Abstract
The Iguaçu River, located at the Southern part of Brazil, has a great socioeconomic and environmental importance due to its high endemic fish fauna and its potential to generate hydroelectric power. However, Iguaçu River suffers intense discharge of pollutants in the origin of the river. In a previous report, the local environmental agency described water quality to improve along the river course. However, no study with integrated evaluation of chemical analysis and biological responses has been reported so far for the Iguaçu River. In the current study, three different Brazilian fish species (Astyanax bifasciatus, Chrenicicla iguassuensis, and Geophagus brasiliensis) were captured in the five cascading reservoirs of Iguaçu River for a multi-biomarker study. Chemical analysis in water, sediment, and muscle indicated high levels of bioavailable metals in all reservoirs. Polycyclic aromatic hydrocarbons (PAHs) were detected in the bile of the three fish species. Integration of the data through a FA/PCA analysis demonstrated the poorest environmental quality of the reservoir farthest from river’s source, which is the opposite of what has been reported by the environmental agency. The presence of hazardous chemicals in the five reservoirs of Iguaçu River, their bioaccumulation in the muscle of fish, and the biological responses showed the impacts of human activities to this area and did not confirm a gradient of pollution between the five reservoirs, from the source toward Iguaçu River’s mouth. Therefore, diffuse source of pollutants present along the river course are increasing the risk of exposure to biota and human populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic ecosystems receive a diverse set of compounds from many anthropogenic sources. Chemical interactions in complex mixtures represent a challenge for assessing the health risks of organisms (Contreras López 2003; Tian et al. 2015), but represent the real situation of exposure.
Human activities have deteriorated water quality in the last century through discharge of poorly treated urban and industrial waste. A critical consequence is the scarcity of potable water even in regions where freshwater is abundant. With the continuous growth of human population, the demand for potable water will increase.
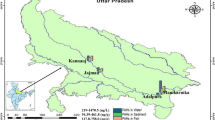
The Iguaçu River is an important river of Southern Brazil (Fig. 1), due to its potential for hydroelectric power generation, human water supply, and the occurrence of many endemic fish species. Nevertheless, Iguaçu River is described as the second most polluted river in Brazil (IBGE, I. B. de G. e E 2010). It rises in the Metropolitan Region of Curitiba (MRC, 1.8 million inhabitants - IBGE 2010) and receives large amounts of urban and industrial wastes without adequate treatment, resulting in frequent cyanobacteria blooms in reservoirs closest to the city (Carneiro et al., 2014) and high concentrations of polycyclic aromatic hydrocarbons (PAHs) in the sediment (Leite et al. 2011).
Approximately 200 km from MRC, there is a sequence of five major cascading reservoirs used for hydropower plants and intense agricultural activity (e.g., soybeans and wheat farms) developed along the riverbank. After crossing Parana State, approximately 1000 km from the MRC, the Iguaçu river reaches the Iguaçu Falls, which works as a natural barrier and is responsible for generating a high endemic fish fauna in the Iguaçu River (about 70 % of fish species) (Baumgartner et al. 2012). The lower part of the Iguaçu river will then flow into the Parana River. This significant ichthyologic endemism represents an important ecologic issue for the Iguaçu River, since susceptibility of extinction increases with the construction of dams, introduction of non-native species, aquaculture, and pollution (Daga and Gubiani, 2012).
The State Environmental Agency described in its most recent online report that an improvement of the water quality index of the studied sites upgraded from moderately to less degraded (IAP 2008). However, these regulatory monitoring programs usually consider only analysis of physicochemical parameters, which are inadequate to predict potential toxic effects on the ecosystem (Dos Santos et al. 2016). Correlations among environmental concentrations of contaminants, bioaccumulation, and early biological responses should be integrated to better comprehend the environmental risks (Van der Oost et al. 2003).
In this context, a multi-biomarker approach is a more suitable analysis to detect earlier biological responses triggered by pollutants (de Andrade Brito et al. 2012; Linde-Arias et al. 2008; Osório et al. 2014). This approach will provide a better ecotoxicological scenario necessary for environmental management (Mills and Chichester, 2005).
Biochemical biomarkers have considerable potential for measuring effects of chemicals under field conditions since they are the earliest sensitive response (Carvalho et al. 2012). Genetic biomarkers inform levels of DNA damage which can be parameter used to assess the susceptibility of an organism to mutations and cancer (Araldi et al. 2015; Collins et al. 2014). Histopathological analysis is another important tool for evaluating lesions in target organs that can detect both acute and chronic exposure (Mela et al. 2007; Oliveira Ribeiro et al. 2006; Rabitto et al. 2005). Evaluation of these biomarkers along with the chemical profile of the water provides a reliable risk assessment for the exposed biota.
In the present study, the water quality of five reservoirs along the Iguaçu River was investigated for the first time through a multi-biomarkers approach in three Brazilian freshwater fish species. Three different fish species were used to compare their specific responses. The fish used are from different trophic levels: the Astyanax bifasciatus (Characidae) or lambari an endemic herbivore and the most abundant fish species, the Chrenicicla iguassuensis (Cichlidae) or joaninha an endemic and carnivorous group, and the Geophagus brasiliensis (Cichlidae) or cará an omnivorous and widely distributed fish (Baumgartner et al. 2012).
Materials and methods
The Iguaçu River is located in Parana, a southern state in Brazil. It extends 1.060 km and is the most important river in a basin of approximately 72.000 km2 (Baumgartner et al. 2012). Along the river, there are five reservoirs that were analyzed in this study (Fig. 1). The Foz do Areia Reservoir (FA, 26° 00′ 60.82″ S 51° 65′ 68.22″ W) has the largest hydropower plant and is the closest to the metropolitan region of Curitiba (MRC). The other reservoirs, toward the river mouth, are Segredo (SE, 25° 79′ 13.36″ S, 52° 11′ 89.36″ W), Salto Santiago (SS, 25° 63′ 73.08″ S, 52° 60′ 20.19″ W), Salto Osório (SO, 25° 53′ 71.06″ S, 53° 00′ 33.31″ W), and Salto Caxias (SC, 25° 53′ 64.43″ S, 53° 49′ 90.70″ W).
Fish sampling
A total of 346 adult fish (A. bifasciatus, C. iguassuensis, and G. brasiliensis) were captured in the reservoirs during summer (January–February) and winter (July–August) of 2013 (Table 1). Fish were captured through fishing nets (4–6 cm mesh) and trawl net with sampling every 4 h during a period of 24 to 48 h. Fish were then anesthetized with 25–30 mg l−1 benzocaine and killed in the field to avoid stress due to fish transportation. The same procedure and capture effort was exerted in all studied reservoirs.
Physicochemical parameters, microcystin-LR, and metals detection
Temperature, pH, and oxygen dissolved data were provided by the State of Parana Electric Company - COPEL and TRACTEBEL Energy companies (Table 2).
Microcystin-LR was extracted according to MATTHIENSEN et al. (1999) with modifications. The intracellular toxin was determined in 1 L of water collected per reservoir and filtered in a glass fiber filter (GF/C 45 mm, Millipore), which was frozen and lyophilized for 24 h. Twenty-five milliliters of pure methanol (HPLC grade, J.T. Baker) was added to extract the toxin at room temperature for 1 h. In order to optimize the extraction, this step was repeated. After the second extraction, the toxin was concentrated with a vacuum rotary evaporator at 80 rpm at 40 °C. The extract was solubilized in 1 ml of methanol and stored at −20 °C. On the other hand, the toxins dissolved in the column of water of the reservoirs were determined in 1 L of filtered water (GF/C 45 mm, Millipore) with addition of 10 % trifluoroacetic acid (TFA) and filtered again. A solid phase column (C18) was used to extract the toxin from the filtered water, which was eluted with 3 ml of 0.1 % methanol/TFA (v/v) at a flux of 10 ml min−1. The collected extracts were dried with nitrogen gas at 45 °C and suspended in methanol. Finally, the extracts were analyzed with a LC-MS ion trap (HPLC Shimadzu Prominence) through electrospray source.
Metals (Ag, Co, Cd, Cr, Cu, Mn, Ni, Pb and Zn) were measured in water, sediment, and fish muscle from the five reservoirs. The water was sampled at 30 cm below the surface and preserved with 0.5 % of HNO3, while the sediment was collected 10 m away from the shore. Samples were protected from light and kept at 4 °C until analysis. For analysis of toxic metals bioaccumulation, muscle samples of all fish were pooled per group and stored at −75 °C until analysis according to Cotta et al. (2006). After acid digestion (Method US EPA 3005A for water; Method US EPA 3050B for sediment and tissue samples), a flame atomic absorption spectrometer was used to detect metals (FAAS, Varian, AA 240FS).
Polycyclic aromatic hydrocarbons detection in bile
The concentration of polycyclic aromatic hydrocarbons (PAHs) in bile was determined following a protocol from Hanson, et al. (2009), with minor modifications. Bile of three individuals was pooled and stored in amber glass vials at −75 °C. Bile samples were diluted (1:1000) in 48 % methanol and added onto 96-well black microplate for PAHs through different excitation/emission wavelengths (288/330, 334/376, 364/406, and 380/422) in spectrophotometer. For quantification, a PAH mix (Cod. 47930-U, SUPELCO) was utilized to establish a PAHs standard curve.
Biological sampling
After anesthesia, some biometric parameters such as total length (TL) and total weight (TW) were obtained for a condition factor of Fulton (K) determination. Blood samples were obtained through caudal vein (for C. iguassuensis and G. brasiliensis) or cardiac puncture (for A. bifasciatus) with heparinized syringes and stored in fetal bovine serum for Comet assay and smeared in a slide for micronucleus counting. After euthanasia, the spinal cord was severed and liver and gill samples were dissected for light and scanning electron microscopy respectively. For biochemical analyses, the liver, muscle, and brain samples were stored at −75 °C. The weight of liver was obtained to determine the hepatosomatic index (HSI) and using the formula HSI = liver mass /(100 × total fish mass).
Biochemical biomarkers
Liver, brain, and muscle samples were thawed and homogenized at 1:10 (w/v) ratio with the following buffers: A Tris–HCl/EDTA buffer (20 mM Tris–HCl, 1 mM ethylenediamine tetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, pH 7.4) was used for the liver samples and a 0.1-M potassium phosphate buffer was used for muscle (pH 7.5) and brain (pH 8.0) samples. Homogenized samples were then centrifuged at 12000g, 4 °C for 30 min; the supernatants were collected and stored at −75 °C. Total protein concentration was determined (Bradford et al., 1976 with minor modifications of volumes for microplates) and utilized to normalize protein concentrations in supernatants. For A. bifasciatus, liver samples of two fish were pooled due to the small size and only catalase, glutathione S-transferase, and lipid peroxidation assays were performed.
Acetylcholinesterase (AChE) activity assay (Ellman et al. 1961) was performed with the muscle and brain supernatants. Catalase (Aebi 1984) and glutathione S-transferase (GST, Keen et al. 1976) activity, non-protein thiols (NPT) / GSH concentration (Sedlak and Lindsay 1968), lipid peroxidation (LPO, Jiang et al. 1991, 1992) and protein carbonylation (PCO, Levine et al. 1994) assays were performed with liver supernatants. Glutathione peroxidase (GPx) activity was analyzed only for G. brasiliensis liver supernatants. Previously published protocols (Costa et al. 2010a, 2010b; de Andrade Brito et al. 2012; Osório et al., 2014) were utilized for analyzing all biochemical biomarkers.
Genotoxicity biomarkers
Comet assay (Speit and Hartmann, 1999) and piscine micronucleus test (Heddle, 1973) were performed with erythrocytes, according to previously published protocols (Cestari et al., 2004; Ferraro et al., 2004; Ramsdorf et al. 2009).
Histopathological biomarkers
Light microscopy
Samples of liver were fixed (70 % ethanol, 4 % formaldehyde, 5 % glacial acetic acid) for 16 h, dehydrated in a graded series of ethanol and xylene, and embedded in Paraplast-Plus (Sigma® St Louis, USA). Sections of 5 μm were obtained using a microtome (Leica), stained with Hematoxylin/Eosin, and mounted with Entellan (Merck®). Analysis was performed according to Bernet et al. (1999) with a few modifications.
Scanning electron microscopy
The second left gill arch was washed with phosphate buffer, fixed (2.5 % glutaraldehyde and 2 % paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.4) for at least 24 h, dehydrated in ethanol series (Merck®) and liquid CO2, and metalized with gold for further analysis in scanning electron microscope JEOL KAL-6360LV.
Statistical analysis
All data were checked for outliers (Grubb’s test), normality (Shapiro’s test), and homoscedasticity (Bartlett’s test) before analysis. Either two-way ANOVA test followed by the Tukey-Kramer post-test (parametric data) or Kruskal-Wallis test followed by the Dunn’s test (nonparametric data) were utilized, with p < 0.05. Correlation analysis (Spearman test) was performed between the variables and age parameters (weight and total length), in order to evaluate the influence of age on biological responses.
Biological data was integrated by two different methods. First, PERMANOVA was applied on biological responses to assess the differences between the two studied seasons (summer and winter). The second integration method was aimed to highlight associations among the variables measured in this study (both biomarker responses and contaminant levels in water, sediment, and body burden) during each of the two seasons (summer and winter) and for the three studied fish species (A. bifasciatus, G. brasiliensis, and C. iguassuensis). Factor analysis with principal component analysis as the extraction method (FA/PCA) was utilized. Associations between the different biomarkers (Comet assay, micronucleus assay, GST, CAT, GSH, GPx, PCO, AChE, LPO, Histopathological index, K, and HSI), metal loads in water (Ni, Cd, Zn, Cr, and Mn), sediment (Ni, Cd, Zn, Cr, Mn, Ag, Pb, and Cu), muscle body burden (Cd, Zn, Cr, Mn, Pb, Cu, and Co), and PAH metabolites in bile were assessed. Three matrices with original data (one for each fish species) were integrated. Since the matrices presented some missing data, the FA/PCA integration for each of the data matrix was performed in two steps: First, the variables showing missing data were omitted, and the FA/PCA was done with all cases; second, the cases showing missing data were omitted, and the analysis was done with all variables.
The variables were auto-scaled (standardized) and treated with equal importance. The selected variables to be interpreted were those associated with the factors with a loading cutoff of at least 0.50, a value which is more conservative than the loading cutoff recommended by Tabachnic and Fidell (1996). The relevance of the observed associations to each of the six sampling stations (cases) was estimated by calculating the factor score from each case for the centroid of all cases for the original data. All the statistical and multivariate analyses were performed using STATISTICA 12 software (StatSoft Inc. USA).
Results
Condition factor (K) and hepatosomatic index (HSI)
Differences of condition factors between groups were observed only for individuals from the species A. bifasciatus. The individuals from SS reservoir presented lower condition factor (1.18) than those of FA (1.31, winter), SE (1.34, summer), and SC (1.33, summer and winter) reservoirs. In general, fish from the five reservoirs had different ages, as estimated by their weight and total length (Table 1). For the three fish species, the weights of the collected fish from SE and SC were higher than those from FA, SS, and SO for the summer samples. Correlation analysis showed a weak negative correlation (r = 0.3–0.6) for parameters of age (weight and total length) only with the response of AChE in the muscle of G. brasiliensis and A. bifasciatus.
The HSIs of G. brasiliensis and A. bifasciatus from the FA reservoir were significantly higher in the fish captured in the winter (3.33 and 0.97, respectively) than in the summer (1.36 and 0.60). G. brasiliensis from the FA reservoir had higher HSI than those from the other reservoirs (SE: 0.58; SS: 1.97).
Physicochemical parameters
Water temperature, pH, dissolved oxygen (DO), and intracellular Microcystin-LR concentrations are summarized in Table 2. The highest registered temperature was 27.5 °C (average of 24.9 °C) in SC reservoir (summer), and the lowest was 7.13 °C (average of 16.3 °C) in FA reservoir (winter). SE reservoir had the lowest pH value (5.90—summer and 4.93—winter). No variation in DO was observed during the seasons for FA and SE reservoirs (around 7 to 8 mg L−1), but remarkable variations were recorded at SS, SO, and SC, with the lowest concentration of DO for the SS reservoir (4.59 mg L−1 summer). Intracellular Microcystin-LR concentrations were higher in FA and SS reservoirs during the summer. In the column of water, Microcystin-LR was only detected in FA (summer) at low concentrations (1.08 ng L−1, data not shown).
Chemical analysis of metals
Water
Chromium was above the levels permitted by Brazilian legislation (CONAMA 2012) in the water from FA, SS and SO (summer), and SS (winter) reservoirs. Cd, Zn and Mn were detected in the water of all reservoirs at both seasons, but at concentrations below the limit established by legislation. The highest concentrations of Ni and Cd were detected in SC reservoir, whereas that of Mn was highest in FA at both studied seasons (Table 3). Other metals such as Ag, Cu, Co and Pb were below the limit of quantification in the water of all reservoirs.
Sediments
Concentrations of Pb and Cu were higher in SO than in the other reservoirs (Table 3).
Fish
From all studied species and reservoirs had high concentrations of Zn in the muscle. Elevated levels of Cr, Cu, Mn, Cd and Pb were also observed in the samples (Table 4). A. bifasciatus and C. iguassuensis from SO reservoir (summer) had the highest Pb concentration. Relatively high concentrations of Cr were also detected in fish from almost all reservoirs, whereas Cd was mainly bioaccumulated in the muscle of fish from SC reservoir (summer).
Chemical speciation of metals was not distinguished by the method of detection performed.
PAHs in bile
The 2-ring PAHs were the ones predominantly detected in the bile of the three species from the five reservoirs. The fish from FA reservoir had the highest amounts of the 2-ring PAHs (Table 5). A. bifasciatus from SS and G. brasiliensis from SO reservoir showed the highest concentrations of larger PAHs.
Biochemical biomarkers
A. bifasciatus showed the more conclusive results when analyzing the livers of individuals from both seasons, but no clear effect of contamination gradient was verified. GST activity was higher in samples from the SE reservoir when compared to SS, SO (winter), and SC (summer) (Fig. 2). Elevated lipid peroxidation (LPO) was observed in A. bifasciatus captured during the winter from the FA and SO reservoirs compared to SS and SC reservoirs. The liver of G. brasiliensis had higher activity of CAT in individuals from the SO reservoir when compared to FA and SS (summer), while for winter it was higher in the individuals from SE (Fig. 2). The GPx activity was enhanced in G. brasiliensis individuals from the SS and SO reservoir compared to individuals from the FA site (summer; data not shown). Corroborating with this result, concentrations of non-protein thiols in individuals from FA reservoir were higher than SS individuals. Protein carbonyls were higher in SE than in FA (summer) and SS (winter). No statistical differences were observed between the groups for GST activity and lipid peroxidation in G. brasiliensis (data not shown). The CAT activity in C. iguassuensis was also higher in individuals from SO than SE and SC (summer), but no significant differences were observed for GST activity, PCO (Fig. 2) and LPO analysis (data not shown).
In general, the AChE activity was lower in individuals from SC reservoir than from the other reservoirs, particularly in the muscle of G. brasiliensis and C. iguassuensis (Fig. 3). For A. bifasciatus, brain AChE activity was the highest in the fish from SO (summer) and SC (winter). AChE enzymatic activity was typically higher in summer than winter (Fig. 3).
Genotoxicity biomarkers
A. bifasciatus and C. iguassuensis from SC reservoir (summer) had the highest scores of DNA damage in red blood cells (Fig. 4). Nuclear morphological alterations (NA), such as notched and micronuclei in erythrocytes, were more frequent in G. brasiliensis fish from SC reservoir (summer). For A. bifasciatus, NA was more frequent in the fish from SE (summer) reservoir.
Histopathological analysis
According to Bernet’s Lesion Index for the liver samples, no differences were observed among individuals captured in the studied sites. However, C. iguassuensis fish from SC reservoir had higher index than those from SO reservoir (summer; data not shown). In general, hepatic lesions were more frequent in G. brasiliensis than in A. bifasciatus and C. iguassuensis. However, the alterations were more frequent in summer than winter (Table 6). Despite of this, some important histopathological findings were observed in individuals from all reservoirs, such as necrosis, inflammatory responses (leukocytes infiltration, perivascular and perinuclear granulomatosis) and presence of parasites (Fig. 5). Other alterations, such as intracellular deposits and vacuolization that were also observed, indicated physiological and circulatory disturbances in the liver.
Liver sections of Astyanax bifasciatus (light microscopy). a Liver without alterations. b Necrosis (arrows head). c Tissue differentiation (arrows head). d Perivascular granulomatosis (arrows head). e Granulomatosis peritubular (arrows head) (scale bar = 50 μm). f Inflammatory response (arrows head) and pre-necrotic area (arrow). g Hemorrhagic area (arrows head). h and i Invasive and encapsulated neoplasic areas, respectively (arrows head). Stain: Hematoxylin and Eosin. Bars: 100 μm (c, i), 50 μm (other images)
Hyperplasia, lamellar fusion, structural alterations of the lamellae and epithelium, and parasites were frequent in the gills of fish from the studied species (Table 6, Fig. 6). The fish captured in FA reservoir had a high incidence of fused lamellae, indicating an alteration in gills normal function. In general, the occurrence of lesions was homogeneously distributed between the species and the reservoirs in the summer, while in winter the variation was restricted between the groups of the same species.
Gills of Astyanax bifasciatus (SEM). a FA winter: fusion of secondary lamellae (arrowhead) and the damages on lamellae epithelia (respectively a1 and a2). b FA summer: secondary lamellae fusion and aneurisms (arrowhead). c SE winter: fusion (arrowhead) and disarrange on secondary lamellae. d SE summer: damages on secondary lamellae (arrowhead), epithelial cell death (d1) and secondary lamellae fusion (d2). e SO winter: secondary lamellae fusion (arrowhead) and epithelial cell death. f SO summer: intense epithelial damages (arrow) as detailed in (f). g SS winter: damages in primary (arrowhead) and secondary lamellae epithelia. h SS summer: few damages are observed, except for the occurrence of epithelial effects on secondary lamellae
Multivariate analysis
The PERMANOVA test revealed significant differences between sites and seasons for the interaction of all measured variables for the studied species. The principal component analysis (PCA), based on individual biomarker values, was performed in order to plot a unified view of the responses of all studied species (Figure S1, ESM 1).
Integrative analyses
Three different datasets were used to perform FA/PCA, metals levels in the water column and sediment, biomarker responses, and contaminant levels (metals and PAHs in the bile) in the three different fish species for an integrated description of environmental quality in the different sampling sites. Tables showing the factor loadings and the estimated scores of the factors for each case are presented in detail in the ESM 1 (Tables S1 and S2).
The total explained variance ranged from 69.03 to 100 % in all analyses. In the summer, the FA, SE, and SC sites showed relevant scores which were associated with the effects seen in the blood tissue, antioxidant responses (induction or inhibition), biomarkers of damage (including increased AChE levels), and low condition factor. In the results obtained for summer, the sites SS and SO showed no relevant effects in the blood for the three studied species. In these sites, antioxidant responses were not found in A. bifasciatus, although G. brasiliensis and C. iguassuensis showed catalase activity relatively higher in SO.
The results obtained from FA/PCA and the estimation of the relevance of factors for each studied site showed that the FA reservoir, followed by SC, presented the highest metal concentrations in water (summer sampling). On the other hand, the SE and SO site showed the highest number of metals in the sediment associated with factors of higher relevance. Different patterns of metals body burden were verified between fish species, with higher bioaccumulation of metals in A. bifasciatus from FA and SC, and G. brasiliensis collected in the SO reservoir.
Regarding the environmental quality in the winter, the results of the FA/PCA also showed a consistency in the damage responses (disturbed AChE activity and higher levels of LPO) and effects in the blood tissue (relatively higher frequencies of blebbed, notched, and mononuclear erythrocytes) in A. bifasciatus and C. iguassuensis. These damage responses were associated with levels of metals in water and sediment from the SC site. In turn, few biological responses of relevance were verified for both A. bifasciatus and G. brasiliensis from SE reservoir, while SS showed a consistency on antioxidant responses during winter regardless the species, suggesting an intermediate environmental quality.
Discussion
The results of the current study show that all of the reservoirs along the Iguaçu River, especially FA, SE, and SC reservoirs, are jeopardized by human activities. The Metropolitan region of Curitiba is a primary source of pollutants while other diffuse sources are also found along the river. The levels of metals detected in water, sediments, and fish muscle from the five reservoirs were either similar or higher than concentrations reported for other impacted aquatic environments (Authman et al. (2012), Maceda-Veiga et al. (2013), Noël et al. (2013) and Voigt et al. (2014), This shows that majority of toxic metals in impacted environments are bioavailable. Furthermore, relevant biological responses observed in fish through the multi-biomarker approach used in this study demonstrate an impact on the health of these organisms.
No clear gradient of pollution was verified along the Iguaçu River course
According to the results obtained from chemical and biological analyses, two aspects are relevant to consider the degree of pollution in the reservoirs: (i) the proximity to the MRC and (ii) the presence of diffuse sources of pollutants along the Iguaçu River. The integrated analysis was able to conclude that there was no clear gradient of pollution between the five reservoirs. The conditions of FA and SE reservoirs confirmed that MRC and others small cities were important sources of pollutants from domestic and industrial wastes to the Iguaçu River. Additionally, the SC reservoir had the poorest environmental quality found possibly due to the intense agricultural activity in that region. The FA/PCA analysis result reinforces that SS and SO reservoirs had the better environmental condition compared to the other reservoirs. However, when considering seasonal effects, the best environmental condition was observed for SE and SS reservoirs in the winter. On the other hand, the worst condition in winter showing greater environmental impact was different between the fish species: FA reservoir for G. brasiliensis, SO reservoir for A. bifasciatus, and SC reservoir C. iguassuensis. These findings indicate a difference in the sensitivity of the different species analyzed for the pollutants present in each reservoir.
Seasonal effects and others factors influencing biological responses of the three fish species
Overall, the integrated set of biomarkers and PERMANOVA analysis confirmed that the biological responses suggest that individuals were more exposed to more pollutants during summer than in winter. The concentration of chemicals could be higher due to the lower water level in the reservoirs during the summer, especially in the FA reservoir. Furthermore, biological activity may also be correlated with the higher temperature during this season. Based on the physicochemical analysis, the five reservoirs presented distinct abiotic factors, such as temperature, that may interfere with chemical bioavailability (Fujita et al. 2001; Malczyk and Branfireun 2015) and affect biological responses (Elahee and Bhagwant 2007; de la Haye et al. 2012; Wäge et al. 2015).
Organic matter content in water, associated with domestic sewage, may also influence these responses, lead to eutrophication, and consequently to cyanobacteria proliferation (Yan et al. 2016; Thevenon et al. 2011). According to a recent report (Ide et al., 2016), organic matter content (dissolved organic carbon, nitrite, nitrate, orthophosphate, total nitrogen, and phosphate) and the density of phytoplankton decreased significantly along the Iguaçu River. This study, therefore, suggests a pollution gradient of domestic waste from the origin toward the river’s mouth. However, significant variation in these parameters was observed only for the first 100 km range of the Iguaçu River from the MRC; between the five reservoirs, there was no longer evident variation. Therefore, the gradient of pollution was verified only before the reservoirs.
Physicochemical parameters are not enough to predict the chemicals effects on fish health
Although physicochemical analysis is utilized to evaluate water quality by environmental agencies in Brazil, they are not enough to predict the real risk of aquatic organisms that are exposed to many pollutants. According to the previous report published by the State Agency for Environment Control - IAP (IAP 2008), the reservoir with the best water quality in Iguaçu River was SC. However, SC reservoir had the worst water quality in the present study. FA reservoir was considered by the agency (IAP) as the worst in terms of water quality, particularly due to the high deficit of oxygen, chlorophyll a, and cyanobacteria blooms (IAP 2008). Water quality gradually improved in the subsequent reservoirs, as these parameters decreased (IAP 2008). However, the current study presents a different view of the water quality in the reservoirs, providing strong evidences that there are other sources of contamination along the river, which refute the hypothesis of a gradient of pollution between the reservoirs.
The five reservoirs are differently impacted by sources of pollution
The FA reservoir is the closest to MRC, the main source of pollutants. Data from this study confirm that the FA site is a highly impacted, showing high levels of metals (Cr, Zn, and Mn) in the water and fish’s muscle, high concentrations of microcystin-LR in the water, and 2-ring PAHs in the fish’s bile. Biological responses, such as high LPO, HSI, and gill lesions in the three fish species, corroborate this condition. Zn and Mn are both ubiquitous elements, essential for normal physiological function and are indicators of human activity. However, deficiency or excess of these metals can be harmful (Giardina et al. 2009). They are known to inhibit antioxidant responses (Qu et al. 2014) and affect the nervous system, including changes of behavior and altered cognitive function (ATSDR - Public Health Statement: Cadmium. 2999). The concentrations of Cr in water were above the established by Brazilian legislation. Cr can bind to biomolecules, resulting in lipid peroxidation, protein carbonylation, and DNA damage (Mattagajasingh et al. 2008; Nickens et al. 2010). Finally, the highest levels of 2-ring PAHs were measured in the three species of fish from FA reservoir, indicating the influence of industrial activities in the MRC, such as crude oil refinement (Gallotta and Christensen, 2012; Leite et al., 2011).
The SE reservoir is the second furthest from MRC and, according to our data, also presented poor water quality. The reservoir had low pH, contained Cd in the water, Mn in the sediment, and elevated carbonylated proteins in the fish’s liver. Acidic condition increases metal solubility in water and can interfere with normal gill osmoregulation, acid–base balance, and uptake/homeostasis of essential elements like Zn, Fe, and Cu in fish (Levit and Bozeman, 2010; Qu et al. 2014).
Metals are ubiquitous elements, but industrial and agricultural activities can increase metal concentration in the environment. Fertilizers can contain Cd (WHO 2011), as well as Co, Cu, Zn, Pb, and Ni as impurities, and herbicides present high levels of Fe, Mn, Pb, and Ni (Gimeno-García et al. 1996). Culture of soybeans, wheat, and corn increase from SE until SC reservoir, representing a potential source of pesticides for these sites.
SS and SO are, respectively, the third and fourth intermediate reservoirs, located in a region of intense agricultural activity. Considering their distances from MRC, other sources of contaminants may be responsible for the low dissolved oxygen, high levels of Cr in the water, low condition factor of the fish in SS reservoir, as well as toxic concentrations of Pb, Cu, and Zn in the sediment, and Pb in the muscle in SO reservoir. These metals may lead to gills dysfunction, oxidative damage (Wei and Yang, 2015), apoptosis (Luzio et al. 2013; Monteiro et al. 2009), and neurotoxicity (White et al. 2007).
SC reservoir is the furthest from MRC, but it has the high concentrations of Cd and Ni in the water, Cd in the muscle, low AChE activity, and high DNA damage in blood cells in all three fish species analyzed. Non-essential metals like Cd can cause deleterious effects on biota even at low concentrations (Satarug et al., 2009). Although the concentration of Cd was below the established level set by the Brazilian legislation, Cd is a human carcinogen, a potent endocrine disruptor, and exerts toxic effects on kidney, skeletal, respiratory, and reproductive systems (Gerbron et al. 2015; Jiménez-Ortega et al. 2012; Wang et al. 2014; WHO 2011). Mn and Pb can inhibit AchE (Santos et al. 2012), and mixtures of Pb + Cd (detected in SC) can induce neuronal degeneration in the brain even at low concentrations (Cobbina et al. 2015).
In summary, independent of the distance from MRC, the studied reservoirs are impacted, at distinct levels, by chemicals from distinct sources.
The presence of complex mixtures in aquatic ecosystems complicate environmental risk assessment
Assessing chemical interactions (e.g., additive, synergistic, or antagonistic effects) is a great challenge for ecotoxicologists (Norberg et al. 2015). According to Gauthier et al. (2014), metal–PAH mixtures can have additive effects, e.g., alteration of membrane permeability to metals and mutual inhibition of detoxification by CYP biotransformation pathway. Mixture of the carcinogens such as Cd and benzo(a)pyrene (B[a]P) can impair the activity of antioxidant enzymes, though these chemicals can induce them when separate (Costa et al. 2010a, 2010b). This scenario illustrates the complexity in establishing the interaction between natural exposure to pollutants and biological effects. Biogeochemistry can only partially explain the differences of metal concentrations in water, sediment, and muscle of fish, whereas different metabolic roles of essential and non-essential elements, environmental factors (Monroy et al. 2014), and food habit (Kalantzi et al. 2013) are related to the distinct bioaccumulation of metals in the three fish species.
A. bifasciatus reflected the environmental quality better than G. brasiliensis and C. iguassuensis
A. bifasciatus represented a good bioindicator of biomonitoring study to the Iguaçu River, since its biological responses reflected the environmental quality better than the other two fish species in the current study. The same conclusion had been reported by Freire et al. (2015) for this species. A. bifasciatus is widely distributed in the Iguaçu River (Daga and Gubiani 2012; Pie et al. 2009), and studies in different regions of Brazil and South America also describe the gender Astyanax sp. as a good bioindicator for biomonitoring (Alberto et al. 2005; de Lemos et al. 2008; Paulino et al. 2014; Prado et al. 2011, 2014).
Different biomarkers showed that the health of fish is compromised
Besides the importance of using different species in biomonitoring programs, it is also crucial to evaluate several responses in target organs to better comprehend the health status of the fish. Biochemical and genotoxicity analysis demonstrated alterations of enzymes involved in antioxidant defenses and nervous system, as well as damages in lipids, proteins, and DNA. These responses were correlated to the bioavailability of metals and PAHs, which induce several lesions and alterations in target tissues, as described by Liu et al. (2010, 2011).
Despite of the similar indexes of lesion among the groups, important pathological changes were observed in the liver and gills of the three fish species. Histopathological findings in target organs represent a strong evidence of chemical exposure in the field. The liver is described as a key organ for biotransformation of chemicals in vertebrates and a potential target for chemical-induced injury. Some chemicals as well as metals can lead to necrosis of hepatocyte cells (de Oliveira Ribeiro et al. 2002; Sánchez-Chardi et al. 2009), DNA fragmentation, and redox unbalance (Proskuryakov et al. 2003). In the gills, some pathological alterations such as primary and secondary lamellae damage, fusion of adjacent lamellae, neoplasia, and aneurisms are characteristic of waterborne exposure to chemicals (Liu et al. 2010; Srivastava et al. 2009). Incidence of lesions in target organs, evidences of neurotoxicity, and macromolecules damage associated with bioaccumulation of toxic metals and PAHs indicated the impact on fish health related to low water quality of the five reservoirs.
Integration of multiple biomarkers and chemical analysis is an efficient strategy for evaluation of water quality
The integration of biomarkers in fish and metal detection represents an efficient strategy for evaluation of water quality and risk assessment of human exposure (Carvalho et al. 2012; Omar et al. 2014; Sakuragui et al. 2013; Gusso-Choueri et al. 2015). An integrated analysis was performed through FA/PCA in order to improve the environmental diagnosis, since it can show the cases when relevant responses are significantly associated with contaminants present in the reservoirs to show the environmental quality of a site.
Final considerations
The results of this study provide strong evidence that human activities greatly impact the five reservoirs of the Iguaçu River. Domestic and industrial wastes from MRC and other small cities were considered as the main responsible to impact FA reservoir even with a considerable distance from this source of pollutants. Water quality of the second reservoir, SE, is impacted by what compounds from the MRC and small cities and agricultural activities. SS and SO reservoirs are primarily subject to pesticides inputs, with less impact from MRC. Finally, SC reservoir had the poorest water quality, indicating that there is no gradient of pollution between the five reservoirs from the origin of the river toward the mouth.
The effects of the chemicals described here demand a risk assessment for the biota and human populations in this area since the Iguaçu basin has a high environmental and socioeconomical importance. These sets of results represent the first study performed in the reservoirs of Iguaçu River showing the impacts in fish health and can be used for its management or to establish conservation politics.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6727660
Alberto A, Camargo AFM, Verani JR, Costa OFT, Fernandes MN (2005) Health variables and gill morphology in the tropical fish Astyanax fasciatus from a sewage-contaminated river. Ecotoxicol Environ Saf 61(2):247–55, doi:10.1016/j.ecoenv.2004.08.009
Araldi RP, de Melo TC, Mendes TB, de Sá Júnior PL, Nozima BHN, Ito ET, de Cassia Stocco R (2015) Using the comet and micronucleus assays for genotoxicity studies: a review. Biomed Pharmacother 72:74–82, doi:10.1016/j.biopha.2015.04.004
ATSDR - Public Health Statement: Cadmium. (n.d.). Retrieved from http://www.atsdr.cdc.gov/phs/phs.asp?id=46&tid=15. Accessed Sept 2012
Authman MMN, Abbas WT, Gaafar AY (2012) Metals concentrations in Nile tilapia Oreochromis niloticus (Linnaeus, 1758) from illegal fish farm in Al-Minufiya Province, Egypt, and their effects on some tissues structures. Ecotoxicol Environ Saf 84:163–172, doi:10.1016/j.ecoenv.2012.07.005
Baumgartner, G., Pavanelli, S. C., Baumgartner, D., Bifi, A. G., Debona, T., & Frana, V. A. (2012). Peixes do baixo rio Iguaçu, 1st edn. Eduem, Maringá (City), p 203
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22(1):25–34, doi:10.1046/j.1365-2761.1999.00134.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248--54. doi:10.1016/0003-2697(76)90527-3
Carneiro, C., Andreoli, C. V., Cunha, C. de L. da N., & Gobbi, E. F. (2014). Reservoir Eutrophication: preventive management: an applied example of Integrated Basin Management Interdisciplinary Research. IWA Publishing. Retrieved from https://www.books.google.com/books?id=afIrBQAAQBAJ&pgis=1
Carvalho CDS, Bernusso VA, De Araújo HSS, Espíndola ELG, Fernandes MN (2012) Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 89(1):60–69, doi:10.1016/j.chemosphere.2012.04.013
Cestari, M. M., Lemos, P. M. M., Ribeiro, C. A. de O., Costa, J. R. M. A., Pelletier, E., Ferraro, M. V. M., … Fenocchio, A. S. (2004). Genetic damage induced by trophic doses of lead in the neotropical fish Hoplias malabaricus (Characiformes, Erythrinidae) as revealed by the comet assay and chromosomal aberrations. Genetics and Molecular Biology, 27(2), 270–274. doi:10.1590/S1415-47572004000200023
Cobbina SJ, Chen Y, Zhou Z, Wu X, Feng W, Wang W, Yang L (2015) Low concentration toxic metal mixture interactions: effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere 132:79–86, doi:10.1016/j.chemosphere.2015.03.013
Collins A, Koppen G, Valdiglesias V, Dusinska M, Kruszewski M, Møller P, Bonassi S (2014) The comet assay as a tool for human biomonitoring studies: the ComNet project. Mutat Res Rev Mutat Res 759:27–39, doi:10.1016/j.mrrev.2013.10.001
Conselho Nacional do Meio Ambiente (CONAMA) (2012) Resoluções do Conama: Resoluções vigentes publicadas entre setembro de 1984 e janeiro de 2012. / Ministério do Meio Ambiente. Brasília:MMA, 2012. p 1126
Contreras López MC (2003) Determination of potentially bioaccumulating complex mixtures of organochlorine compounds in wastewater: a review. Environ Int 28(8):751–9, doi:10.1016/S0160-4120(02)00120-4
Costa DDM, Neto FF, Costa MDM, Morais RN, Garcia JRE, Esquivel BM, Ribeiro C a O (2010a) Vitellogenesis and other physiological responses induced by 17-β-estradiol in males of freshwater fish Rhamdia quelen. Comp Biochem Physiol Part C: Toxicol Pharmacol 151(2):248–257, doi:10.1016/j.cbpc.2009.11.002
Costa PM, Chicano-Gálvez E, López Barea J, DelValls TA, Costa MH (2010b) Alterations to proteome and tissue recovery responses in fish liver caused by a short-term combination treatment with cadmium and benzo[a]pyrene. Environ Pollut (Barking, Essex : 1987) 158(10):3338–46, doi:10.1016/j.envpol.2010.07.030
Cotta JAO, Rezende MOO, Piovani MR (2006) Evaluation of metal content in sediments of the Betari River in the Parque Estadual Turístico do Alto Ribeira -PETAR-, São Paulo, Brazil. Quimica Nova, doi:10.1590/S0100-40422006000100009
Daga VS, Gubiani É a (2012) Variations in the endemic fish assemblage of a global freshwater ecoregion: associations with introduced species in cascading reservoirs. Acta Oecologica 41:95–105, doi:10.1016/j.actao.2012.04.005
de Andrade Brito I, Arruda Freire C, Yamamoto FY, Silva de Assis HC, Rodrigues Souza-Bastos L, Cestari MM, de Oliveira Ribeiro CA (2012) Monitoring water quality in reservoirs for human supply through multi-biomarker evaluation in tropical fish. J Environ Monit 14(2):615, doi:10.1039/c2em10461j
de la Haye KL, Spicer JI, Widdicombe S, Briffa M (2012) Reduced pH sea water disrupts chemo-responsive behaviour in an intertidal crustacean. J Exp Mar Biol Ecol 412:134–140, doi:10.1016/j.jembe.2011.11.013
de Lemos CT, Iranço FDA, de Oliveira NCDÁ, de Souza GD, Fachel JMG (2008) Biomonitoring of genotoxicity using micronuclei assay in native population of Astyanax jacuhiensis (Characiformes: Characidae) at sites under petrochemical influence. Sci Total Environ 406(1–2):337–343, doi:10.1016/j.scitotenv.2008.07.006
de Oliveira Ribeiro, C. A., Belger, L., Pelletier, E., & Rouleau, C. (2002). Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpinus). Environmental Research, 90(3), 217–25. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12477467
Dos Santos DR, Yamamoto FY, Filipak Neto F, Randi MAF, Garcia JE, Costa DDM, de Oliveira Ribeiro CA (2016) The applied indicators of water quality may underestimate the risk of chemical exposure to human population in reservoirs utilized for human supply-Southern Brazil., Environmental Science and Pollution Research International, doi:10.1007/s11356-015-5995-0
Elahee KB, Bhagwant S (2007) Hematological and gill histopathological parameters of three tropical fish species from a polluted lagoon on the west coast of Mauritius. Ecotoxicol Environ Saf 68(3):361–71, doi:10.1016/j.ecoenv.2006.06.003
ELLMAN, G. L., COURTNEY, K. D., ANDRES, V., & FEATHER-STONE, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88–95. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13726518
Ferraro, M. V. M., Fenocchio, A. S., Mantovani, M. S., Ribeiro, C. de O., & Cestari, M. M. (2004). Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genetics and Molecular Biology, 27(1), 103–107. doi:10.1590/S1415-47572004000100017
Freire CA, Souza-bastos LR, Chiesse J, Tincani FH, Piancini LDS, Randi MAF, De Oliveira-ribeiro CA (2015) RESEARCH ARTICLE A multibiomarker evaluation of urban, industrial, and agricultural exposure of small characins in a large freshwater basin in southern Brazil, 13263–13277., doi:10.1007/s11356-015-4585-5
Fujita, T., Nishimura, K., Takayama, C., Yoshida, M., & Uchida, M. (2001). Handbook of Pesticide Toxicology. Handbook of Pesticide Toxicology. Elsevier. doi:10.1016/B978-012426260-7.50032-X
Gallotta FDC, Christensen JH (2012) Source identification of petroleum hydrocarbons in soil and sediments from Igua??u River Watershed, Paran?? Brazil using the CHEMSIC method (CHEMometric analysis of Selected Ion Chromatograms). J Chromatogr A 1235:149–158, doi:10.1016/j.chroma.2012.02.041
Gauthier PT, Norwood WP, Prepas EE, Pyle GG (2014) Metal-PAH mixtures in the aquatic environment: a review of co-toxic mechanisms leading to more-than-additive outcomes. Aquat Toxicol 154:253–269, doi:10.1016/j.aquatox.2014.05.026
Gerbron M, Geraudie P, Xuereb B, Marie S, Minier C (2015) In vitro and in vivo studies of the endocrine disrupting potency of cadmium in roach (Rutilus rutilus) liver., Marine Pollution Bulletin, 10.1016/j.marpolbul.2015.03.043
Giardina A, Larson SE, Wisner B, Wheeler J, Chao M (2009) Long-term and acute effects of zinc contamination of a stream on fish mortality and physiology. Environ Toxicol Chem 28(2):287–95, doi:10.1897/07-461.1
Gimeno-García E, Andreu V, Boluda R (1996) Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ Pollut 92(1):19–25, doi:10.1016/0269-7491(95)00090-9
Gusso-Choueri, P. K., Choueri, R. B., de Araújo, G. S., Cruz, A. C. F., Stremel, T., Campos, S., … Ribeiro, C. A. O. (2015). Assessing pollution in marine protected areas: the role of a multi-biomarker and multi-organ approach. Environmental Science and Pollution Research International. doi:10.1007/s11356-015-4911-y
Hanson N, Persson S, Larsson A (2009) Analyses of perch (Perca fluviatilis) bile suggest increasing exposure to PAHs and other pollutants in a reference area on the Swedish Baltic coast. J Environ Monit : JEM 11(2):389–93, doi:10.1039/b817703a
Heddle, J. A. (1973). A rapid in vivo test for chromosomal damage. Mutation Research, 18(2), 187–90. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4351282
IAP, I. A. do P. (2008). Qualidade das aguas dos Reservatórios do Estado do Paraná 2005 a 2008.
IBGE, I. B. de G. e E. (2010). IBGE | Censo 2010. Retrieved September 29, 2015, from http://www.censo2010.ibge.gov.br/
Ide, A., Osawa, R., Marcante, L., Pereira, J., & Azevedo, J. C. (2016). Occurrence of pharmaceutical products, female sex hormones and caffeine in a subtropical region in Brazil. Clean Soil Air Water, 1st edn. Eduem, Maringá (City), p 203
Jiang, Z. Y., Hunt, J. V, & Wolff, S. P. (1992). Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Analytical Biochemistry, 202(2), 384–9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1519766
Jiang, Z. Y., Woollard, A. C., & Wolff, S. P. (1991). Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids, 26(10), 853–6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1795606
Jiménez-Ortega V, Cano Barquilla P, Fernández-Mateos P, Cardinali DP, Esquifino AI (2012) Cadmium as an endocrine disruptor: correlation with anterior pituitary redox and circadian clock mechanisms and prevention by melatonin. Free Radic Biol Med 53(12):2287–97, doi:10.1016/j.freeradbiomed.2012.10.533
Kalantzi I, Black KD, Pergantis SA, Shimmield TM, Papageorgiou N, Sevastou K, Karakassis I (2013) Metals and other elements in tissues of wild fish from fish farms and comparison with farmed species in sites with oxic and anoxic sediments. Food Chem 141(2):680–94, doi:10.1016/j.foodchem.2013.04.049
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S transferases. J Biol Chem 251(20):6183–6188
Leite, N. F., Peralta-Zamora, P., & Grassi, M. T. (2011). Distribution and origin of polycyclic aromatic hydrocarbons in surface sediments from an urban river basin at the Metropolitan region of Curitiba, Brazil. Journal of Environmental Sciences (China), 23(6), 904–11. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22066212
Levine, R. L., Williams, J. A., Stadtman, E. R., & Shacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology, 233, 346–57. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8015469
Levit, S. M., & Bozeman, M. (2010). A Literature Review of Effects of Cadmium on Fish, (November), 16. Retrieved from http://www.nature.org/wherewework/northamerica/states/alaska/files/cadmium_literature_review_dec_2010.pdf
Linde-Arias AR, Inácio AF, Novo L a, de Alburquerque C, Moreira JC (2008) Multibiomarker approach in fish to assess the impact of pollution in a large Brazilian river, Paraiba do Sul. Environ Pollut 156(3):974–979, doi:10.1016/j.envpol.2008.05.006
Liu XJ, Luo Z, Li CH, Xiong BX, Zhao YH, Li XD (2011) Antioxidant responses, hepatic intermediary metabolism, histology and ultrastructure in Synechogobius hasta exposed to waterborne cadmium. Ecotoxicol Environ Saf 74(5):1156–1163, doi:10.1016/j.ecoenv.2011.02.015
Liu XJ, Luo Z, Xiong BX, Liu X, Zhao YH, Hu GF, Lv GJ (2010) Effect of waterborne copper exposure on growth, hepatic enzymatic activities and histology in Synechogobius hasta. Ecotoxicol Environ Saf 73(6):1286–1291, doi:10.1016/j.ecoenv.2010.06.019
Luzio, A., Monteiro, S. M., Fontaínhas-Fernandes, A. A., Pinto-Carnide, O., Matos, M., & Coimbra, A. M. (2013). Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquatic Toxicology (Amsterdam, Netherlands), 128–129, 183–9. doi:10.1016/j.aquatox.2012.12.018
Maceda-Veiga A, Monroy M, Navarro E, Viscor G, de Sostoa A (2013) Metal concentrations and pathological responses of wild native fish exposed to sewage discharge in a Mediterranean river. Sci Total Environ 449:9–19, doi:10.1016/j.scitotenv.2013.01.012
Malczyk, E. A., & Branfireun, B. A. (2015). Mercury in sediment, water, and fish in a managed tropical wetland-lake ecosystem. The Science of the Total Environment, 524-525C, 260–268. doi:10.1016/j.scitotenv.2015.04.015
Mattagajasingh SN, Misra BR, Misra HP (2008) Carcinogenic chromium(VI)-induced protein oxidation and lipid peroxidation: Implications in DNA-protein crosslinking., Journal of Applied Toxicology, doi:10.1002/jat.1364
MATTHIENSEN A, YUNES JS, CODD GA (1999) Ocorrência, distribuição e toxicidade de cianobactérias no estuário da Lagoa dos Patos, RS. Rev Bras Biol 59(3):361–376, doi:10.1590/S0034-71081999000300002
Mela M, Randi MAF, Ventura DF, Carvalho CEV, Pelletier E, Oliveira Ribeiro CA (2007) Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68(3):426–35, doi:10.1016/j.ecoenv.2006.11.013
Mills LJ, Chichester C (2005) Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci Total Environ 343(1–3):1–34, doi:10.1016/j.scitotenv.2004.12.070
Monroy M, Maceda-Veiga A, de Sostoa A (2014) Metal concentration in water, sediment and four fish species from Lake Titicaca reveals a large-scale environmental concern. Sci Total Environ 487:233–44, doi:10.1016/j.scitotenv.2014.03.134
Monteiro SM, dos Santos NMS, Calejo M, Fontainhas-Fernandes A, Sousa M (2009) Copper toxicity in gills of the teleost fish, Oreochromis niloticus: effects in apoptosis induction and cell proliferation. Aquat Toxicol (Amsterdam, Netherlands) 94(3):219–28, doi:10.1016/j.aquatox.2009.07.008
Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: A double-edged sword. Chem Biol Interact 188(2):276–88, doi:10.1016/j.cbi.2010.04.018
Noël L, Chekri R, Millour S, Merlo M, Leblanc J-C, Guérin T (2013) Distribution and relationships of As, Cd, Pb and Hg in freshwater fish from five French fishing areas. Chemosphere 90(6):1900–10, doi:10.1016/j.chemosphere.2012.10.015
Norberg, G. F., Gerhadsson, L., Mumtaz, M., Ruiz, P., & Fowler, B. A. (2015). Handbook on the toxicology of metals. Chemosphere (Fourth Edi, Vol. 9). Elsevier. doi:10.1016/0045-6535(80)90137-X
Oliveira Ribeiro CA, Filipak Neto F, Mela M, Silva PH, Randi MAF, Rabitto IS, Pelletier E (2006) Hematological findings in neotropical fish Hoplias malabaricus exposed to subchronic and dietary doses of methylmercury, inorganic lead, and tributyltin chloride. Environ Res 101(1):74–80, doi:10.1016/j.envres.2005.11.005
Omar W a, Saleh YS, Marie M-AS (2014) Integrating multiple fish biomarkers and risk assessment as indicators of metal pollution along the Red Sea coast of Hodeida, Yemen Republic. Ecotoxicol Environ Saf 110:221–231, doi:10.1016/j.ecoenv.2014.09.004
Osório FHT, Silva LFO, Piancini LDS, Azevedo ACB, Liebel S, Yamamoto FY, de Oliveira Ribeiro CA (2014) Water quality assessment of the Tubarão River through chemical analysis and biomarkers in the Neotropical fish Geophagus brasiliensis. Environ Sci Pollut Res 21(15):9145–9160, doi:10.1007/s11356-013-1512-5
Paulino, M. G., Benze, T. P., Sadauskas-Henrique, H., Sakuragui, M. M., Fernandes, J. B., & Fernandes, M. N. (2014). The impact of organochlorines and metals on wild fish living in a tropical hydroelectric reservoir: bioaccumulation and histopathological biomarkers. The Science of the Total Environment, 497-498C, 293–306. doi:10.1016/j.scitotenv.2014.07.122
Pie MR, Baggio RA, Boeger WA, Patella LA, Ostrensky A, Vitule JRS, Abilhoa V (2009) Molecular data reveal a diverse Astyanax species complex in the upper Iguaçu River. J Fish Biol 75(9):2357–62, doi:10.1111/j.1095-8649.2009.02438.x
Prado PS, Pinheiro APB, Bazzoli N, Rizzo E (2014) Reproductive biomarkers responses induced by xenoestrogens in the characid fish Astyanax fasciatus inhabiting a South American reservoir: an integrated field and laboratory approach. Environ Res 131:165–73, doi:10.1016/j.envres.2014.03.002
Prado PS, Souza CC, Bazzoli N, Rizzo E (2011) Reproductive disruption in lambari Astyanax fasciatus from a Southeastern Brazilian reservoir. Ecotoxicol Environ Saf 74(7):1879–1887, doi:10.1016/j.ecoenv.2011.07.017
Proskuryakov SY, Konoplyannikov AG, Gabai VL (2003) Necrosis: a specific form of programmed cell death? Exp Cell Res 283(1):1–16, doi:10.1016/S0014-4827(02)00027-7
Qu, R., Feng, M., Wang, X., Qin, L., Wang, C., Wang, Z., & Wang, L. (2014). Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquatic Toxicology (Amsterdam, Netherlands), 150, 9–16. doi:10.1016/j.aquatox.2014.02.008
Rabitto IS, Alves Costa JRM, Silva de Assis HC, Pelletier EE, Akaishi FM, Anjos A, Oliveira Ribeiro CA (2005) Effects of dietary Pb(II) and tributyltin on neotropical fish, Hoplias malabaricus: histopathological and biochemical findings. Ecotoxicol Environ Saf 60(2):147–56, doi:10.1016/j.ecoenv.2004.03.002
Ramsdorf, W. A., Guimarães, F. de S. F., Ferraro, M. V. M., Gabardo, J., Trindade, E. da S., & Cestari, M. M. (2009). Establishment of experimental conditions for preserving samples of fish blood for analysis with both comet assay and flow cytometry. Mutation Research, 673(1), 78–81. doi:10.1016/j.mrgentox.2008.11.010
Sakuragui MM, Paulino MG, Pereira CDS, Carvalho CS, Sadauskas-Henrique H, Fernandes MN (2013) Integrated use of antioxidant enzymes and oxidative damage in two fish species to assess pollution in man-made hydroelectric reservoirs. Environ Pollut 178:41–51, doi:10.1016/j.envpol.2013.02.032
Sánchez-Chardi A, Ribeiro CAO, Nadal J (2009) Metals in liver and kidneys and the effects of chronic exposure to pyrite mine pollution in the shrew Crocidura russula inhabiting the protected wetland of Doñana. Chemosphere 76(3):387–94, doi:10.1016/j.chemosphere.2009.03.036
Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP (2012) The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 292(2–3):90–8, doi:10.1016/j.tox.2011.11.017
Satarug S, Garrett SH, Sens MA, Sens DA (2009) Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 118(2):182–190, doi:10.1289/ehp.0901234
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205, doi:10.1016/0003-2697(68)90092-4
Speit, G., & Hartmann, A. (1999). The comet assay (single-cell gel test). A sensitive genotoxicity test for the detection of DNA damage and repair. Methods in Molecular Biology (Clifton, N.J.), 113, 203–12. doi:10.1385/1-59259-675-4:203
Srivastava R, Srivastava SM, Abidi R, Das MK (2009) Cellular level effects of metal toxicity on gills and liver of fishes. Comp Biochem Physiol A Mol Integr Physiol 154(1):S19–S20, doi:10.1016/j.cbpa.2009.05.071
Tabachnic BG, Fidell LS (1996) Using multivariate statistics. Harper Collins College Publishers, New York
Thevenon F, Graham ND, Herbez A, Wildi W, Poté J (2011) Spatio-temporal distribution of organic and inorganic pollutants from Lake Geneva (Switzerland) reveals strong interacting effects of sewage treatment plant and eutrophication on microbial abundance. Chemosphere 84(5):609–17, doi:10.1016/j.chemosphere.2011.03.051
Tian D, Zheng W, He G, Zheng Y, Andersen ME, Tan H, Qu W (2015) Predicting cytotoxicity of complex mixtures in high cancer incidence regions of the Huai River Basin based on GC-MS spectrum with partial least squares regression. Environ Res 137:391–7, doi:10.1016/j.envres.2014.12.027
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149, doi:10.1016/S1382-6689(02)00126-6
Voigt, C. L., da Silva, C. P., Doria, H. B., Randi, M. A. F., de Oliveira Ribeiro, C. A., & de Campos, S. X. (2014). Bioconcentration and bioaccumulation of metal in freshwater Neotropical fish Geophagus brasiliensis. Environmental Science and Pollution Research International. doi:10.1007/s11356-014-3967-4
Wäge, J., Hardege, J. D., Larsson, T. A., Simakov, O., Chapman, E. C., Arendt, D., & Rotchell, J. M. (2015). Effects of low seawater pH on the marine polychaete Platynereis dumerilii. Marine Pollution Bulletin. doi:10.1016/j.marpolbul.2015.04.027
Wang HJ, Liu ZP, Jia XD, Chen H, Tan YJ (2014) Endocrine disruption of cadmium in rats using the OECD enhanced TG 407 test system. BiomedEnviron Sci : BES 27(12):950–9, doi:10.3967/bes2014.135
Wei K, Yang J (2015) Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish Procambarus clarkii. Ecotoxicol Environ Saf 113:446–53, doi:10.1016/j.ecoenv.2014.12.032
White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Basha MR (2007) New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol 225(1):1–27, doi:10.1016/j.taap.2007.08.001
Who. (2011). Cadmium in Drinking-water, 16. Retrieved from http://www.who.int/water_sanitation_health/dwq/chemicals/cadmium.pdf
Yan HY, Zhang XR, Dong JH, Shang MS, Shan K, Wu D, Wang GY (2016) Spatial and temporal relation rule acquisition of eutrophication in Da’ning River based on rough set theory. Ecol Indic 66:180–189, doi:10.1016/j.ecolind.2016.01.032
Acknowledgments
The authors thank COPEL and TRACTEBEL energy companies, professor Gilmar Baumgartner (Unioeste, Toledo City, Parana State) and MSc Ana Carolina B. Azevedo for assistance in catching and sampling of fish, Graciel Diamante and Hovik Gasparyan for English review, and Fundação Araucaria for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Kenneth Mei Yee Leung
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 801 kb)
Rights and permissions
About this article
Cite this article
Yamamoto, F., Pereira, M., Lottermann, E. et al. Bioavailability of pollutants sets risk of exposure to biota and human population in reservoirs from Iguaçu River (Southern Brazil). Environ Sci Pollut Res 23, 18111–18128 (2016). https://doi.org/10.1007/s11356-016-6924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6924-6