Abstract
Benthic algae or microphytobenthos (MPB) in intertidal flats play an important role in the sediment and overlying water ecosystems. We hypothesize that there are effects of sediment texture on the vertical distribution of MPB using chlorophyll a (chl a) as a proxy for MPB biomass and present results over a 2.5-year period. Four sites were sampled monthly: two sandy sites (A10 and A12) and two muddy sites (A0 and A14) on the intertidal flats of the Fraser River Estuary. At the two sandy sites, pigments were distributed down to 10 cm. High ratios of depth-integrated chl a to phaeopigments suggest that the chl a had been recently buried. In contrast, at the muddy sites, pigments were limited to the top 4 cm, with MBP in the top 1 cm contributing up to 60 % of the whole sediment core pigments. As a result, the depth-integrated chl a values were on average 2,044 mg m–2 (160–4,200) at A10 and 882 mg m−2 (183–2,569) at A12, the two sandy sites, and much higher than at the two muddy sites where averages of 84 mg m−2 (41–174) and 235 mg m−2 (77–854) were measured at A0 and A14, respectively. Despite these lower concentrations at the muddy sites than at the sandy sites, particulate organic carbon (POC) and nitrogen (PON) concentrations showed a homogenous vertical distribution at the two sandy sites. Such a homogeneous vertical distribution of chl a, POC, and PON suggests that vertical transport mechanisms were actively transporting organic material into and out of the sediment. These results suggest that MBP on sandy sediments play a very active role in providing food for herbivores and are interacting with the overlying water column in the sediment-water exchange processes during tidal cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tidal flats provide a rich habitat for wildlife. Intertidal benthic microalgae or microphytobenthos (MPB) on tidal flats are important primary producers (MacIntyre et al. 1996; Pinckney and Zingmark 1993a; Underwood and Kromkamp 1999), an integral component of inorganic nutrient cycles in the estuary (Guarini et al. 2008; Krom 1991; Rizzo 1990), and highly influential on sediment stability (Defew et al. 2002; Le Hir et al. 2007; Underwood and Paterson 1993). The high biological production of intertidal flats is due to the presence of high MPB biomass (e.g., pennate diatoms) (Light and Beardall 1998). The biomass and productivity of MPB in the top few millimeters of the sediment can exceed those of the whole euphotic zone in the water column (MacIntyre et al. 1996; Cartaxana et al. 2006). These few millimeters of MPB represent a thin interface between the water and sediment, which is strongly influenced by tidal cycles (Blanchard et al. 2001). The spatial distribution of MPB, both in the horizontal and vertical, is highly heterogeneous and dictated by both active as well as passive factors (Chennu et al. 2013; Du et al. 2010; Mitbavkar and Anil 2004). Active factors may include grazing by other organisms on MPB (Hagerthey et al. 2002), bioturbation (Defew et al. 2002; Le Hir et al. 2007), and vertical migration, i.e., the movement of cells down to avoid photoinhibition and up to obtain sunlight (Consalvey et al. 2004; Mitbavkar and Anil 2004), while passive factors include salinity, nutrient availability, and sediment type (Du et al. 2009), but importantly also hydrodynamic exposure, which can influence the effects of resuspension, mixing, and burial processes (Du et al. 2010). Resuspension of MPB has been described to be important in contributing to the overlying water column primary productivity (Cartaxana et al. 2006; Shaffer and Sullivan 1988; Wainright 1990). On the other hand, the effects of tidal cycles on the transport of MPB into deeper sediments (Pinckney and Zingmark 1993b) may be important in biogeochemical processes in the sediment (Barranguet et al. 1997; Huettel and Rusch 2000). Estuarine intertidal sediment is a large reservoir for organic matter and inorganic nutrients and hence can act as a source or sink of nutrients into or out of the water column. In particular, MPB can be a strong influence on nutrient cycling and a good source of labile organic material (Hardison et al. 2013).
In a previous study, we found a decrease in chl a in the water column over the sediment of one of our study sites on Sturgeon Bank, which was thought to be likely due to the feeding by benthic organisms (Yin and Harrison 2000). At the same time, ammonium concentrations were almost always higher during ebb tides than flood tides, indicating a release of NH4 + from the sediment. Therefore, the intertidal flat of Sturgeon Bank appears to be a sink for chl a and a source of NH4 + to the Strait of Georgia beyond the intertidal flat. Particulate C/N ratios were always >7, indicating a predominantly terrestrial origin of particulate organics. Particulate C/N ratios were also found to be higher during ebb tides than flood tides, indicating preferential utilization of particulate nitrogen when organic matter was deposited onto the sediment.
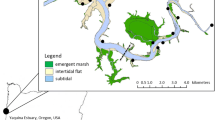
Like many other intertidal regions, the Sturgeon Bank ecosystem in the Fraser River Estuary near Vancouver, BC, Canada, is biologically productive and supports extensive fish and juvenile salmon as well as waterfowl (Sutherland et al. 2013) and forms a productive component of the Strait of Georgia ecosystem (Masson and Perry 2013). Sturgeon Bank is a 6 km wide tidal flat with an intertidal area of 83 km2, located on the western edge of the Fraser River delta (Fig. 1). Sturgeon Bank is bordered by the Steveston Jetty to the south that separates the Fraser River Main Arm from the Bank and is divided by the Iona Jetty in the northern part which was built to prevent the direct spillage of sewage effluent from the Iona Sewage Treatment Plant (Iona STP), situated on Iona Island, onto the public beaches in the north (Fig. 1). The intertidal area on Sturgeon Bank was exposed to primary treated sewage effluent (about 23,000 kg of suspended solids per day) from the Iona STP between 1962 and 1988 (Harrison et al. 1999). Sewage effluent was discharged into an open ditch on the south side of the Iona jetty, and it was pushed onto the surrounding intertidal area during flood tides. Earlier environmental studies conducted in this region prior to the sewage diversion include sediment distribution (Luternauer and Murray 1973), erosion of sediments (Amos et al. 1997), benthic invertebrates (Bawden et al. 1973; Levings et al. 1983; Otte and Levings 1975), and contamination by heavy metals (Birtwell et al. 1983; Bendell-Young et al. 2004). Beginning in 1989, the primary treated sewage was discharged through a pipe (7 km long) at a depth of 100 m in the Strait of Georgia. The study of the vertical distribution of MPB in this region not only helps us to understand the mechanisms of the MPB communities in sandy vs. muddy sediments, but also allows us to evaluate their response to an anthropogenic impact such as sewage discharge and its abatement. While a number of studies have investigated effects of eutrophication gradients from sewage discharge on macrobenthic communities of tidal areas (e.g., Arvai et al. 2002; Moore and Rodger 1991; Underwood and Chapman 1997), changes within benthic microalgal communities to such inputs are less well studied (e.g., Agatz et al. 1999; JayaPrada 2013).
The objectives of this study were to examine: (1) the spatial and seasonal distribution of intertidal microphytobenthic biomass (MPB) using chl a as a proxy of their biomass in the Fraser River Estuary, and (2) the effects of muddy vs. sandy sediments on the vertical distribution of pigments. The effect of the remaining sewage contamination on MPB biomass was also evaluated.
Materials and methods
Study site characteristics and sampling
Four sites A0, A10, A12, and A14 (Fig. 1) were selected for sediment coring on the tidal flats of Sturgeon and Roberts Banks. A wide variety of information for these particular sites is available from previous studies (Ross 1998; Thomas and Bendell-Young 1998; Yin and Harrison 2000; Bendell-Young et al. 2004). A0 was located approximately 300 m from the former outfall of the Iona Sewage Treatment Plant (STP) and was considered to be the most contaminated site of the four stations (Otte and Levings 1975; Birtwell et al. 1983; Thomas and Bendell-Young 1998). A fourth station, A14 (Fig. 1), was chosen as the reference station for A0 since it had a similar sediment composition. Like A14, A10, and A12 did not receive effluent from the Iona STP due to the presence of the jetties and the tidal current regime deflecting sewage northward into the Strait of Georgia (Otte and Levings 1975; Arvai et al. 2002). A14 was located in the intertidal zone on Roberts Bank, which is separated from Sturgeon Bank by the Steveston Jetty and the Fraser River. The four stations have a similar elevation above the chart datum (0–0.3 m, Feeney 1995). Tides in the Strait of Georgia are mixed semidiurnal (LeBlond 1983) with tidal ranges of about 2 to 5 m. All four stations were exposed to similar waves and currents and were located approximately −0.3 m relative to mean sea level.
The sediment on the tidal flat mainly consists of well-sorted sand (>90 %), except in the high intertidal zone (Feeney 1995; Luternauer and Murray 1973). A10 and A12 are good representatives of this large sandy bank, while A0 near the STP was mostly mud (>90 %; Feeney 1995); A14 on Roberts Bank was similar to A0, but with a lower mud content. All four sites were marked with concrete blocks for the identification of sampling.
Between May 1994 and September 1996, samples were taken biweekly (usually during spring tides) from May to August and then once every 4 to 6 weeks in the other months. Sites were accessed using a hovercraft after the ebb tide retreated from the sites. Samples were always taken around the approximate same time after the ebb tide had retreated. Hourly tidal heights at Sand Heads were used to determine the tidal heights for Sturgeon Bank and were provided by the Tides and Current Section, Institute of Ocean Sciences, Sidney, BC (Canada).
Measurements of pigments
Chl a was used as an indicator of MPB biomass. Pigments in the sediment were sampled with a cylinder cut from a 60-ml syringe (21 mm in diameter) for A0 and A14, and with plastic tubes (12 mm in diameter) for the sandy stations A10 and A12. The cylinders or tubes were carefully inserted 10 cm (A0, A14) or 20 cm (A10, A12) into the sediment. Coring locations were randomly selected in sediments that were not covered with a visible MPB surface aggregation. Five replicate cores were taken randomly at each site within a 1-m radius, frozen immediately on dry ice and returned to the lab within a few hours. Two cores were used for whole core extraction, while three cores were used for vertical profiling of pigments in the sediment. For the three cores (only one core in Aug 1994 to Feb 1995) used to obtain a vertical profile, each core was sliced at 1-cm intervals and each slice placed in a plastic bottle for pigment extraction. Results were also averaged to obtain depth-integrated values comparable to the measurements obtained from the whole core extractions. For the whole core extractions, the top 4-cm section of each core for the muddy sediments of A0 and A14 was placed in a bottle and extracted, since anoxic conditions dominated the sediment below this depth. For the sandy sediments of A10 and A12, a deeper 10- to 16-cm section was extracted in full since preliminary measurements showed active chlorophyll a down to these deeper depths, presumably pulled down through the larger interstitial spaces in these sandy sites during the ebbing tide.
Acetone (95 %) was added to the samples, and after hand-shaking for a few minutes, they were stored in the dark at 4 °C and extracted for 24 h. The supernatant was taken for fluorescence readings on a fluorometer (Turner Designs® Model 10). Chlorophyll a and chlorophyllide a, if present, were measured as “chlorophyll a” (hereafter referred to as “chl a”). Concentrations (g m−3) were calculated using the same equation as for the water column in the method of Parsons et al. (1984), except for the volume (V), which was replaced with the volume of sediment extracted. Chl a concentrations of depth-integrated values and whole core extractions (together hereafter referred to as “depth-integrated” values for the sake of clarity) are given in milligrams per square meter. The concentrations of phaeopigments (which can include both phaeophytin a as well as pheophorbide a) were also calculated according to Parsons et al. (1984). To ensure that the interference by other degradation pigments did not affect our previous chl a measurements, we took duplicate sediment cores from the same four sites on July 4, 2000, and used the method of Whitney and Darley (1979), in which chl a was extracted in hexane. This method determines chl a concentrations in sediment corrected for all three degradation products (phaeophytin a, pheophorbide a, and chlorophyllide a). The vertical patterns of pigment concentrations between our method and theirs were comparable.
Measurements of particulate organic carbon and nitrogen
A small portion of the sliced sediment sample for chl a was placed in a 10-ml glass vial and freeze-dried for storage for later analysis of particulate organic carbon and nitrogen (POC and PON). Samples were ground into a homogeneous powder, treated with dilute acids to remove inorganic carbonates and a small amount (∼20–30 mg) weighed and wrapped in a tin cup ready for analysis. POC and PON in the sediment sample were analyzed with a Carlo Erba model NA 1500 NCS elemental analyzer, using the dry combustion method described by Sharp (1974).
Statistical analyses
The data fulfilled all criteria to perform parametric tests; one-way analysis of variances (ANOVAs) were performed using Statistica® for Windows with a level of significance of p < 0.05 to investigate differences among the four sites. ANOVAs were performed individually on each sampling date. In order to indicate which pairs of sites specifically were different, multiple comparisons were performed using the Tukey test and Pearson correlation analyses were also carried out using MS Excel to test for significant differences between sites based on the r-critical values and degrees of freedom.
Results
Spatial and temporal variability of chl a and phaeopigments
At the two muddy sites (A0 and A14), the depth-integrated MPB chl a generally fluctuated between approximately 40 and 300 mg m−2. Mean biomass at A0 was 84 mg m−2 and there was no clear seasonal pattern during 1994–1996 (Fig. 2). The highest biomass at A0 (174 mg chl a m−2) occurred in October and November 1994. At A14, chl a was significantly higher (p < 0.05) than at A0 and ranged between 70 and 864 mg m−2 with a mean of 235 mg m−2 during the 2.5-year study (Fig. 2).
Depth-integrated benthic microalgal chl a for a top 4 cm of sediment cores at A0 and A14, b top 10–15 cm at A10 and A12, and depth-integrated benthic phaeopigment concentrations at c A0 and A14, and d A10 and A12, during 1994–96. Error bars are mean ± SE (n = 5). Note the difference in scale between the top and bottom graphs, respectively, as well as between chl a and phaeopigment concentrations
At the sandy sites A10 and A12, MPB chl a fluctuated between 200 and 4,000 mg m−2 and there was no clear seasonal pattern. The average was 2,044 and 882 mg m−2 at A10 and A12, respectively (Table 1). Therefore, MPB chl a at the sandy stations (A10 and A12) was nearly an order of magnitude higher than at the muddy stations, A0 and A14 (Table 1).
Depth-integrated phaeopigments were similar at A0 and A14 and ranged from 59 to 583 mg m−2 (Fig. 2c, d) with no clear seasonal pattern. Mean phaeopigment concentrations at A10 and A12 were 346 and 284 mg m−2, respectively (Table 1), and significantly higher (p < 0.05) than at A0 (146 mg m−2) and A14 (209 mg m−2). Phaeopigments were similar to chl a concentrations at A0 and A14, but significantly lower (p < 0.05) than chl a at A10 and A12 (Fig. 2).
The spatial distribution of MPB biomass was A10 > A12 > A14 > A0. In most cases, the differences in chl a among the four sites were statistically significant (p < 0.001) (Fig. 3). This indicated that the chl a levels at the four sites varied independently from each other. Further, multiple comparisons between paired sites revealed that chl a concentrations between A0 and A14 were generally not significantly different (p > 0.05), and only 3 days were significantly different (p < 0.05) (Fig. 3d). In contrast, comparisons between all other pairs of stations (Fig. 3b, c, e-g) were significantly different throughout most of the study period (p < 0.05). May and June samples in 1994 were, however, quite low and also similar for all stations, thus giving a mostly non-significant result in the multiple comparisons (Fig. 3).
One-way (single factor) analysis of variance (ANOVA) among a the four sites and b-g multiple comparisons of benthic microalgal biomass (chl a) between a pair of different sites performed for each sampling day. Different levels of significance are represented by dashed lines: Points above the p = 0.05 line indicate non-significant differences, while any points below indicate that differences are significant at least at p < 0.05, if not at p < 0.01 or p < 0.001 if found below the respective dashed lines
Correlation analysis revealed that there was a significant correlation (p < 0.05) in chl a concentrations between A0 and A14 (muddy sites) and between A10 and A12 (sandy sites). Similarly, the correlation for phaeopigments was also significant for the two pairs of stations. However, correlations between muddy and sandy stations, for example, between A0 and A10, or A0 and A12 for both chl a and phaeopigments, were not significant (p > 0.05).
The ratio of depth-integrated chl a to phaeopigments was significantly higher (p < 0.05) at A10 and A12 than at the muddy sites A0 and A14 (Fig. 4), generally indicating more freshly buried algal biomass at A10 and A12 relative to A0 and A14. However, in some cases, the higher ratio may be due to higher surface chl a (Fig. 5). The 2.5-year average ratio was 6.6 and 3.5 for A10 and A12, respectively, and 0.6 and 1.1 for A0 and A14, respectively. Although the ratios fluctuated, there were significant correlations (p < 0.01) between the depth-integrated chl a and phaeopigments for each site.
Ratios of depth-integrated chl a to phaeopigments at A0, A10, A12, and A14 during 1994–1996 (data are from Fig. 2). The mean ratio for A0, A10, A12, and A14 was 0.60, 6.58, 3.48, and 1.09, respectively
The concentration (left panel) and percent (right panel) of chl a and phaeopigments in the top 1 cm of sediment at A0, A10, A12, and A14 between May 1994 and Feb. 1995 (data from Figs. 4 and 5). Percent of total pigments represent the amount of pigments in the top 1 cm compared to total pigments in the whole sediment core. Error bars are the mean ± SE (n = 5), and if error bars for May–July 1994 are not visible, they are smaller than the symbol. There are no replicates from Aug. 1994 to Feb. 1995
Vertical distribution of chl a and phaeopigments
Vertical profiles of chl a concentrations at A0 showed that chl a was highest in the top 1 cm and decreased with depth, particularly between Sep. 16 and Nov. 29, 1994 (Fig. 6), but chl a concentrations at A0 were generally <15 g m−3 at all core depths. Phaeopigment concentrations followed the chl a distribution and were mostly significantly higher (p < 0.05) than chl a, and often higher at a core depth of 4 cm or deeper (Fig. 6a). At A14, the vertical distribution of chl a (Fig. 6b) was similar to A0. The distribution of phaeopigments was mostly parallel to chl a, but concentrations of phaeopigments were generally significantly higher (p < 0.05) than for chl a (Fig. 6b).
Vertical profiles of chl a and phaeopigment concentrations in sediments at a A0 and b A14 between Jun. 1994 and Jan. 1995. Note the difference in scale. Error (horizontal) bars are mean ± SE (n = 3) and shown only for Jun. 23 and Jul. 11. If error bars are not visible on these dates, they were smaller than the symbol. There were no replicates on other dates. Data on August 9 at A0 (a) were missing
The vertical distribution of chl a at A10 and A12 was significantly different (p < 0.05) from that at A0 and A14. Chl a content at depth in the core was generally significantly higher (p < 0.05) than in surface samples on many sampling days (e.g., at A10 on Jun. 23, Aug. 19, and Sep. 16, 1994, and at A12 on Aug. 9 and Oct. 14) (Fig. 7). Chl a even reached 42 g m−3 at 4-cm depth at A10 on Nov. 29. In comparison, phaeopigments were low and relatively homogeneously distributed at both sites (Fig. 7). Chl a at depth was several times higher at A10 and A12 than at A0 and A14.
Vertical profiles of chl a and phaeopigment concentrations in sediments at a A10 and b A12 between Jun. 1994 and Jan. 1995. Note the difference in scale. Error (horizontal) bars are mean ± SE (n = 3) and shown only for Jun. 23 and Jul. 11. If error bars are not visible on these dates, they were smaller than the symbol. There were no replicates on other dates. Cores could not be sampled on Aug. 9 at A0
Surface (top 1 cm) chl a and phaeopigment concentrations were reasonably similar among the four sites (Fig. 5). Surface chl a was generally <100 mg m−2 at all four stations during summer, possibly due to high sediment temperatures and high light exposure. During the fall and winter months, chl a was higher (<200 mg m−2) at A0 and A14, but significantly higher (100 to 600 mg m−2) at A12 and A10 than during other months. Surface chl a made up about 50 % of the total sediment core chl a content at A0 and A14, but only 20 % of the total sediment core chl a content at A10 and A12 (Fig. 5).
Vertical distribution of POC and PON
Particulate organic carbon (POC) and nitrogen (PON) at A0 were approximately 10 and 0.8 mg g−1 in the top 1 cm, respectively, and decreased with depth to ∼4 and 0.2–0.4 mg g−1, respectively (Fig. 8). The vertical distribution pattern did not change much during the period from May 24, 1994–Mar. 22, 1995. At A14, POC and PON were much lower than at A0 in the top 6 cm, and the vertical decreasing trend was less pronounced except for Mar. 22, 1995. In contrast, POC and PON concentrations at the two sandy sites A10 and A12 were low and almost homogeneously distributed with depth during this period (Fig. 8). Concentrations were lower than those measured at both A14 and A0.
The respective POC/PON ratios were >11 at A0 and A14 but <10 at A10 and A12. Vertical profiles of POC/PON ratio appeared to show higher values in the middle depths at A0 and A14 but a slight increasing trend with depth at A10 and A12 (Fig. 9).
Vertical profiles of POC/chl a ratios showed very high values exceeding 1000:1 at A0 and A14 with the former being several times higher than the latter. At A10 and A12, POC/chl a ratios were much smaller than those at A0, although they were greater than 100:1 in the top 6 cm (Fig. 10).
Discussion
Spatial and seasonal distribution of MPB biomass
The temporal and spatial distribution of MPB biomass and production is regulated by different natural factors. These factors include light (Blanchard and Montagna 1992; MacIntyre and Cullen 1996; Pinckney and Zingmark 1993b), nutrient supply (Burford et al. 2012; Admiraal 1984), grazing (Arvai et al. 2002; Hagerthey et al. 2002; Sundbäck et al. 1996), physical processes mainly associated with water and current movement (Blanchard et al. 2001; Huettel et al. 2007; Pinckney and Zingmark 1991; Grangere et al. 2012), and sediment composition (Cartaxana et al. 2006; Jesus et al. 2009; Du et al. 2009, 2010; Ubertini et al. 2015). MPB biomass at all four sites was highly variable throughout the 2.5-year study period and did not reveal clear seasonal patterns. This agrees with other studies that have found that MPB is highly variable within and between years (Brito et al. 2012; MacIntyre et al. 1996; Underwood and Kromkamp 1999; Thornton et al. 2002). Frequently, increases in benthic diatom biomass are observed during late spring to summer months (Colijn and Dijkema 1981; Underwood and Kromkamp 1999; Montani et al. 2003), which agrees with our results, particularly from spring 1995 onwards (Fig. 2). Less clear signals were observed in 1994, with high MPB biomass being observed in the autumn at A14. Although conditions were similar between A0 and A14, the location of A14 south of the Fraser River South Arm on Roberts Bank may have subjected it to different light and nutrient conditions compared to the sites on Sturgeon Bank (Arvai et al. 2002). A mosaic of water masses is known to occur on Sturgeon Bank (Yin and Harrison 2000) with most of the runoff discharge from the Fraser River occurring in late spring to early summer, along with a secondary surge in late autumn (Burd et al. 2008). The discharge affects the estuarine circulation in the Strait of Georgia and subsequently the influence of the sediment-laden plume on benthic and pelagic environments on these intertidal banks.
MPB biomass has typically been measured in the top mm to 2-cm depth in intertidal mudflats and ranges from 1 to 560 mg m−2 (MacIntyre et al. 1996). This agrees with values observed in our study of around 40–300 mg m−2 throughout the study period at the muddy sites, while our estimates of only the top 1 cm were also comparable to previous measurements at our sites (Bawden et al. 1973; BC Research 1975) and other areas such as Netarts Bay and Ems-Dollard estuary (Davis and McIntire 1983; Colijn and de Jonge 1984).
Effect of sandy and muddy sediment on vertical pigment distribution
We observed nearly an order of magnitude higher values for depth-integrated biomass at the sandy sites compared to the muddy sites (up to 4000 mg m−2 at A10). We also found a biomass signal to much greater depths at the sandy sites (A10, A12) compared to the muddy sites (A0 and A14), where chl a content was concentrated in the first few centimeters. The presence of phytoplankton cells beyond the upper few millimeters of sediment is known for muddy sites in other studies (Brito et al. 2012), although highest concentrations are normally found in the first millimeter (de Brouwer and Stal 2001). Chl a usually decreases rapidly with depth due to its degradation into breakdown products such as phaeopigments (Baulch et al. 2009; Du et al. 2010; Grinham et al. 2007). The vertical distribution of chl a and phaeopigments at A0 and A14 agrees with these observations, but not the vertical profiles at A10 and A12 where high chl a values relative to phaeopigments exist throughout the core profiles, even at a depth of 10 cm (Figs. 5, 6, and 7). This supports the findings by Evrard et al. (2008) where depth-integrated MPB production in permeable (sandy) sediments can significantly exceed that of intertidal muddy areas. Sandy sediments have larger interstitial spaces allowing cells to be present at much greater depths in the sediment. Since shallow cores miss the “buried” chlorophyll, especially in sandy/fine-grained sediments, Grinham et al. (2007) emphasized that core tube diameter, core depth, and other factors must be considered when making comparisons between various studies.
Several studies have found significant chlorophyll concentrations down to 10 and even 25-cm depth in sandy sediments (Barranguet et al. 1997; d’Andrea et al. 2002; Zetsche et al. 2012). Several mechanisms can contribute to large amounts of chlorophyll in deeper sediments including biological factors such as the vertical migration of cells (Consalvey et al. 2004; MacIntyre et al. 1996) and the bioturbation by infauna (Defew et al. 2002; Le Hir et al. 2007; Yamaguchi et al. 2007). Physical factors include wind events (Plante et al. 1986; Cartaxana et al. 2006), sediment relocation and resuspension (Barranguet et al. 1997; Delgado et al. 1991), advective transport mechanisms (Huettel and Rusch 2000; Huettel et al. 2007), and tidal drainage (Billerbeck et al. 2006). Vertical migration, however, generally occurs only within the top 1 cm for sandy sediments (Du et al. 2010; Mitbavkar and Anil 2004). On the other hand, porewater advection is driven by pressure gradients caused by changes in density or wave and current interactions with sediment topography (Huettel et al. 2007; Zetsche et al. 2012) and has been shown to transport particles such as algal cells into and out of the sediment at much greater depths (Huettel et al. 2007). Permeable sediments not only enhance the transport of particles such as algal cells through the sediment, but also affect remineralization rates and nutrient fluxes of the sediment (d’Andrea et al. 2002; Huettel and Rusch 2000). Sediment relocation can efficiently remove dead MPB by resuspending previously trapped particles into the water column (Delgado et al. 1991; Schallenberg and Burns 2004) and may explain the low phaeopigment content that we observed at the sandy sites. This seems more plausible than a rapid degradation of the cells, since the chl a/phaeopigment ratios were >1 at the sandy sites, which suggests recently buried chlorophyll. Lower POC/chl a ratios at the sandy sites than at the muddy sites (Fig. 10) indicate a relatively larger chl a contribution to POC at the former sites, which suggests that organic matter at the sandy sites was more recent and likely from phytoplankton as C/N ratios were close to 7:1 at the sandy sites (Fig. 9).
The vertical depth profiles for pigments at A10 and A12 suggest that one or several of these mechanisms allow the penetration as well as the removal of algal cells through the relatively large interstitial spaces of sandy sediments deeper into the sediments. POC and PON concentrations support the observation of sediment substrate effects, with sandy sediments showing almost homogeneous distributions throughout the sediment column compared to muddy sediments. Muddy sediments such as those at A14 always had higher POC and PON concentrations than at A10 and A12.
Long time recovery from sediment contamination
Comparing the two muddy sites, A0 exhibited lower chl a values, possibly due to the remaining effects from the previous sewage outfall. However, there was evidence of a positive response to the sewage abatement at A0 when MPB chl a values in this study were compared with previous studies at muddy sites on Sturgeon Bank (Otte and Levings 1975; B.C. Research 1975). Chl a concentrations at A0 were significantly lower, but POC and PON concentrations were significantly higher than at the other three sites (Fig. 2) 5 years after the diversion. Higher C/N ratios at A0 and A14 than at A10 and A12 also indicated a more terrestrial origin of the organic matter at the former sites and a more marine origin, i.e., phytoplankton signature at the latter sites. The POC/chl a ratio was also much higher at A0 than all the other three sites, further confirming the continuation of sewage contamination effects. Other studies on the same tidal flat also showed relatively high concentrations of metals such as Hg, Ag, Pb, Zn, Cu, and Cr still present from the earlier contamination (Thomas and Bendell-Young 1998) although there was a considerable improvement in the environment at the most contaminated site at A0 (Arvai et al. 2002).
The recovery from muddy sediment contamination by sewage effluent may take a decade or longer due to both organic and inorganic (e.g., Hg; Thomas and Bendell-Young 1998) contamination. We took sediment samples at four sites, 11 years (2000) after the diversion. There was a five-fold increase in chl a at the surface at A0 (Figs. 4 and 11) and deeper penetration of active chl a beyond 4 cm at A0 (Fig. 11). This suggests that MBP biomass accumulation at the site was still stressed by contamination 11 years after sewage abatement. The comparison between 5 and 11 years highlighted that the recovery from the sewage contamination requires a long time, especially in muddy/anoxic sediments as found in previous studies (Peletier 1996; Larson and Sundbӓck 2004).
Vertical profiles of chl a concentrations in sediments at A0, A10, A12, and A14 on 4 July 2000 using the hexane method of Whitney and Darley (1979) with two replicates (open and filled circles)
Conclusion
The intertidal flat is an important part of the dynamic coastal ecosystem, and interstitial spaces in sandy sediments play an important role in the vertical distribution of microphytobenthos in the sediment. The increase in the vertical distribution of MPB biomass can provide more food for benthic grazers, enhance the biogeochemical cycling of organic matter and nutrients, and may have aided the recovery from sewage contamination after the sewage abatement. For example, in a parallel study to our chlorophyll distribution, the amphipod Corophium salmonis was shown to have recolonized A0 (the most contaminated site) after sewage abatement (Arvai et al. 2002). Although we cannot rule out natural temporal or spatial variability, given the increase in chl a at all four sites, it has taken the system many years to recover from the effects of sewage pollution, especially at the most contaminated muddy site, A0. To fully understand complex coastal systems, detailed long-term studies need to include the monitoring of microphytobenthic communities. Presently, few functional indices exist to routinely monitor coastal benthic habitats, and the role of MPB and benthic diatoms as ecological indicators has been seldom explored despite their potential (Desrosiers et al. 2013).
References
Admiraal W (1984) The ecology of estuarine sediment-inhabiting diatoms. Prog Phycol Res 3:269–322
Agatz M, Asmus RM, Deventer B (1999) Structural changes in the benthic diatom community along a eutrophication gradient on a tidal flat. Helgol Mar Res 53:92–101
Amos CL, Feeney TD, Sutherland TF, Luternauer JL (1997) The stability of fine-grained sediments from the Fraser River delta. Estuar Coast Shelf Sci 45:507–524
Arvai JL, Levings CD, Harrison PJ, Neill WE (2002) Improvement of the sediment ecosystem following diversion of an intertidal sewage outfall at the Fraser River estuary, Canada, with emphasis on Corophium salmonis (Amphipoda). Mar Poll Bull 44:511–519
Barranguet C, Herman PMJ, Sinke JJ (1997) Microphytobenthos biomass and community composition studied by pigment biomarkers: importance and fate in the carbon cycle of a tidal flat. J Sea Res 38:59–70
Baulch HM, Turner MA, Findlay DL, Vinebrooke RD, Donahue WF (2009) Benthic algal biomass – measurements and errors. Can J Fish Aquat Sci 66:1–13
Bawden CA, Heath WA, Norton AB (1973) A preliminary baseline study of Roberts and Sturgeon Banks. Westwater Research Centre Tech Rep No 1, University of British Columbia
BC Research Inc (1975) Environmental studies at Iona Island. Report No 2 Project No 1511/2, Great Vancouver Regional District, Burnaby, British Columbia, Canada
Bendell-Young L, Yin K, Thomas C, Harrison PJ, Feeney TD, Arvai JL, Levings CD, Ross L (2004) Biogeochemistry of the intertidal area of the Fraser River estuary, In: Groulx BJ, Mosher DC, Luternauer JL, Bilderback DE (eds) Fraser River Delta, British Columbia: issues of an urban estuary. Geol Survey Can Bull 567:189–212
Billerbeck M, Røy H, Bosselmann K, Huettel M (2006) Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuar Coast Shelf Sci 71:704–716
Birtwell IK, Greer GL, Nassichuk MD, Rogers IH (1983) Studies of the impact of municipal sewage discharge onto an intertidal area within the Fraser River Estuary, British Columbia. Can Tech Rep Fish Aquat Sci 1170
Blanchard GF, Guarini JM, Orvain F, Sauriau PG (2001) Dynamic behavior of benthic microalgal biomass in mudflats. J Exp Mar Biol Ecol 264:85–100
Blanchard GF, Montagna PA (1992) Photosynthetic response of natural assemblages of marine benthic microalgae to short- and long-term variations of incident irradiance in Baffin Bay, Texas. J Phycol 28:7–14
Brito AC, Fernandes TF, Newton A, Facca C, Tett P (2012) Does microphytobenthos resuspension influence phytoplankton in shallow systems? A comparison through a Fourier series analysis. Estuar Coast Shelf Sci 110:77–84
Burd BJ, Barnes PAG, Wright CA, Thomson RE (2008) A review of subtidal benthic habitats and invertebrate biota of the Strait of Georgia. Mar Environ Res 66:S3–S38
Burford MA, Revill AT, Smith J, Clementson L (2012) Effect of sewage nutrients on algal production, biomass and pigments in tropical tidal creeks. Mar Poll Bull 64:2671–2680
Cartaxana P, Mendes CR, van Leeuwe MA, Brotas V (2006) Comparative study on microphytobenthic pigments of muddy and sandy intertidal sediments of the Tagus estuary. Estuar Coast Shelf Sci 66:225–230
Chennu A, Färber P, Volkenborn N, Al-Najjar MAA, Janssen F, de Beer D, Polerecky L (2013) Hyperspectral imaging of the microscale distribution and dynamics of microphytobenthos in intertidal sediments. Limnol Oceanogr Methods 11:511–528
Colijn F, Dijkema KS (1981) Species composition of benthic diatoms and distribution of chlorophyll a on an intertidal flat in the Dutch Wadden Sea. Mar Ecol Prog Ser 4:9–21
Colijn F, de Jonge VN (1984) Primary production of microphytobenthos in the EMS-Dollard estuary. Mar Ecol Prog Ser 14:185–196
Consalvey M, Paterson D, Underwood G (2004) The ups and downs of life in a benthic biofilm: migration of benthic diatoms. Diatomol Res 18:181–202. doi:10.1080/0269249X.2004.9705870
Davis MW, McIntire CD (1983) Effects of physical gradients on the production of sediment-associated algae. Mar Ecol Prog Ser 13:103–114
d’Andrea AF, Aller RC, Lopez GR (2002) Organic matter flux and reactivity on a South Carolina sandflat: the impacts of porewater advection and macrobiological structures. Limnol Oceanogr 47:1056–1070
de Brouwer JFC, Stal LJ (2001) Short-term dynamics in microphytobenthos distribution and associated extracellular carbohydrates in surface sediments of an intertidal mudflat. Mar Ecol Prog Ser 218:33–44
Defew EC, Tolhurst TJ, Paterson DM (2002) Site-specific features influence sediment stability of intertidal flats. Hydrol Earth Syst Sci 6:971–981
Delgado M, de Jonge VN, Peletier H (1991) Experiments on resuspension of natural microphytobenthos populations. Mar Biol 108:321–328
Desrosiers C, Leflaive J, Eulin A, Ten Hage L (2013) Bioindicators in marine waters: benthic diatoms as a tool to assess water quality from eutrophic to oligotrophic coastal ecosystems. Ecol Indic 32:25–34
Du GY, Son M, Yun M, An S, Chung IK (2009) Microphytobenthic biomass and species composition in intertidal flats of the Nakdong River estuary, Korea. Estuar Coast Shelf Sci 82:663–672
Du GY, Son M, An S, Chung IK (2010) Temporal variation in the vertical distribution of microphytobenthos in intertidal flats of the Nakong River estuary, Korea. Estuar Coast Shelf Sci 86:62–70
Evrard V, Cook PLM, Veuger B, Huettel M, Middelburg JJ (2008) Tracing carbon and nitrogen incorporation and pathways in the microbial community of a photic subtidal sand. Aquat Microb Ecol 53:257–269
Feeney TD (1995) Physical controls on the distribution of contaminants on Sturgeon Bank, Fraser River delta, British Columbia. MSc thesis, University of British Columbia, Canada
Grangere K, Ubertini M, Lefebvre S, Gangnery A, Le Gendre A, Orvain F (2012) Spatial variability of benthic-pelagic coupling in an estuary ecosystem: consequences for microphytobenthos resuspension phenomenon. PLoS ONE 7:1–17
Grinham AR, Carruthers TJB, Fisher PL, Udy JW, Dennison WC (2007) Accurately measuring the abundance of benthic microalgae in spatially variable habitats. Limnol Oceanogr Methods 5:119–125
Guarini JM, Sari N, Moritz C (2008) Modelling the dynamics of the microalgal biomass in semi-enclosed shallow-water ecosystems. Ecol Model 211:267–278
Hagerthey SE, Defew EC, Paterson DM (2002) Influence of Corophium volutator and Hydrobia ulvae on intertidal benthic diatom assemblages under different nutrient and temperature regimes. Mar Ecol Prog Ser 245:47–59
Hardison AK, Canuel EA, Anderson IC, Tobias CR, Veuger B, Waters MN (2013) Microphytobenthos and benthic macroalgae determine sediment organic matter composition in shallow photic sediments. Biogeosciences 10:5571–5588
Harrison PJ, Yin K, Ross L, Arvai J, Gordon K, Bendell Young L, Thomas C, Elner R, Sewell M, Shepherd P (1999) The delta foreshore ecosystem: past and present status of geochemistry, benthic community production and shorebird utilization after sewage diversion. In: Gray C, Tuominen T (eds) Health of the Fraser River Aquatic Ecosystem. Environment Canada, Vancouver, BC, pp 189–210
Huettel M, Cook P, Janssen F, Lavik G, Middelburg JJ (2007) Transport and degradation of a dinoflagellate bloom in permeable sublittoral sediment. Mar Ecol Prog Ser 340:139–153
Huettel M, Rusch A (2000) Transport and degradation of phytoplankton in permeable sediment. Limnol Oceanogr 45:534–549
JayaPrada C (2013) Impact of pollution on epilithic benthic microalgal community of Visakhapatnam harbour, east coast of India. Int J Environ Anim Conserv 2:35–49
Jesus B, Brotos V, Ribeiro L, Mendes CR, Cartaxana P, Paterson DM (2009) Adaptations of microphytobenthos assemblages to sediment type and tidal position. Cont Shelf Res 29:1624–1634
Krom MD (1991) Importance of benthic productivity in controlling the flux of dissolved inorganic nitrogen through the sediment-water interface in a hypertrophic marine ecosystem. Mar Ecol Prog Ser 78:163–172
Larson F, Sundbӓck K (2004) Role of microphytobenthos in recovery of functions in a shallow-water sediment system after hypoxic events. Mar Ecol Prog Ser 357:1–16
LeBlond PH (1983) The Strait of Georgia: functional anatomy of a coastal sea. Can J Fish Aquat Sci 40:1033–1063
Le Hir P, Monbet Y, Orvain F (2007) Sediment erodability in sediment transport modelling: can we account for biota effects? Cont Shelf Res 27:1116–1142
Levings CD, Foreman RE, Tunnicliffe VJ (1983) Review of the benthos of the Strait of Georgia and contiguous fjords. Can J Fish Aquat Sci 40:1120–1141
Light BR, Beardall J (1998) Distribution and spatial variation of benthic microalgal biomass in a temperate, shallow-water marine system. Aquat Bot 61:39–54
Luternauer JL, Murray JW (1973) Sedimentation on the western delta-front of the Fraser River, British Columbia. Can J Earth Sci 10:1642–1663
MacIntyre HL, Cullen JJ (1996) Primary production by suspended and benthic microalgae in a turbid estuary: time-scales of variability in San Antonio Bay, Texas. Mar Ecol Prog Ser 145:245–268
MacIntyre HL, Geider RJ, Miller DC (1996) Microphytobenthos: the ecological role of the “secret garden” of unvegetated shallow-water marine habitats: I. Distribution, abundance and primary production. Estuaries 19:186–201
Masson D, Perry RI (2013) The Strait of Georgia ecosystem research initiative; an overview. Prog Oceanogr 115:1–5
Mitbavkar S, Anil A (2004) Vertical migratory rhythms of benthic diatoms in a tropical intertidal sand flat: influence of irradiance and tides. Mar Biol 145:9–20
Montani S, Magni P, Abe N (2003) Seasonal and interannual patterns of intertidal microphytobenthos in combination with laboratory and areal production estimates. Mar Ecol Prog Ser 249:79–91
Moore DC, Rodger GK (1991) Recovery of a sewage sludge dumping ground. 2. Macrobenthic community. Mar Ecol Prog Ser 75:301–308
Otte G, Levings CD (1975) Distribution of macroinvertebrates communities on a mudflat influenced by sewage. Fraser River Estuary, British Columbia. Fish Mar Ser Tech Rep 476
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Peletier H (1996) Long-term changes in intertidal estuarine diatom assemblages related to reduced input of organic waste. Mar Ecol Prog Ser 137:265–271
Pinckney JL, Zingmark RG (1991) Effects of tidal stage and sun angles on intertidal benthic microalgal productivity. Mar Ecol Prog Ser 76:81–89
Pinckney JL, Zingmark RG (1993a) Biomass and production of benthic microalgal communities in estuarine habitats. Estuaries 16:887–897
Pinckney JL, Zingmark RG (1993b) Modeling the annual production of intertidal benthic microalgae in estuarine ecosystems. J Phycol 29:396–407
Plante R, Plante S, Cuny MR, Reys JP (1986) Photosynthetic pigments of sandy sediments on the north Mediterranean coast: their spatial distribution and its effect on sampling strategies. Mar Ecol Prog Ser 34:133–141
Rizzo WM (1990) Nutrient exchange between the water column and a subtidal benthic microalgal community. Estuaries 13:219–226
Ross L (1998) Intertidal benthic primary productivity following sewage effluent diversion on Sturgeon Bank, Fraser River delta. M.Sc. Thesis, University of British Columbia, Canada
Schallenberg M, Burns CW (2004) Effects of sediment resuspension on phytoplankton production: teasing apart the influences of light, nutrients and algal entrainment. Freshw Biol 49:143–159
Shaffer G, Sullivan MJ (1988) Water column productivity attributable to displaced benthic diatoms in well-mixed shallow estuaries. J Phycol 24:132–140
Sharp JH (1974) Improved analysis of particulate organic carbon and nitrogen from seawater. Limnol Oceanogr 19:984–989
Sundbäck K, Nilsson P, Nilsson C, Jönsson B (1996) Balance between autotrophic and heterotrophic components and processes in microbenthic communities of sandy sediments: a field study. Estuar Coast Shelf Sci 43:689–706
Sutherland TF, Elner RW, O’Neill JD (2013) Roberts Bank: ecological crucible of the Fraser estuary. Prog Oceanogr 115:171–180
Thomas CA, Bendell-Young L (1998) Linking the sediment geochemistry of an intertidal region to metal bioavailability in the deposit feeder Macoma balthica. Mar Ecol Prog Ser 173:197–213
Thornton DCO, Dong LF, Underwood GJC, Nedwell DB (2002) Factors affecting microphytobenthic biomass, species composition and production in the Colne Estuary (UK). Aquat Microb Ecol 27:285–300
Ubertini M, Lefebvre S, Rakotomalala C, Orvain F (2015) Impact of sediment grain-size and biofilm age on epipelic microphytobenthos resuspension. J Exp Mar Biol Ecol 467:52–64
Underwood AJ, Chapman MG (1997) Subtidal assemblages on rocky reefs at a cliff-face sewage outfall (North Head, Sydney, Australia): what happened when the outfall was turned off? Mar Pollut Bull 33:293–302
Underwood GJC, Kromkamp J (1999) Primary production by phytoplankton and microphytobenthos in estuaries. Adv Ecol Res 29:95–153
Underwood GJC, Paterson D (1993) Seasonal changes in diatom biomass, sediment stability and biogenic stabilization in the Severn Estuary. J Mar Biol Assoc UK 73:871–887
Wainright SC (1990) Sediment-to-water fluxes of particulate material and microbes by resuspension and their contribution to the planktonic food web. Mar Ecol Prog Ser 62:271–281
Whitney DE, Darley WM (1979) A method for the determination of chlorophyll a in samples containing degradation products. Limnol Oceanogr 24:183–186
Yamaguchi H, Montani S, Tsutsumi H, Hamada K, Ueda N, Tada K (2007) Dynamics of microphotobenthos biomass in a coastal area of western Seto Inland Sea, Japan. Estuar Coast Shelf Sci 75:423–432
Yin K, Harrison PJ (2000) Influences of flood and ebb tides on nutrient fluxes and chlorophyll on an intertidal flat. Mar Ecol Prog Ser 196:75–85
Zetsche E, Bulling MT, Witte U (2012) Permeability of intertidal sandflats: impact of temporal variability on sediment metabolism. Cont Shelf Res 42:41–50
Acknowledgments
This research was funded by Fraser River Action Plan sponsored by Environment Canada and the Department of Fisheries and Oceans through Dr. Colin Levings. We thank Dr. Colin Gray of Environment Canada for providing funding. We thank Leo Rebele, Joe Arvai, Amy Hillaby, and Mingxin Guo who provided assistance with field sampling. Comments on an earlier draft of the manuscript by Dr. Terri Sutherland were very useful. We thank the Canadian Coast Guard for providing the hovercraft time, and the officers and crew of Richmond Hovercraft Base for their assistance. Kedong Yin acknowledges the continuing support by NSFC 91328203, 31511130135, and GD2013B051000042.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Hein
Rights and permissions
About this article
Cite this article
Yin, K., Zetsche, EM. & Harrison, P.J. Effects of sandy vs muddy sediments on the vertical distribution of microphytobenthos in intertidal flats of the Fraser River Estuary, Canada. Environ Sci Pollut Res 23, 14196–14209 (2016). https://doi.org/10.1007/s11356-016-6571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6571-y