study in only 7 days. This rapid removal is likely a result of a significant biomass of active VC degraders. VC degradation occurred in both the labeled and the unlabeled VC amended microcosms but not in the abiotic controls, confirming biological removal (Fig. 1). The DNA samples extracted on days 3 and 7 were subject to ultracentrifugation. Following this, the DNA quantity in each fraction was determined (Fig. 2). The presence of DNA in the lighter BD fractions from the unlabeled VC amended cultures and DNA in the heavier fractions from the labeled VC-amended cultures confirms the uptake of labeled carbon by the microorganisms in the mixed community.
Percent VC remaining in cultures amended with 13C vinyl chloride (open triangles and squares), 12C vinyl chloride (closed triangles and squares) and in the abiotic controls (closed diamonds). Arrows indicate when DNA was extracted. The bars represent standard deviations from triplicates (samples) or replicates (controls)
DNA concentration (ng/μL) at day 3 (A) and day 7 (B) in fractions obtained from the labeled VC and unlabeled VC amended cultures. The complete and dashed lines represent DNA concentrations from the unlabeled and labeled cultures, respectively. Replicate DNA measurements are shown. Arrows indicate samples selected for sequencing
The DNA in these heavier fractions (indicated with arrows in Fig. 2) was sequenced. After mothur analysis, the final numbers of sequences obtained were 2,010,477 and 2,073,705 for days 3 and 7, respectively. The chimeric percentage was 1.32 and 1.93 % on days 3 and 7, respectively. Among the final sequences, <2 % were unique at both time points.
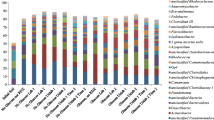
Interestingly, at both time points, five genera were dominant in the heavy fractions, including Nocardioides (Actinobacteria), Sediminibacterium (Bacteroidetes), Aquabacterium (Proteobacteria), Variovorax (Proteobacteria), and, to a lesser extent, Pseudomonas (Proteobacteria) (Fig. 3). The level of enrichment (% relative abundance) was similar in all four heavy fractions at both time points. The data indicates that these microorganisms are responsible for label uptake from VC. In particular, Nocardioides, Sediminibacterium, and Aquabacterium were the three most abundant at both time points. It is uncertain if the enriched phylotypes were responsible for the initial transformation of this chemical, or if they were assimilating VC metabolites.
Primer design, specificity test, and 16S rRNA abundance in SIP fractions
To provide further evidence of label uptake, specific primers were designed toward three of the five dominant genera in the heavy fractions: Sediminibacterium, Aquabacterium, and Variovorax. Nocardioides was not targeted, as this genus has already been linked to VC metabolism (Paes et al. 2015). The primers were utilized to compare gene abundance across the BD profile between the samples amended with labeled VC and those amended with unlabeled VC.
First, the primer specificity was tested by amplifying DNA, creating 16S rRNA gene clone libraries, and sequencing these clones. For this, 16 clones were sequenced for each primer set. The primer sets illustrated a satisfactory level of specificity for their targets. For Sediminibacterium, 14 of 16 clones were 100 % identical with the Sediminibacterium sequence, and 2 of 16 aligned at a 99 % identity level. For Aquabacterium, 13 of 16 aligned at 100 %, 2 of 16 aligned at 99 %, and 1 of 16 aligned at 98 % with the Aquabacterium sequence. For Variovorax, 8 of 13 aligned at 100 % and 5 of 13 aligned at 98.8 % with the Variovorax sequence.
Following the specificity testing, qPCR assays for Sediminibacterium, Aquabacterium, and Variovorax were performed on all of the fractions across the BD gradient at both time points. As expected, maximum gene copy values resided in higher BD fractions for labeled VC amended samples compared to unlabeled VC amended samples, again indicating label uptake by these phylotypes (Fig. 4).
Quantitative PCR has emerged as the method of choice for enumerating targeted genes. Dehalococcoides 16S rRNA primers have been tested and used for site assessment and bioremediation implementation for chlorinated pollutants under anaerobic conditions (Hatt and Loffler 2012; Kanitkar et al. 2016; Stedtfeld et al. 2014). In the present study, the 16S rRNA primers were designed for the phylotypes associated with carbon uptake from VC (Sediminibacterium, Aquabacterium, and Variovorax). These were used to provide additional evidence that these microorganisms were involved in label uptake. The shifts observed in the 16S rRNA gene abundance peaks toward heavier fractions during the VC degradation process indicate the accumulation of 13C in the 16S rRNA genes of Sediminibacterium, Aquabacterium, and Variovorax. Similar shifts have been observed by other authors who combined SIP and qPCR for toluene-degrading cultures with the gene bssA (Sun and Cupples 2012).
To date, known aerobic VC-assimilating microorganisms belong to the phyla Proteobacteria and Actinobacteria. Within these two phyla, several isolates have been associated with aerobic VC assimilation, including those in the genera Mycobacterium (Coleman et al. 2002a; Fathepure et al. 2005; Guan et al. 2013; Hartmans et al. 1985; Jin et al. 2010; Jin and Mattes 2008; Taylor et al. 2007), Pseudomonas (Coleman and Spain 2003; Verce et al. 2000), Nocardioides (Coleman et al. 2002a), Ochrobactrum (Danko et al. 2004), and Ralstonia (Elango et al. 2006).
In agreement with previous work (Coleman et al. 2002a), the current research indicates that the genus Nocardioides is associated with VC assimilation. Interestingly, both Pseudomonas and Ralstonia were enriched to a lesser extent in the heavy fractions for the labeled VC amended cultures (Fig. 3) and both have previously been linked to aerobic VC removal (Danko et al. 2004; Elango et al. 2006; Verce et al. 2000). Isolates within the genus Sediminibacterium have not previously been linked to aerobic VC degradation, although this genus has been found at groundwater sites contaminated with chlorinated aliphatic hydrocarbons (Guan et al. 2013). The genera Aquabacterium and Variovorax have not previously been associated with VC degradation. However, Aquabacterium has been observed at sites contaminated with chlorinated aliphatic hydrocarbons (Guan et al. 2013).
To our knowledge, only one other report exists on identifying the bacteria that are able to assimilate carbon from VC in a mixed microbial community (Paes et al. 2015). In our previous study, Nocardioides was also a dominant VC assimilator and with other phylotypes (Brevundimonas, Tissierella, and Rhodoferax), which is being associated with minor levels of label uptake. Taken collectively, Nocardioides is clearly an important phylotype for aerobic VC degradation in mixed communities. As both cultures were derived from contaminated site groundwater, it is possible that this genus is also important for in situ VC degradation.
Functional gene (etnC and etnE) abundance in SIP fractions
Quantitative PCR assays for the functional genes etnC and etnE were also performed for BD fractions from both the labeled and the unlabeled VC amended cultures. Overall, the highest unlabeled peaks were at lighter BD fractions (BD = 1.720–1.760 g mL−1), and the highest labeled peaks were at heavier BD fractions (BD = 1.740–1.780 g mL−1) (Fig. 5). The genes etnC and etnE are involved in VC metabolism and are, therefore, useful biomarkers for VC degradation potential. Peak shifts of etnC and etnE gene abundance toward heavier fractions were observed indicating uptake of 13C into the microorganisms harboring these genes. The data generated here are in agreement with previous research, which has indicated the importance of etnC and etnE in the VC biodegradation pathway of Nocardioides sp. (such as strain JS614) (Mattes et al. 2005; Paes et al. 2015).
Microbial community changes during VC degradation
As expected, in the experiment to examine microbial community changes over time, degradation of VC occurred in the cultures but not in the abiotic control (Fig. 6), with a VC half-life of 8.1 ± 1.3 days. DNA extracted during VC removal (days 2, 9, 10, 13, 14 and 16) was examined with high-throughput sequencing to determine the relative abundance of the phylotypes identified as being enriched in the heavy fractions (Figs. 3 and 7). The data indicate that the relative abundance of Nocardioides was high initially and remained high during VC degradation (18–33 % relative abundance). Surprisingly, Sediminibacterium, Aquabacterium, and Pseudomonas were present at less than 1 % relative abundance, and Variovorax was <0.0017 % in these cultures. However, the abundance of the three phylotypes did increase with time, suggesting that they experienced a growth benefit from VC degradation.
Conclusions
The microorganisms responsible for assimilating 13C from VC during aerobic VC degradation included Nocardioides (Actinobacteria), Sediminibacterium (Proteobacteria), Aquabacterium (Proteobacteria), Variovorax (Proteobacteria) and, to a lesser extent, Pseudomonas (Proteobacteria). Hence, both previously reported VC assimilators (Nocardioides, Pseudomonas), and novel phylotypes (Sediminibacterium, Aquabacterium, Variovorax) were involved in the label uptake from VC. Additional research is needed to determine if these microorganisms are using carbon from VC directly or VC metabolites. The overall microbial community analysis of this culture indicated that Nocardioides was dominant, although, the relative abundance of the other phylotypes increased as VC was degraded, suggesting a growth benefit from VC degradation. SIP on two functional genes involved in the VC biodegradation pathway (etnE and etnC) indicated that the microorganisms harboring these genes were involved in the label uptake. The specific primers designed toward the putative VC degraders may be of use for investigating VC degradation potential at contaminated groundwater sites.
References
Bradley PM (2003) History and ecology of chloroethene biodegradation: a review. Bioremed J 7:81–109
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS 108:4516–4522
Coleman NV, Spain JC (2003) Distribution of the coenzyme m pathway of epoxide metabolism among ethene- and vinyl chloride-degrading Mycobacterium strains. Appl Environ Microb 69:6041–6046
Coleman NV, Mattes TE, Gossett JM, Spain JC (2002a) Phylogenetic and kinetic diversity of aerobic vinyl chloride-assimilating bacteria from contaminated sites. Appl Environ Microb 68:6162–6171
Coleman NV, Mattes TE, Gossett JM, Spain JC (2002b) Biodegradation of cis-dichloroethene as the sole carbon source by a beta-proteobacterium. Appl Environ Microb 68:2726–2730
Cupples AM (2011) The use of nucleic acid based stable isotope probing to identify the microorganisms responsible for anaerobic benzene and toluene biodegradation. J Micro Meth 85:83–91
Cupples AM, Spormann AM, McCarty PL (2003) Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl Environ Microb 69:953–959
Danko AS, Luo MZ, Bagwell CE, Brigmon RL, Freedman DL (2004) Involvement of linear plasmids in aerobic biodegradation of vinyl chloride. Appl Environ Microb 70:6092–6097
Davis J, Carpenter C (1990) Aerobic degradation of vinyl chloride in groundwater samples. Appl Environ Microb 56:3878–3880
Elango VK, Liggenstoffer AS, Fathepure BZ (2006) Biodegradation of vinyl chloride and cis-dichloroethene by a Ralstonia sp. strain TRW-1. Appl Micro Biotec 72:1270–1275
Fathepure BZ, Elango VK, Singh H, Bruner MA (2005) Bioaugmentation potential of a vinyl chloride-assimilating Mycobacterium sp., isolated from a chloroethene-contaminated aquifer. FEMS Micro Lett 248:227–234
Findlay M, Smoler DF, Fogel S, Mattes TE (2016) Aerobic vinyl chloride metabolism in groundwater microcosms by methanotrophic and etheneotrophic bacteria. Environ Sci Technol 50:3617–3625
Fullerton H, Rogers R, Freedman D, Zinder S (2014) Isolation of an aerobic vinyl chloride oxidizer from anaerobic groundwater. Biodegradation 25:893–901
Gossett JM (1987) Measurement of Henrys law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol 21:202–208
Guan XY, Liu F, Xie YX, Zhu LL, Han B (2013) Microbiota associated with the migration and transformation of chlorinated aliphatic hydrocarbons in groundwater. Environ Geochem Health 35:535–549
Hartmans S, Debont JAM (1992) Aerobic vinyl chloride metabolism in Mycobacterium aurum L1. Appl Environ Microb 58:1220–1226
Hartmans S, Debont JAM, Tramper J, Luyben K (1985) Bacterial degradation of vinyl chloride. Biotechnol Lett 7:383–388
Hatt JK, Loffler FE (2012) Quantitative real-time PCR (qPCR) detection chemistries affect enumeration of the Dehalococcoides 16S rRNA gene in groundwater. J Micro Meth 88:263–270
He J, Ritalahti KM, Yang K-L, Koenigsberg SS, Löffler FE (2003) Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62–65
Jin YO, Mattes TE (2008) Adaptation of aerobic, ethene-assimilating Mycobacterium strains to vinyl chloride as a growth substrate. Environ Sci Technol 42:4784–4789
Jin YO, Mattes TE (2010) A quantitative PCR assay for aerobic, vinyl chloride- and ethene-assimilating microorganisms in groundwater. Environ Sci Technol 44:9036–9041
Jin YO, Cheung S, Coleman NV, Mattes TE (2010) Association of missense mutations in epoxyalkane coenzyme M transferase with adaptation of Mycobacterium sp. strain JS623 to growth on vinyl chloride. Appl Environ Microb 76:3413–3419
Kanitkar Y, Stedtfeld RD, Steffan RJ, Hashsham SA, Cupples AM (2016) Development of loop mediated isothermal amplification (LAMP) for rapid detection and quantification of Dehalococcoides spp. biomarker genes in commercial reductive dechlorinating cultures KB-1 and SDC-9. Appl Environ Microb, In Press
Loffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Muller JA, Fullerton H, Zinder SH, Spormann AM (2013) Dehalococcoides mccartyi gen. nov., sp nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Micr 63:625–635
Mattes TE, Coleman NV, Spain JC, Gossett JM (2005) Physiological and molecular genetic analyses of vinyl chloride and ethene biodegradation in Nocardioides sp. strain JS614. Arch Micro 183:95–106
Mattes TE, Alexander AK, Coleman NV (2010) Aerobic biodegradation of the chloroethenes: pathways, enzymes, ecology, and evolution. FEMS Micro Rev 34:445–475
Paes F, Liu X, Mattes TE, Cupples AM (2015) Elucidating carbon uptake from vinyl chloride using stable isotope probing and Illumina sequencing. Appl Micro Biotec 99:7735–7743
Patterson BM, Aravena R, Davis GB, Furness AJ, Bastow TP, Bouchard D (2013) Multiple lines of evidence to demonstrate vinyl chloride aerobic biodegradation in the vadose zone, and factors controlling rates. J Contam Hydrol 153:69–77
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Gloeckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Radajewski S, Ineson P, Parekh NR, Murrell JC (2000) Stable-isotope probing as a tool in microbial ecology. Nature 403:646–649
Ritalahti KM, Amos BK, Sung Y, Wu QZ, Koenigsberg SS, Loffler FE (2006) Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl Environ Microb 72:2765–2774
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75:7537–7541
Stedtfeld RD, Stedtfeld TM, Kronlein M, Seyrig G, Steffan RJ, Cupples AM, Hashsham SA (2014) DNA extraction-free quantification of Dehalococcoides spp. in groundwater using a hand-held device. Environ Sci Technol 48:13855–13863
Sun WM, Cupples AM (2012) Diversity of five anaerobic toluene-degrading microbial communities investigated using stable isotope probing. Appl Environ Microb 78:972–980
Taylor AE, Dolan ME, Bottomley PJ, Semprini L (2007) Utilization of fluoroethene as a surrogate for aerobic vinyl chloride transformation. Environ Sci Technol 41:6378–6383
Verce MF, Ulrich RL, Freedman DL (2000) Characterization of an isolate that uses vinyl chloride as a growth substrate under aerobic conditions. Appl Environ Microb 66:3535–3542
Acknowledgments
A collaborative NSF Grant (number 1233154) awarded to Timothy E. Mattes and Alison M. Cupples funded this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work did not involve research with animals or humans.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Gerald Thouand
Rights and permissions
About this article
Cite this article
Wilson, F.P., Liu, X., Mattes, T.E. et al. Nocardioides, Sediminibacterium, Aquabacterium, Variovorax, and Pseudomonas linked to carbon uptake during aerobic vinyl chloride biodegradation. Environ Sci Pollut Res 23, 19062–19070 (2016). https://doi.org/10.1007/s11356-016-7099-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7099-x