Abstract
Tidal flat is often the dominant habitat type in US Pacific Northwest (PNW) estuaries. This research examined environmental factors that explain the spatial patterns of microphytobenthos (MPB) biomass and community structure in a PNW estuary with complex nutrient dynamics. MPB biomass, individual species abundances, diatom community metrics, and diatom community structure were strongly correlated with distance from the estuary mouth, salinity, substrate composition, and pore water-soluble reactive phosphorus (SRP). The microphytobenthos was dominated by diatoms and diatom diversity and species richness were negatively associated with salinity, substrate sand content, and pore water SRP. Diatom community structure varied with estuary position, with the epipsammic taxa Catenula adhaerens, Opephora spp. 1, and Planothidium delicatulum dominating the assemblage at sites near the estuary mouth and epipelic taxa, such as Nitzschia frustulum and Nitzschia palea, being more abundant at sites in the middle and upper estuary. Distance from the estuary mouth and salinity were the most important predictors of diatom assemblage structure, based on CCA analysis. MPB biomass was highest at sites in the lower estuary, characterized by higher salinities, SRP, and substrate sand composition. Pore water nitrogen and surrounding land use were not important predictors of MPB biomass or community structure. We attribute our findings to the nutrient dynamics of Yaquina estuary, which is high in nutrients due to both coastal upwelling and watershed-derived nitrate and exhibits an increasing gradient of phosphorus towards the estuary mouth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are among the most productive and valuable ecosystems on Earth. They provide a wide variety of ecosystem services, including carbon sequestration, biodiversity maintenance, nutrient cycling, water purification, fisheries maintenance, and coastal protection (MEA 2005a, b; Barbier et al. 2011). Currently, over 40% of the world’s population lives within 80 km of the coast (MEA 2005a, b) resulting in estuaries being among the ecosystems most impacted by human activities (Lotze et al. 2006; Worm et al. 2006; Halpern et al. 2008). Human activities, such as shipping and aquaculture, have direct impacts on estuarine habitats, while activities in the surrounding watershed may have indirect impacts on estuarine habitats by increasing nutrient inputs or introducing invasive species. While many estuaries in the Pacific Northwest USA (PNW) have historically experienced fewer anthropogenic impacts than those in southern California, the Atlantic coast, and Europe, coastal populations in this region are projected to rise, air and ocean temperatures to warm, and erosion and flooding are expected to increase due to stronger storms (NOAA 2013; Retallack et al. 2016; Mote et al. 2019), potentially resulting in dramatic changes to estuarine ecosystems.
The estuarine food web is supported by a complex combination of autochthonous and allochthonous carbon sources. Autochthonous carbon can be derived from vegetated salt marshes, seagrass beds, and both pelagic and benthic microscopic primary producers, while allochthonous carbon is derived primarily from the estuary watershed. Microphytobenthos (MPB) can be a conspicuous and important component of the unvegetated intertidal tidal flat (McGlathery et al. 2013), occupying the top few centimeters of substrate where there is sufficient light for photosynthesis. Historically, the contribution of carbon from MPB to the larger estuarine food web has been ignored. However, recent studies have increasingly demonstrated the importance of MPB-derived carbon as a food source for higher trophic levels (Currin et al. 1995; Herman et al. 2000; Middleburg et al. 2000; van Oevelen et al. 2006; Christianen et al. 2017). Annual rates of MPB primary production are highly variable, with rates as high as 2500 g C m−2 year−1 being reported (Valiela 1995). Although rates in temperate regions usually range from < 10–500 g C m−2 year−1 (Hargrave et al. 1983; Thom and Albright 1990; Brotas and Catarino 1995; review in Cahoon et al. 1999). MPB can constitute a substantial component of total estuarine production, especially in areas where vegetated salt marshes and seagrass beds are not abundant. MPB accounts for anywhere from 20 to 80% of total primary production in estuaries, higher than pelagic plankton (Thom and Albright 1990; Cahoon et al. 1999; Underwood and Kromkamp 1999; Longphuirt et al. 2007). For example, Cahoon et al. (1999) estimated that 80% of chlorophyll related biomass in Onsow Bay, NC, USA, was found within the sediment as opposed to the water column. Several studies have demonstrated that MPB is an important energy source for many consumers at higher trophic levels (MacIntyre et al. 1996; Kang et al. 2006; Christianen et al. 2017). Finally, in addition to providing carbon directly to higher trophic levels, MPB can also contribute carbon to the estuarine food web via detrital pathways and dissolved organic carbon leaks and via resuspension (see review in McGlathery et al. 2013).
The tidal cycle of estuaries creates one of the most dynamic habitats that microalgal experience, with light, temperature, salinity, moisture, and nutrient availability in continual flux. Estuarine MPB are thought to experience a much more variable environment than their pelagic counterparts (da Silva et al. 2017). Despite this dynamic environment, coastal areas often exhibit high rates of MPB primary production due to high levels of available nutrients (Cadée and Hegemann 1974; Admiraal 1984). The environmental factors shaping MPB abundance, distribution, and community structure both within estuaries and across estuaries have received much attention in the literature (see review in Trobajo and Sullivan 2010). Early studies demonstrated the importance of salinity (e.g., Admiraal and Peletier 1979, 1980; Juggins 1992; Underwood et al. 1998), nutrients (e.g., Admiraal 1977; Admiraal and Peletier 1979, 1980; Brotas and Catarino 1995; Underwood et al. 1998), inundation (Brotas and Catarino 1995), light (Admiraal 1976; Admiraal and Peletier 1980; MacIntyre et al. 1996), and sediment composition (Colijn and Dijekma 1981; Oh and Koh 1995) in regulating MPB biomass and species composition. These ideas were also demonstrated for PNW estuaries (Riznyk and Phinney 1972; Riznyk et al. 1978; Davis and McIntire 1983; Whiting and McIntire 1985). More recent studies spanning broader geographic and environmental gradients from around the globe have reinforced these ideas (e.g., Sawai et al. 2016; Semcheski et al. 2016; Costa-Böddeker et al. 2017; Desianti et al. 2019; Kim et al. 2019). Most studies that have examined the estuarine gradient have tended to focus on the importance of local site conditions (e.g., Underwood 1994; Wachnicka et al. 2010; Sawai et al. 2016; Costa-Böddeker et al. 2017) and not included landscape level parameters. However, Cooper (1995) demonstrated that long-term changes in diatom communities in the Chesapeake Bay were brought on, in part, by major changes in land use and research on MPB in freshwater habitats (e.g., Pan et al. 2004; Walker and Pan 2006; Hill and Kurtenbach 2009) and previous work on tidal channel MPB in the PNW (Weilhoefer et al. 2015) has demonstrated the importance of both within site and surrounding land use in shaping the MPB community. More recently, work in Korean estuaries demonstrated the impact of land use on estuarine MPB (Kim et al. 2015).
In some estuaries, the intertidal tidal flat can constitute at large portion of the estuary area. In the PNW, the large tidal amplitude results in a large proportion of the total estuarine area being intertidal. Intertidal habitat constitutes 52% of estuarine area on average in PNW estuaries, reaching as much as 90% in some systems (Lee et al. 2006). In addition, in many areas of the PNW, upwards of 70% of intertidal wetlands have been lost (Brophy 2005). Thus, tidal flat MPB is an important primary producer in this region and understanding the environmental factors shaping MPB abundance and community structure is essential to understanding overall estuary function. Finally, while human impacts are often less in PNW estuaries compared with European estuaries and US estuaries along the Atlantic and southern Pacific coasts, nutrient dynamics in PNW estuaries are complex. Oceanic upwelling in the spring and summer months is an important source of nutrients to the lower portions of estuaries, while riverine-derived nutrients, stemming largely from nitrogen-fixing symbioses with red alder in watersheds, can be important in the upper estuary (Hickey and Banas 2003; Brown et al. 2007; Brown and Ozretich 2009).
The goal of our research was to examine the environmental factors that explain the spatial patterns of MPB biomass and community structure in a PNW estuary with complex nutrient dynamics. We examined the effects of both local environmental factors and landscape-level parameters in shaping the distribution and abundance of MPB. This information will be useful in forecasting how the MPB community will respond to stressors, such as anthropogenic nutrient loading associated with coastal population growth and sea level rise, that are predicted for this region.
Materials and Methods
This study was conducted in the Yaquina estuary located on the central Oregon coast of the USA, a typical small (15.8 km2) PNW estuary (Hickey and Banas 2003; Kentula and DeWitt 2003). Yaquina estuary formed as a drowned river mouth and contains large areas of tidal flats and wetlands. There are approximately 534 acres of intertidal tidal flat within the estuary (ORDEQ 2005). The tidal regime of Yaquina estuary is characterized as mixed semi-diurnal, with a tidal range of 1.9–2.5 m daily (NOAA 2012). Flow in the Yaquina River ranges from 1.3 m3 s−1 in late summer to 87 m3 s−1 in the winter, and water temperatures are fairly consistent throughout the year (low 10’s °C; ORDEQ 2005). During the summer months, the estuary is considered ocean-dominated, with the salt water wedge reaching 21.8 km upstream of the estuary mouth (Lemagie and Lercazk 2014). In the rainy season (November–April), the estuary is river-dominated. The dominant source of nutrients to Yaquina Bay varies seasonally; during the wet season, nitrate derived from nitrogen-fixing bacteria living in association with red alder trees in the watershed is the dominant nutrient source. During the dry season (May–October), nitrate from oceanic upwelling is the dominant nutrient source (Brown and Ozretich 2009). Twenty-two kilometers upstream of the estuary mouth, Yaquina estuary receives discharged waste water from the city of Toledo, OR (population 3600), in addition to natural nutrient inputs from the river and ocean inflow (Brown and Ozretich 2009). The watershed of Yaquina estuary is primarily forested.
Fifteen sites within the Yaquina Bay estuary were randomly selected to cover the estuary gradient on both the north and south sides of the estuary (Fig. 1). Distance between sites averaged 1.6 ± 1.6 km (range 0.38–6.3 km). In many areas of the estuary, the banks are lined with steep slopes and site selection was limited by safe access to the tidal flat. At each site, replicate samples were collected randomly within a 25-m × 25-m area. All samples from all sites were taken at similar tidal elevations to limit the confounding effects of duration of inundation and exposure to air. All samples were collected within 2 h after a daytime low tide.
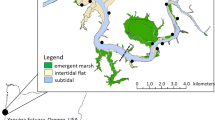
Map of the Yaquina estuary, OR, USA, showing intertidal habitat types (based on National Wetlands Inventory classification; FGDC 2013). Sampling locations are denoted by darkened circles. The dashed line denotes break between marine-dominated and riverine-dominated segments of the Yaquina estuary (from Brown et al. 2007 based on Lee et al. 2006)
Field Sampling
During the last week of June 2016, surface sediment samples were collected from the 15 sites for MPB biomass and community composition analysis. Sampling locations were devoid of macrophytes at low tide. Surface sediment samples were collected by lightly pressing a 2-cm diameter, 0.5 mm thick delimiter onto the sediment surface to create an impression outline. The top surface layer (~ 3–4 cm) of the encircled area of sediment was scraped out using a metal spatula. Three replicate samples were collected at each site and subsampled for MPB biomass and community composition analysis. All samples were collected during low tide between the hours of 10:00–13:00 to allow for maximum diel diatom migration to the sediment surface. Samples for MPB species identification were fixed with buffered formalin for a final concentration of 4%.
Salinity of the pore water was measured using a refractometer at each location where surface sediment samples were collected. At each sampling location, three sediment cores (top 2 cm saved) were collected with a modified 50-cc syringe for laboratory determination of dissolved nitrogen and phosphorus in the pore water. In addition, three sediment cores were collected with a modified 50-cm3 syringe for laboratory determination of sediment grain size.
Laboratory Analysis
MPB biomass was quantified as chlorophyll-a per gram of sediment, corrected for phaeopigments. Sediment samples were extracted in 15 ml of 90% acetone for 24 h. Samples were centrifuged and the supernatant was used for chlorophyll analysis using the equations of Lorenzen (1967).
Samples for MPB species identification were scanned under the light microscope upon returning to the laboratory. On average, greater than 95% of the living algal cells were diatom species so samples were prepared for diatom identification. Surface sediment samples were homogenized and approximately 10 g of homogenized sediment were heat-digested for 30–45 min using concentrated nitric acid. No evidence of diatom frustule damage from the digestion process was observed. Samples were repeatedly rinsed with deionized water until their pH was approximately neutral. Resultant diatom material was mounted on glass slides with Naphrax high-resolution mounting media (refractive index 1.73). Transects were scanned until at least 500 diatom valves were identified and enumerated to the species level using a Zeiss Axio Scope microscope at 1000× magnification. Diatom identification was based on the volumes of Krammer and Lange-Bertalot (1986, 1988, 1991a, 1991b, 2000). Further reference was also made to Riznyk (1973), Sundbäck (1987), Cooper (1995), Sabbe and Vyverman (1995), and Sawai and Nagumo (2003). Shannon–Weaver diversity (H′), dominance, evenness, and richness were calculated for each sampling plot at each site and the three samples from each site were averaged to produce a site mean for each metric. Taxon dominance was calculated as the relative abundance of the most common taxa at each site. Diatom taxa that could be identified to species level were classified in terms of life form (Denys 1991; Vos and de Wolf 1993) and salinity tolerance (Denys 1991; van dam et al. 1994). Based on this classification, we calculated the percentage of taxa that were classified as each life form, the percentage of total valves counted that were classified as each life form, the average relative abundance of each life form at each site, and the average abundance of each life form in the lower, middle, and upper estuary.
Sediment cores collected for pore water chemistry analysis were spun in a centrifuge at 1000×g for 20 min to extract pore water. Samples were filtered through a 0.45-μm GF/F filter to remove particulate matter. Extracted pore water samples were analyzed for soluble reactive phosphate (SRP) by the ascorbic acid method (Wetzel and Likens 1991), nitrate and nitrite (NN) by the cadmium reduction method (Clesceri et al. 1998), and ammonium nitrogen (AM) was measured by the phenol-hypochlorite method (Wetzel and Likens 1991). Sediment grain size analysis was used to determine the fraction of silt and clay (< 0.63 μm), sand (> 0.63 μm, < 2 mm), and gravel (> 2 mm) at the site. Cores were homogenized by kneading and a subsample of each core was dried for 24 h at 100 °C. Dried sediment samples were weighed and passed through sieves (#10 mesh to separate gravel from sand; #230 mesh to separate sand from silt and clay). The samples that passed through each sieve were weighed to determine the fraction in each sediment class.
The landscape characteristics in the 100-m buffer surrounding each site were evaluated using a geographic information system (GIS). Land cover was calculated using the following GIS data: Coastal Landscape Analysis and Modeling Study 1996 gradient nearest neighbor vegetation classes (Ohmann and Gregory 2002), multi-resolution landscape characteristics 2001 land cover (MRLC 2001), Scranton (2004) tidal wetland classification for Oregon coastal wetlands, and USGS 10-m digital elevation models (USGS 2012).
Data Analysis
Principle component analysis (PCA) was used to characterize the environmental variability in the study area using the dataset of four water quality, three substrate, and two land use variables. MPB biomass as chlorophyll and diatom community metrics were used as passive variables in PCA analysis to examine the associations between these variables and environmental variables. Unconstrained ordination (non-metric multi-dimensional scaling (NMDS; Bray–Curtis distance measure) was used to examine variations in diatom composition (arcsine transformed relative abundance data) at each site. The 47 taxa with a relative abundance greater than 1% at one or more sites were included in the NMDS analysis. Relationships between the diatom assemblage composition and observed environmental variables were explored using ENVFIT analysis. ENVFIT analysis overlays the environmental data matrix on the unconstrained ordination of diatom assemblages and uses permutation tests to determine the significance of the relationship between each environmental parameter and the diatom ordination (Oksanen 2010). Constrained ordination (stepwise canonical correspondence analysis (CCA) with backward and forward selection) was used to examine the variability in the diatom community structure that could be explained by measured environmental variables. Environmental variables were log or arcsine square root transformed for normality prior to all analyses. All data analyses and visualizations were performed using R-version 3.6.1 (R Development Core Team 2018), using packages “corrplot” (Wei et al. 2017), “factoextra” (Kassambara and Mundt 2020), “FactoMineR” (Husson et al. 2020), “ggplot2” (Wickham 2009), “MASS” (Ripley et al. 2018), and “vegan” (Oksanen et al. 2013).
Results
Environmental Conditions
The Yaquina estuary exhibited strong gradients in several environmental variables, including pore water salinity, sediment composition, and pore water SRP, while pore water NN and AM were highly variable among the sites (Table 1). There were no trends in buffer land use with distance from the estuary mouth. Forested land use dominated the 100-m buffer at most sites (mean: 74% ± 26%). However, there were two sites where developed buffer comprised over 50% of the 100-m buffer. The first two axes of the PCA of site environmental characteristics explained 57.1% of the total variance in environmental data, with axes 1 and 2 explaining 35.5% and 22.1% of the total variance, respectively (Fig. 2). Results of the PCA demonstrated a strong environmental gradient within Yaquina estuary related to estuary position. Salinity, sandy substrates, and SRP were highly correlated and highest at sites within the lower estuary (right side of PCA diagram). Sites in the upper estuary had finer substrates and lower SRP concentrations (left side of the ordination diagram). Land use in the 100-m buffer and nitrogen were a secondary gradient, with forested land use being correlated with pore water AM and developed land use being correlated with NN.
PCA ordination of environmental variables measured at 15 sites in Yaquina estuary, OR, USA. Solid line vectors indicate environmental variables used in PCA (AM: pore water ammonium concentration (μg L−1), clay: % clay in sediment, DEV: % developed land in the 100-m buffer, DIS: distance from estuary mouth, FOR: % forested land use in the 100-m buffer, gravel: % gravel in sediment, NN: pore water nitrate + nitrite concentration (μg L−1), SAL: pore water salinity, sand: % sand in sediment, SRP: pore water phosphate concentration (μg L−1). Dashed line vectors are supplementary variables (CHL: algal biomass (μg chlorophyll g sediment−1), DOM: % dominance by a single taxa, ES: % epipsammic taxa, H: Shannon diversity, S: species richness). Polygons enclose sites located in the same position of the estuary
MPB Biomass and Community Composition
MPB biomass was variable throughout the estuary, ranging from 3 to 60 μg chlorophyll g sediment−1 with a mean of 21.9 ± 11.4 μg chlorophyll g sediment−1 (Table 1). MPB biomass was highest at the sites closest to the estuary mouth and generally decreased as you moved up the estuary (Fig. 3). MPB biomass was strongly associated with substrate composition, being highest at sites with sandier substrates (Fig. 2). Higher MPB biomass was also associated with higher pore water salinities and SRP. The MPB community was dominated by diatoms (Bacillariophyta), with over 95% of cells at all sites being diatoms. Cyanophyta made up a small fraction of the MPB community at 4 sites (< 5%). There was no relationship between % Cyanophyta and distance to estuary mouth.
A total of 178 diatom taxa were identified from the surface sediment at the 15 tidal flat sites (Fig. 4). Of these, 104 could confidently be identified to species level. Taxa richness ranged between 21 and 46 per site, with an average of 34.1 ± 5.9 per site. Alpha diversity of diatom species (H′) averaged 2.6 ± 0.3 per site, with a maximum of 3.0. The most abundantly occurring taxa were Catenula adhaerens (Mereschkowsky) Mereschkowsky, Opephora spp. 1, Planothidium delicatulum (Kützing) Round & Bukhtiyarova, Navicula gregaria Donkin, Navicula spp. 1, Nitzschia palea (Kützing) W. Smith, Cocconeis placentula Ehrenberg, Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot, and Pseudostaurosira perminuta (Grunow) Sabbe & Vyverman. Six taxa occurred at all 15 sites (Catenula adhaerens, Opephora spp. 1, Planothidium delicatulum, Navicula gregaria, Navicula spp. 1, Nitzschia palea; Table 2, Fig. 5). However, 70% of taxa occurred at fewer than 10 sites. In general, individual taxa did not dominate the assemblage at any site (mean dominance 20.4% ± 9.8, maximum dominance 38.6%), with the number of taxa with relative abundance greater than 10% ranging between 1 and 4 per site. The majority of taxa that could be identified were highly motile, epipelic taxa (60% of taxa), while 28% were epipsammic taxa and 3.5% were tychoplanktonic. However, epipsammic taxa made 52% of the total valves counted, comprising on average 51% ± 28% of the diatoms at a site, while epipelic taxa made up 44% of total valves counted, comprising on average 43% ± 26% of the diatoms found at a site. Tychoplanktonic taxa were 3% of total valves counted (mean per site: 3% ± 3%).
The 15 most abundant diatom species (based on total number of valves counted) in Yaquina estuary, OR, USA. a Catenula adhaerens valve view, b Catenula adhaerens girdle view, c Pseudostaurosira perminuta valve view, d Pseudostaurosira perminuta girdle view, e Opephora spp. 1 valve view, f Opephora spp. 1 girdle view, g Cocconeis spp. 1 pseudoraphe valve (PRV), (h) Cocconeis spp. 1 raphe valve (RV), i Cocconeis placentula PRV, j Cocconeis disculus PRV, k Nitzschia inconspicua, l Planothidium delicatulum PRV, m Planothidium delicatulum RV, n Planothidium lanceolatum PRV, o Planothidium lanceolatum RV, p Nitzschia pellucida, q Halamphora coffeaeformis, r Navicula gregaria, s Navicula spp. 1, t Nitzschia palea, u Nitzschia frustulum
Association with Environmental Variables
The MPB diatom assemblage composition was strongly related to location in the estuary, with assemblages at lower estuary sites being distinct from middle and upper estuary sites (Fig. 6; two-dimensional solution, stress = 0.07). Sites in the lower estuary clustered on the negative end of NMDS axis 1, while sites in the upper estuary clustered at the positive end of this axis. Sites in the lower estuary were dominated by epipsammic taxa with marine and brackish preferences, including Catenula adhaerens, Opephora spp. 1, and Planothidium delicatulum. These sites were characterized by higher salinities, higher SRP concentrations, and sandier substrates. The dominant diatom taxa in middle and upper estuary sites were highly motile taxa with more brackish-fresh to fresh salinity preferences, such as Navicula gregaria, Navicula spp. 1, Nitzschia frustulum (Kützing) Grunow, and Nitzschia palea. These sites were characterized by finer substrates. Pseudostaurosira perminuta was also abundant at middle and upper estuary sites. This tychoplanktonic species can tolerate salinities from brackish to fresh (Sabbe and Vyverman 1995). The results of ENVFIT analysis showed that distance from the estuary mouth (R2 = 0.89, p = 0.001) and SRP (R2 = 0.68, p = 0.002) were the environmental variables most strongly correlated to the overall diatom assemblage in Yaquina estuary (Fig. 6). Pore water salinity (R2 = 0.46, p = 0.049), substrate sand content (R2 = 0.42, p = 0.04), and substrate clay content (R2 = 0.44, p = 0.04) were also significantly related to the diatom assemblage, while pore water nitrogen and buffer land use were not.
Results of CCA confirmed that distance from the estuary mouth had the strongest influence on MPB diatom assemblages, followed by pore water salinity and the highly intercorrelated nature of the environmental variables. The final CCA model, selected after backwards and forward selection, accounted for 35.0% of the variability in the diatom assemblage, with distance (F = 3.99, p = 0.005) and salinity (F = 1.68, p = 0.049) being the two significant predictors in the model.
Discussion
The estuarine MPB biomass and diatom community structure in Yaquina estuary respond to the strong gradients in salinity, sediment composition, and SRP found within the estuary. Sites in the lower estuary, characterized by higher salinities, SRP, and more coarse-grained substrates, had higher MPB biomass, lower diversity, and distinct diatom communities compared to middle and upper estuary sites. These findings are similar to several studies from estuaries worldwide that have pointed to the importance of sediment composition, salinity, and nutrients together for shaping tidal flat MPB community structure (e.g., Admiraal 1984; Desianti et al. 2017; Desianti et al. 2019). As these three environmental gradients are highly correlated with distance from the estuary mouth in Yaquina estuary, it is impossible to extricate their individual effects on MPB biomass and community composition. This is a familiar problem in estuarine diatom research, pointed out by Underwood et al. (1998) regarding salinity and nutrients and repeatedly confirmed by other researchers for variables including sediment composition (e.g., Kim et al. 2015; Desianti et al. 2017; Desianti et al. 2019).
MPB biomass was more variable across the Yaquina estuary than within a single site. This finding is consistent with other studies of tidal flat MPB biomass which report great variability within estuaries due to environmental gradients established by tidal forcing (Underwood 1998; Janousek 2009; Janousek et al. 2009; Semcheski et al. 2016). While light, temperature, and nutrient concentrations have all been implicated in limiting MPB production (see review in McGlathery et al. 2013), we detected a strong relationship between MPB biomass and the gradients in substrate, SRP, and salinity within the estuary. MPB biomass was greatest at sites with sandy substrates near the estuary mouth. This finding contrasts studies that have documented lower MPB biomass on sandy substrates due to the lower nutrient supply available in sandy compared to organic sediments (Underwood and Kromkamp 1999; Billerbeck et al. 2007) and higher likelihood of biomass turnover and loss due to tidal resuspension in sandy habitats (Middelburg et al. 2000; McGlathery et al. 2013). However, Cahoon et al. (1999) and Semcheski et al. (2016) reported a positive correlation between sediment grain size and MPB biomass. MPB biomass was positively related to pore water salinity, differing from work that found no relationship between MPB biomass and salinity (Janousek et al. 2009; Semcheski et al. 2016). Finally, while primary production in oceanic and estuarine systems has traditionally been thought to be nitrogen limited and studies have noted the importance of pore water nitrogen for increased MPB biomass (Nilsson et al. 1991; Underwood et al. 1998), we did not detect a correlation between MPB biomass and pore water nitrogen. Rather, pore water SRP showed a positive correlation with MPB biomass in our study. A possible explanation for the differences in the relationships between nutrients and MPB biomass detected in our study is the unique nutrient dynamics of Yaquina estuary. In most estuaries, there is a decreasing gradient in nutrient concentrations as you move towards the estuary mouth (Nedwell and Trimmer 1996; Ogilvie et al. 1997; Underwood et al. 1998). In contrast, we detected a strong gradient of increasing pore water SRP moving towards the estuary mouth and did not detect a gradient in pore water nitrogen, neither NN nor AM. While our study represents only a one-time sampling event, in a much more detailed study of seasonal patterns of water column nutrients in Yaquina estuary, Brown and Ozretich (2009) demonstrated the importance of oceanic-derived nutrients, detecting a similar pattern of decreasing phosphate concentrations moving up the estuary during the dry season (summer). This, coupled with possible increased release of phosphates from terrigenous sediments at higher salinities (Jordan et al. 2008), sets up a gradient of decreasing phosphorus with distance from the ocean. The Yaquina estuary can also be characterized as naturally high in nitrogen as it receives high nitrogen loads from both coastal upwelling in the summer and winter inputs from symbiotic nitrogen-fixing associations of red alder trees in the surrounding watershed (Naymik et al. 2005; Brown and Ozretich 2009). This abundant nitrogen supply may explain the lack of relationship between nitrogen and MPB biomass.
The Yaquina estuary tidal flat supports a diverse community of benthic microalgae, overwhelmingly dominated by diatoms. The estuarine MPB is recognized as a diverse assemblage composed of various algal groups (Sullivan and Moncreiff 1990; Pinckney and Zingmark 1993; Janousek 2009) but dominance of the MPB community by diatoms is commonly reported in the literature (e.g., Sullivan 1999; Janousek 2009; McGlathery et al. 2013; Manoylov et al. 2016). While Amspoker and McIntire (1978) also reported a low percentage of allochthonous diatoms on the tidal flats in Yaquina estuary, the predominance of living, benthic, autochthonous diatoms within our samples differs from what has been reported in some estuaries (Vos and de Wolf 1988; Costa-Böddeker et al. 2017; Desianti et al. 2019). Previous studies on tidal flat diatoms have shown that planktonic diatoms, diatoms from allochthonous sources, and a high proportion of dead taxa are often abundant in the collected tidal flat assemblages (Vos and De Wolf 1988; Bárcena and Abrantes 1998; Manoylov et al. 2016; Costa-Böddeker et al. 2017), having the potential to obscure relationships between tidal flat diatoms and surrounding environmental conditions. The diatom assemblages found at our sites appear to be predominantly resident assemblages and we detected strong relationships to environmental gradients within the estuary.
Tidal flat diatom assemblages in Yaquina estuary were dominated by benthic, pennate taxa and tolerances to salinities ranging from fresh-brackish to brackish-marine. The tidal flat diatom flora of Yaquina estuary is largely similar to that reported in previous work from Oregon and Washington estuaries (McIntire and Overton 1971; McIntire 1973; McIntire 1978; Whiting and McIntire 1985; Sawai et al. 2016). Tidal flat diatom assemblages are often dominated by pennate forms (Underwood et al. 1998; Wachnicka et al. 2010; Manoylov et al. 2016; Sawai et al. 2016; Semcheski et al. 2016). Of the taxa that could be identified to species level, 60% were classified epipelic taxa and 28% were classified as epipsammic taxa. However, epipsammic taxa made 52% of the total valves counted, while epipelic taxa made up 44%. Epipsammic taxa growing attached to sand grains with brackish to marine salinity preferences, including Catenula adhaerens, Opephora spp., and Planothidium delicatulum, overwhelmingly dominated the tidal flat assemblages at sites in the lower estuary (mean epipsammic taxa abundance in lower estuary: 84 ± 10%), reflecting the strong environmental gradient of salinity and substrate type within Yaquina estuary. These three species are common in tidal flat epipsammon assemblages in estuaries around the world, indicating a cosmopolitan distribution of these species (e.g., Sundbäck and Medlin 1986; de Jonge and van den Bergs 1987; Denys 1991; Sawai et al. 2016). Nitzschia sensu lato and Navicula sensu lato taxa were abundant in middle and upper estuary sites, constituting 46 ± 22% and 60 ± 9% on average of the diatom assemblage at these sites, respectively. These epipelic taxa are highly motile and commonly found in silty sediment like that which dominates this part of Yaquina estuary (Denys 1991; Janssen et al. 1999). While pore water nitrogen shows no relationship with distance from estuary mouth and SRP concentrations are actually lower in the middle and upper estuary, these dominant Navicula and Nitzschia taxa can all be classified as having eutrophic nutrient preferences. Again, the Yaquina estuary has high background levels of nutrients (Brown and Ozretich 2009) and the abundance of these taxa may reflect the overall nutrient enriched state of this estuary. Finally, the 15 most frequently occurring taxa are classified as euryhaline (Denys 1991), a common occurrence in intertidal habitats where salinities can vary widely on a daily basis due to tides (McKew et al. 2011; Yamamoto et al. 2017).
MPB diatom species richness and diversity in our study were similar to previous work in the Yaquina estuary (McIntire and Overton 1971; Amspoker and McIntire 1978). MPB diatom richness and diversity varies widely within the literature, with some studies reporting similar values (e.g., Underwood 1994; Wachnicka et al. 2010; Kim et al. 2015; Semcheski et al. 2016), while others report much higher values (e.g., Amspoker 1977; Oh and Koh 1995; Manoylov et al. 2016; Desianti et al. 2017; Desianti et al. 2019). A possible explanation for the lower richness and diversity in our study is that we sampled a single habitat, the tidal flat, in a single estuary, while other studies often sample multiple habitats across larger geographic areas. Estimates of richness and diversity in estuarine systems are also complicated by the fact that there is no comprehensive published flora for brackish taxa, resulting in species often being misidentified as they are forced into the existing descriptions of marine and freshwater floras. We encountered numerous taxa, mostly small, and mostly encountered in only a few samples at low abundances, that we were unable to identify to species level. Similarly, 45% of taxa in Desianti et al. (2017) could not be identified to species level. Diversity metrics were highly associated with the major environmental gradients of salinity, substrate, and SRP within Yaquina estuary (PCA), with lower species richness and diversity and higher dominance at sites in the lower estuary with higher salinities, coarser substrates, and higher SRP. While many studies have examined the relationship between overall diatom assemblage and environmental parameters, fewer have examined the relationships specifically between diversity parameters and environmental characteristics. In contrast to our findings, higher diversity values have been reported in sandy substrates, possibly due to constant sediment reworking keeping any one taxa from becoming dominant (Amspoker 1977; Vilbaste et al. 2000; Ribeiro et al. 2013). Sediment mixing in the Yaquina estuary is thought to be lower than other estuaries (Riznyk and Phinney 1972), which might help to explain why we found lower diversity values at sandier sites. Whitfield et al. (2012) developed a general model of the relationship between estuarine species diversity and salinity that predicts lowest diversity at intermediate salinities. However, findings from other studies have been mixed. Wachnicka et al. (2010) found a positive relationship between salinity and the diversity of the tidal flat diatom assemblage. And while Semcheski et al. (2016) found a positive correlation between estuarine phytoplankton diversity and salinity, no relationships were detected between salinity and MPB diversity. Finally, studies covering larger geographic areas have not detected significant relationships between MPB diatom diversity and nutrients (Wachnicka et al. 2010; Weilhoefer et al. 2015). Clearly, the diversity of estuarine MPB diatoms is influenced by a complex array of environmental factors and relationships between diversity and specific environmental factors may depend on the individual characteristics of the estuary and the strength of the environmental gradients within the estuary.
The distribution of the MPB diatom community in Yaquina estuary also responded to the strong gradients in salinity, sediment composition, and SRP found within the estuary. Sites in the lower estuary had distinct communities from middle and upper estuary sites, again making it difficult to separate out the individual effects of each of these variables on the diatom assemblage. CCA analysis confirmed the importance of distance from the estuary mouth and, to a lesser extent salinity, in accounting for the variability in the diatom assemblage. Our findings are consistent with numerous studies that demonstrated the importance of salinity in structuring the MPB diatom assemblage (Underwood et al.1999; Wachnicka et al. 2010; Costa-Böddecker et al. 2017; Desianti et al. 2019). However, not all studies on estuarine MPB have found significant relationships between salinity and the MPB community. For example, Janousek (2009), Janousek et al. (2009), and Semcheski et al. (2016) found that salinity was responsible for little of the variation in tidal flat MPB community composition. While salinity was a significant predictor of diatom assemblage structure in Yaquina estuary based on CCA, distance from the estuary mouth accounted for more explained variability in the diatom assemblage. Within estuaries, salinity at a site can be highly variable, being influenced by tides, seasons, and localized freshwater inputs. For example, Nelson and Kashima (1993) determinized that localized freshwater inputs from the marsh slope influenced tidal flat diatom assemblages in coastal Oregon. In Yaquina estuary, the steep topography surrounding the sites results in many small, localized freshets that influence one-time salinity readings taken at sites. In addition, evaporation during low tide may affect pore water salinity readings, particularly in sandy substrates where larger pore spaces result in higher evaporation rates. Thus, distance from the estuary mouth may be a better proxy for long-term site salinity and may explain why distance from the estuary mouth explained more variability in diatom assemblages than did salinity.
While not a significant predictor in the final CCA model due to high correlations with distance from the estuary mouth, ENVFIT analysis detected significant relationships between substrate type and overall diatom assemblage. As mentioned above, sites in the lower estuary are dominated by epipsammic taxa, indicating that substrate is key in determining the diatoms living at a site. While individual diatom taxa have preferences for certain substrate type, there is no consistent trend in what other studies have found regarding the influence of substrate on MPB community composition. For example, sediment grain size was an important determinant of diatom species composition in tidal environments in Oregon and Washington, USA (Whiting and McIntire 1985; Sawai et al. 2016), the Chesapeake Bay, USA (Semcheski et al. 2016), Europe (Sabbe and Vyverman 1991; Ribeiro et al. 2013), and Korea (Oh and Koh 1995). In contrast, sediment grain size was not important for determining diatom composition in mid-Atlantic coastal areas (Desianti et al. 2019) and major algal taxonomic groups in a California, USA, estuary (Janousek 2009).
We also observed a gradient of increasing pore water phosphate moving closer to the estuary mouth, while no similar gradient was observed in nitrogen. While again it is impossible to tease part the effects of salinity, substrate, and SRP on the diatom assemblage in our study, and SRP was not a significant predictor in the final CCA model, SRP was significantly correlated to the overall diatom assemblage based on ENVFIT analysis. Early work on nutrients in estuarine systems demonstrated the importance of nitrogen in shaping MPB community composition and biomass (Admiraal and Peletier 1979, 1980; Admiraal 1984; Nilsson et al. 1991; Peletier 1996). Recent studies by Desianti et al. (2017, 2019) demonstrated strong associations between MPB assemblage structure and total nitrogen but not total phosphorus. However, when strong phosphorus gradients do exist, they can be very important. For example, total phosphorus was one of the most significant variables determining tidal flat MPB composition in estuaries and mangrove forests of Vietnam (Costa-Böddecker et al. 2017). The importance of both phosphorus and nitrogen in shaping MPB community composition was demonstrated for tidal wetlands in Yaquina estuary (Weilhoefer et al. 2015) and estuaries throughout the Korean peninsula by Kim et al. (2015, 2019).
Land use in the 100-m buffer surrounding the site was not associated with MPB biomass, diatom community metrics, or overall MPB diatom community composition. While the influence of both buffer and watershed land use can be an important determinant of diatom community composition in lakes, streams, and wetlands (Pan et al. 2004; Walker and Pan 2006; Hill and Kurtenbach 2009), it is has not historically been included in the study of estuarine tidal flat MPB. We included it in this study because land use at multiple scales was a significant determinant of tidal channel diatom assemblage in Oregon tidal wetlands (Weilhoefer et al. 2015) and recent studies in Korean estuaries have demonstrated the influence of land cover on diatom assemblages (Kim et al. 2015; Kim et al. 2019). While a few sites in our study did have urban, agricultural, and industrial uses in the buffer surrounding the site, the land use immediately surrounding most of our sites was predominantly forested. Studies that have demonstrated the importance of land use (e.g., Weilhoefer et al. 2015; Kim et al. 2015; Kim et al. 2019) have covered a wider variability in land uses than our current study which might account for the relationships detected.
While the importance of salinity, nutrients, and sediment composition in structuring the MPB diatom community have been demonstrated in many studies, there are no consistent trends across estuaries in how these variables affect the MPB. These studies have varied over geographic areas and scales, species composition, length of environmental gradients, intercorrelations of environmental gradients, and field methodologies. In our study, we detected strong relationships between several environmental variables (salinity, substrate, SRP) and MPB biomass, individual species abundances, diatom community metrics, and diatom community structure in a single habitat type within a single estuary. While it is impossible to tease out the effects of salinity, substrate, and SRP in Yaquina estuary, it is clear that tidal flat diatoms are responding to several environmental factors, reflecting the unique hydrologic and nutrient regime of Yaquina estuary. Despite the many challenges of working in estuarine systems, Desianti et al. (2019) demonstrated the utility of diatoms as bioindicators across of range of estuarine habitats and our study lends support to the idea of MPB being used as indicators of environmental conditions in estuarine environments. However, there is a need for more research before generalizations can be made about the fundamental factors affecting MPB distribution and abundance and to predict how this valuable community will respond to changing environmental conditions.
References
Admiraal, W. 1976. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar. Biol. 39: 1–9.
Admiraal, W. 1977. Tolerance of estuarine benthic diatoms to high concentrations of ammonia, nitrite ion, nitrate ion and orthophosphate. Mar. Biol. 43 (4): 307–315.
Admiraal, W. 1984. The ecology of estuarine sediment inhabiting diatoms. Prog Phycol Res 3: 269–270.
Admiraal, W., and H. Peletier. 1979. Influence of organic compounds and light limitation on the growth rate of estuarine benthic diatoms. Br J Phycol 14 (3): 197–206.
Admiraal, W., and H. Peletier. 1980. Influence of seasonal variations of temperature and light on the growth rate of cultures and natural populations of intertidal diatoms. Mar. Ecol. Prog. Ser. 2: 35–43.
Amspoker, M.C. 1977. The distribution of intertidal epipsammic diatoms on Scripps Beach, La Jolla, California, USA. Botanica Marina XX: 227–232.
Amspoker, M.C., and C.D. McIntire. 1978. Distribution of intertidal diatoms associated with sediments in Yaquina estuary, Oregon. J. Phycol. 14 (4): 387–295.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.M. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81 (2): 169–193.
Bárcena, M.A., and F. Abrantes. 1998. Evidence of a high-productivity area off the coast of Málaga from studies of diatoms in surface sediments. Mar. Micropaleontol. 35 (1-2): 91–103.
Billerbeck, M., H. Røy, K. Bosselmann, and M. Huettel. 2007. Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuar. Coast. Shelf Sci. 71 (3-4): 704–716.
Brophy, L. 2005. Tidal wetland prioritization for the Siuslaw River estuary. Oregon: Prepared for Siuslaw Watershed Council.
Brotas, V., and F. Catarino. 1995. Microphytobenthos primary production of Tagus estuary intertidal flats (Portugal). Neth. J. Aquat. Ecol. 29 (3-4): 333–339.
Brown, C.A., W.G. Nelson, B.L. Boese, T.H. DeWitt, P.M. Eldridge, J.E. Kaldy, H. Lee II, J.H. Power, and D.R. Young. 2007. An approach to developing nutrient criteria for Pacific Northwest estuaries: a case study of Yaquina estuary, Oregon. USEPA Office of Research and Development, National Health and Environmental Effects Laboratory, Western Ecology Division. EPA/600/R-07/046.
Brown, C.A., and R.J. Ozretich. 2009. Coupling between the coastal ocean and Yaquina bay, Oregon: Importance of oceanic inputs relative to other nitrogen sources. Estuar. Coasts 32 (2): 219–237.
Cadée, G.C., and J. Hegeman. 1974. Primary production of the benthic microflora living on tidal flats in the Dutch Wadden Sea. Neth. J. Sea Res. 8 (2-3): 260–291.
Cahoon, L.B., J.E. Nearhoof, and C.L. Tilton. 1999. Sediment grain size effect on benthic microalgal biomass in shallow ecosystems. Estuaries 22 (3): 735–741.
Cahoon, L.B., R.S. Redman, and C.R. Tronzo. 1999. Benthic microalgal biomass in the sediments of Onslow Bay, North Carolina. Estuar. Coast. Shelf Sci. 31: 805–816.
Christianen, M.J.A., J.J. Middelburg, S.J. Holtuijsen, J. Jouta, T.J. Compton, T. Van Der Heide, T. Piersma, J.S. Sinninghe Damsté, H.W. Van Der Veer, S. Schouten, and H. Olff. 2017. Benthic primary producers are key to sustain the Wadden Sea food web: stable carbon isotope analysis at landscape scale. Ecology 98 (6): 1498–1512.
Clesceri, L.S., A.E. Greenberg, and A.D. Eaton. 1998. Standard methods for the examination of water and wastewater. Twentieth ed. Maryland: American Public Health Association.
Colijn, F., and K.S. Dijkema. 1981. Species composition of benthic diatoms and distribution of chlorophyll a on an intertidal flat in the Dutch Wadden Sea. Mar. Ecol. Prog. Ser. 4: 9–21.
Cooper, S.R. 1995. A 2,500-year history of anoxia and eutrophication in Chesapeake Bay. Estuaries 16: 617–626.
Costa-Böddeker, S., L.X. Thuyên, A. Schwarz, H.D. Huy, and A. Schwalb. 2017. Diatom assemblages in surface sediments along nutrient and salinity gradients of Thai Vai estuary and can Gio mangrove forest, southern Vietnam. Estuar. Coasts 40 (2): 479–492.
Currin, C.A., S.Y. Newell, and H.W. Paerl. 1995. The role of standing dead Spartina alterniflora and benthic microalgae in salt-marsh food webs–considerations based on multiple stable isotope analysis. Mar. Ecol. Prog. Ser. 121: 99–116.
da Silva, M.J., S. Cruz, and P. Cartaxana. 2017. Inorganic carbon availability in benthic diatom communities: photosynthesis and migration. Philos. Trans. R. Soc. B 372: 1728. https://doi.org/10.1098/rstb.2016.039820160398.
Davis, M.W., and C.D. McIntire. 1983. Effects of physical gradients on the production dynamics of sediment-associated algae. Mar. Ecol. Prog. Ser. 13: 103–114.
de Jonge, V.N., and J. van den Bergs. 1987. Experiments on the resuspension of estuarine sediments containing benthic diatoms. Estuar. Coast. Shelf Sci. 24 (6): 725–740.
Denys, L. 1991. A check-list of the diatoms in the Holocene deposits of the western Belgian coastal plain with a survey of their apparent ecological requirements. I. Introduction, ecological code and complete list. Ministère des Affaires Economiques – Service Géologique de Belgique.
Desianti, N., M.D. Enache, M. Griffiths, K. Bisku, A. Degan, M. DaSilva, D. Millemann, L. Lippincott, E. Watson, A. Gray, D. Nikitina, and M. Potapova. 2019. The potential and limitations of diatoms as environmental indicators in mid-Atlantic coastal wetlands. Estuaries Coasts 42 (6): 1440–1458. https://doi.org/10.1007/s12237-019-00603-4.
Desianti, N., M. Potapova, M. Enache, T.J. Belton, D.J. Velinsky, R. Thomas, and J. Mead. 2017. Sediment diatoms as environmental indicators in New Jersey coastal lagoons. J. Coast. Res. 78: 127–140.
FGDC (Federal Geographic Data Committee). 2013. Classification of wetlands and deepwater habitats of the United States. FGDC-STD-004-2013. Second edition. Wetlands subcommittee, Federal Geographic Data Committee and U.S. Fish and Wildlife Service, Washington, DC.
Halpern, B.S., S. Walbridge, K.A. Selkoe, and C.V. Kappel. 2008. A global map of human impact on marine ecosystems. Science 319 (5865): 948–952.
Hargrave, B.T., N.J. Prouse, G.A. Phillips, and P.A. Neame. 1983. Primary production and respiration in pelagic and benthic communities at two intertidal sites in the upper bay of Fundy. Can. J. Fish. Aquat. Sci. 40: 229–243.
Herman, P.M.J., J.J. Middelburg, J. Widdows, C.H. Lucas, and C.H.R. Heip. 2000. Stable isotopes as trophic tracers: combining field sampling and manipulative labelling of food resources for macrobenthos. Mar. Ecol. Prog. Ser. 204: 79–92.
Hickey, B.M., and N.S. Banas. 2003. Oceanography of the US Pacific northwest coastal ocean and estuaries with application to coastal ecology. Estuaries 26 (4): 1010–1031.
Hill, B.H., and J.P. Kurtenbach. 2009. Correlations of sedimentary diatoms with watershed land use and limnological conditions in northern New Jersey Lakes. Lake Reservoir Manage. 17: 105–120.
Husson, F., J. Josse, S. Le, and J. Mazet. 2020. Package ‘FactoMineR”. https://cran.r-project.org/web/packages/FactoMineR/FactoMineR.pdf
Janousek, C.N. 2009. Taxonomic composition and diversity of microphytobenthos in southern California marine wetland habitats. Wetlands 29 (1): 163–175.
Janousek, C.N., C.A. Currin, and L.A. Levin. 2009. Succession of microphytobenthos in a restored coastal wetland. Estuar. Coasts 30: 265–276.
Janssen, M., M. Hurst, E. Rhiel, and W.E. Krumbein. 1999. Vertical migration behaviour of diatom assemblages of Wadden Sea sediments (Dangast, Germany): a study using cryo-scanning electron microscopy. Int. Microbiol. 2 (2): 103–110.
Jordan, T.E., J.C. Cornwell, W.R. Boyton, and J.T. Anderson. 2008. Changes in phosphorus biogeochemistry along an estuarine salinity gradient: the iron conveyer belt. Limnol. Oceanogr. 53 (1): 172–184.
Juggins, S. 1992. Diatoms in the Thames estuary, England: ecology, paleoecology, and salinity transfer function. In Biblotheca Diatomologica band 25, ed. H. Lange-Bertalot. Berlin: J. Cramer.
Kang, C.K., Y.W. Lee, E.J. Choy, J.K. Shin, I.S. Seo, and J.S. Hong. 2006. Microphytobenthos seasonality determines growth and reproduction in intertidal bivalves. Mar. Ecol. Prog. Ser. 315: 113–127.
Kassambara, A., and F. Mundt. 2020. Package ‘factoextra’. https://cran.r-project.org/web/packages/factoextra/factoextra.pdf
Kentula, M.E., and T.H. DeWitt. 2003. Abundance of seagrass (Zostera marina L.) and macroalgae in relation to the salinity-temperature gradient in Yaquina Bay, Oregon, USA. Estuaries 26 (4): 1130–1141.
Kim, H.-K., I.-H. Cho, E.-A. Hwang, Y.-J. Kim, and B.-H. Kim. 2019. Benthic diatom communities in Korean estuaries: species appearances in relation to environmental variables. Int. J. Environ. Res. Public Health 16 (15). https://doi.org/10.3390/ijerph16152681.
Kim, H.-K., Y.-S. Kwon, Y.-J. Kim, and B.-H. Kim. 2015. Distribution of epilithic diatoms in estuaries of the Korean peninsula in relation to environmental variables. Water 7 (12): 6702–6718.
Kramer, K., and H. Lange-Bertalot. 1986. Susswasserflora von mitteleuropa: Bacillariophyceae, part 1. Naviculaceae. Heidelberg: Spektrum Akademischer Verlag.
Kramer, K., and H. Lange-Bertalot. 1988. Susswasserflora von mitteleuropa: Bacillariophyceae, part 2. Epithemiaceae, bacillariophyceae, surirellaceae. Heidelberg: Spektrum Akademischer Verlag.
Kramer, K., and H. Lange-Bertalot. 1991a. Susswasserflora von mitteleuropa: Bacillariophyceae, part 3. Centrales, fragilariaceae, eunotiaceae, achnanthaceae. Heidelberg: Spektrum Akademischer Verlag.
Kramer, K., and H. Lange-Bertalot. 1991b. Susswasserflora von mitteleuropa: Bacillariophyceae, part 4. Achnanthaceae. Heidelberg: Spektrum Akademischer Verlag.
Kramer, K., and H. Lange-Bertalot. 2000. Susswasserflora von mitteleuropa: Bacillariophyceae, part 5. English and French translation of keys. Heidelberg: Spektrum Akademischer Verlag.
Lee, H. II, C.A. Brown, B.L. Boese, and D.R. Young, eds. 2006. Proposed classification scheme for coastal receiving waters based on SAV and food web sensitivity to nutrients, volume 2: nutrient drivers, seagrass distributions, and regional classifications of Pacific Northwest estuaries. USEPA Office of Research and Development, National Health and Environmental Effects Laboratory. Internal Report.
Lemagie, E.P., and J.A. Lerczak. 2014. A comparison of bulk estuarine turnover timescales to particle tracking timescales using a model of the Yaquina Bay estuary. Estuar. Coasts 38: 1797–1814.
Longphuirt, S.N., J. Clavier, J. Grall, L. Chauvaud, F. Le Loch, I. Le Berre, J. Flye-Sainte-Marie, J. Richard, and A. Leynaert. 2007. Primary production and spatial distribution of subtidal microphytobenthos in a temperate coastal system, the bay of Brest, France. Estuar. Coast. Shelf Sci. 74 (3): 367–380.
Lorenzen, C.J. 1967. Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol. Oceanogr. 12 (2): 343–346. https://doi.org/10.4319/lo.1967.12.2.0343.
Lotze, H.K., H.S. Lenihan, B.J. Bourque, R.H. Bradbury, R.G. Cooke, M.C. Kay, S.M. Kidwell, M.X. Kirby, C.H. Peterson, and J.B.C. Jackson. 2006. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312 (5781): 1806–1809.
MacIntyre, H.L., R.J. Geider, and D.C. Miller. 1996. Microphytobenthos: the ecological role of the “secret garden” of un-investigated shallow water marine habitats. I. Distribution, abundance and primary production. Estuaries 19 (2): 186–201.
Manoylov, K.M., Y.E. France, A. Geletu, and J.N. Dominy Jr. 2016. Algal community membership of estuarine mudflats from the Savannah River, United States. J. Mar. Sci. Eng. 4 (1). https://doi.org/10.3390/jmse4010011.
McGlathery, K.J., K. Sundbäck, and P. Fong. 2013. Estuarine benthic algae. In Estuarine ecology, ed. J.W. Day Jr., B.C. Crump, W.M. Kemp, and A. Yáńez-Arancibia. Hoboken: Wiley-Blackwell.
McIntire, C.D. 1973. Diatom associations in Yaquina estuary, Oregon: a multivariate analysis. J. Phycol. 9: 254–259.
McIntire, C.D. 1978. The distribution of estuarine diatoms along environmental gradients: a canonical correlation. Estuar. Coast. Mar. Sci. 6 (5): 447–457.
McIntire C.D., and W. S. Overton. 1971. Distributional patterns in assemblages of attached diatoms from Yaquina Estuary, Oregon. Ecology 52: doi:https://doi.org/10.2307/1936024
McKew, B.A., J.D. Taylor, T.J. McGenity, and G.J.C. Underwood. 2011. Resistance and resilience of benthic biofilm communities from a temperate saltmarsh to desiccation and rewetting. ISME J 5 (1): 30–41.
Middelburg, J.J., C. Barranguet, H.T.S. Boschker, P.M.J. Herman, T. Moens, and C.H.R. Heip. 2000. The fate of intertidal microphytobenthos carbon: an in situ C-13-labeling study. Limnol. Oceanogr. 45: 224–1234.
Millennium Ecosystems Assessment. 2005a. Ecosystems and human well-being: wetlands and water synthesis. Washington D.C.: World Resources Institute.
Millennium Ecosystems Assessment. 2005b. Ecosystems and human well-being: synthesis. Washington D.C.: Island Press.
MLRC. 2001. NLCD land cover (CONSUS) 2001 data. https://www.mrlc.gov/data. Accessed 2010.
Mote, P.W., J. Abatzoglou, K.D. Dello, K. Hegewisch, and D.E. Rupp. 2019. Fourth Oregon climate assessment report. Oregon Climate Change Research Institute. occri.net/ocar4.
Naymik, J., Y. Pan, and J. Ford. 2005. Diatom assemblages as indicators of timber harvest effects in coastal Oregon streams. J. N. Am. Benthol. Soc. 24 (3): 569–584.
Nedwell, D.B., and M. Trimmer. 1996. Nitrogen fluxes through the upper estuary of the great Ouse, England: the role of the bottom sediments. Mar. Ecol. Prog. Ser. 142: 273–286.
Nelson, A.R., and K. Kashima. 1993. Diatom zonation in southern Oregon tidal marshes relative to vascular plants, foraminifera, and sea level. J. Coast. Res. 9: 673–697.
Nilsson, P., B. Jönsson, I.L. Swanberg, and K. Sundbäck. 1991. Response of a marine shallow-water sediment system to an increased load of inorganic nutrients. Mar. Ecol. Prog. Ser. 71: 275–290.
NOAA. 2012. Tide tables 2012 - west coast of north and south American including the Hawaiian islands. US Department of Commerce.
NOAA. 2013. National coastal population report: Population trends from 1970–2020. National Oceanographic and Atmospheric Administration.
Ogilvie, B., D.B. Nedwell, R.M. Harrison, A. Robinson, and A. Sage. 1997. High nitrate, muddy estuaries as nitrogen sinks: the nitrogen budget of the river Colne estuary (United Kingdom). Mar. Ecol. Prog. Ser. 150: 217–228.
Oh, S.-H., and C.-H. Koh. 1995. Distribution of diatoms in the surficial sediments of the Mangyung-Dongjin tidal flat, west coast of Korea (eastern Yellow Sea). Mar. Biol. 122 (3): 487–496.
Ohmann, J.L., and M.J. Gregory. 2002. Predictive mapping for forest composition and structure with direct gradient analysis and nearest-neighbor imputation in coastal Oregon, U.S.A. Can. J. For. Res. 32: 725–741.
Oksanen, J. 2010. Multivariate analysis of ecological communities in R: vegan tutorial. http://phylodiversity.net/azanne/csfar/images/8/85/Vegan.pdf
Oksanen, J., F.G. Blanchet, R. Kindt, P. Legendre, P.R. Minchin, R.B. O’Hara, G.L. Simpson, P. Solymos, M. Henry, H. Stevens, and H. Wagner. 2013. Package ‘Vegan’. ISBN 0-387-95457-0.
ORDEQ. 2005. Yaquina Bay. Oregon geographic response plan. State of Oregon Department of Environmental Quality.
Pan, Y., A. Herlihy, P. Kaufmann, J. Wigington, J. van Sickle, and T. Moser. 2004. Linkages among land-use, water quality, physical habitat conditions and lotic diatom assemblages: a multispatial scale assessment. Hydrobiologia 515 (1-3): 59–73.
Peletier, H. 1996. Long-term changes in intertidal estuarine diatom assemblages related to reduced input of organic waste. Mar. Ecol. Prog. Ser. 137: 265–271.
Pinckney, J., and R.G. Zingmark. 1993. Biomass and production of benthic microalgae communities in estuarine habitats. Estuaries 16 (4): 887–897.
R Core Development Team. 2018. R: a language and environment for statistical computing. R Foundation for statistical computing. Vienna: R Foundation for Statistical Computing. URL http://www.R-project.org/
Retallack, G.J., D.G. Gavin, E.B. Davis, N.D. Sheldon, J.M. Erlandson, M.H. Reed, E.A. Bestland, J.J. Roering, R.J. Carson, and R.B. Mitchells. 2016. Oregon 2100: projected climatic and ecological changes. Bulletin no. 26, Museum of Natural History, University of Oregon.
Ribeiro, L., V. Brotas, Y. Rincé, and B. Jesus. 2013. Structure and diversity of intertidal benthic diatom assemblages in contrasting shores: a case study from the Tagus estuary. J. Phycol. 49 (2): 258–270.
Ripley, B., B. Venables, D.M. Bates, K. Hornik, A. Gebhardt, and D. Firth. 2018. Package ‘MASS’. http://www.stats.ox.ac.uk/pub/MASS4/.
Riznyk, R.Z. 1973. Interstitial diatoms from two tidal flats in Yaquina estuary, Oregon, USA. Botanica Marina XVI: 113–138.
Riznyk, R. 1978. Interstitial diatoms from two tidal flats in Yaquina estuary, Oregon, USA. Botanica Marina XVI: 113–138.
Riznyk, R.Z., and H.K. Phinney. 1972. The distribution of intertidal phytopsammon in an Oregon estuary. Mar. Biol. 13 (4): 318–324.
Sabbe, K., and W. Vyverman. 1991. Distribution of benthic diatom assemblages in the Westerschelde (Zeeland, the Netherlands). Belg. J. Bot. 124: 91–101.
Sabbe, K., and W. Vyverman. 1995. Taxonomy, morphology and ecology of some widespread representatives of the diatom genus Opephora. Eur. J. Phycol. 30 (4): 235–249.
Sawai, Y., B.P. Horton, A.C. Kemp, A.D. Hawkes, T. Nagumo, and A.R. Nelson. 2016. Relationships between diatoms and tidal environments in Oregon and Washington, USA. Diatom Res 31 (1): 17–38.
Sawai, Y., and T. Nagumo. 2003. Diatoms from Alsea Bay, Oregon, USA. Diatom 19: 33–46.
Scranton, R. 2004. The application of geographic information systems for delineation and classification of tidal wetlands for resources management of Oregon’s coastal watersheds. Corvallis: Oregon State University’s Master’s Thesis.
Semcheski, M.R., T.A. Egerton, and H.G. Marshall. 2016. Composition and diversity of intertidal microphytobenthos and phytoplankton in Chesapeake Bay. Wetlands 36 (3): 483–496.
Sullivan, M. 1999. Applied diatom studies in estuaries and shallow coastal environments. In The diatoms: applications for the environmental and earth sciences, ed. E. Stoermer and J. Smol. Cambridge: Cambridge University Press.
Sullivan, M.J., and C.A. Moncreiff. 1990. Edaphic algae are an important component of salt marsh food-webs: Evidence from multiple stable isotope analyses. Mar. Ecol. Press Ser 62: 149–159.
Sundbäck, K. 1987. The epipsammic marine diatom Opephora olsenii Möller. Diatom Res 2 (2): 241–249.
Sundbäck, K., and L.K. Medlin. 1986. A light and electron microscopic study of the epipsammic diatom Catenula adhaerens Mereschkowsky. Diatom Res 1 (2): 283–290.
Thom, R.M., and R.G. Albright. 1990. Dynamics of benthic vegetation standing-stock, irradiance, and water properties in Central Puget Sound. Mar. Biol. 104 (1): 129–141.
Trobajo, R., and M.J. Sullivan. 2010. Applied diatom studies in estuaries and shallow coastal environments. In Diatoms: application for the environmental and earth sciences, ed. E.F. Stoermer and J.P. Smol, 2nd ed., 309–319. Cambridge: Cambridge University Press.
Underwood, G.J.C. 1994. Seasonal and spatial variation in epipelic diatom assemblages in the Severn estuary. Diatom Res 9 (2): 451–472.
Underwood, G.J.C., and J.C. Kromkamp. 1999. Primary production by phytoplankton and microphytobenthos in estuaries. Adv. Ecol. Res. 29: 93–153.
Underwood, G., J. Phillips, and K. Saunders. 1998. Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur. J. Phycol. 32: 173–183.
USGS. 2012. National elevation dataset – NAVD88 meters – 1/3rd-arc-second (approx. 10m). https://gisdata.nd.gov/Metadata/ISO/html/metadata_DEM_NED_10m.html#ID0EKNA. Accessed 2012.
Valiela, I. 1995. Marine ecological processes. New York: Springer-Verlag.
van Dam, H., A. Mertens, and J.A. Sinkeldam. 1994. A coded check list and ecological indicator values of freshwater diatoms from the Netherlands. Aquat. Ecol. 28: 117–133.
van Oevelen, D., K. Soetaert, J.J. Middelburg, P.M.J. Herman, L. Moodley, I. Hamels, T. Moens, and C.H.R. Heip. 2006. Carbon flows through a benthic food web: Integrating biomass, isotope and tracer data. J. Mar. Res. 64 (3): 453–482.
Vilbaste, S., K. Sundbäck, C. Nilsson, and J. Truu. 2000. Distribution of benthic diatoms in the littoral zone of the Gulf of Riga, the Baltic Sea. Eur. J. Phycol. 35 (4): 373–385.
Vos, P.C., and H. de Wolf. 1988. Methodological aspects of paleo-ecological diatom research in coastal areas of the Netherlands. Geol. Mijnb. 67: 31–40.
Vos, P.C., and H. de Wolf. 1993. Diatoms as a tool for reconstructing sedimentary environments in coastal wetlands, methodological aspects. Hydrobiologia 269–270: 285–296.
Wachnicka, A., E. Gaiser, L. Collins, T. Frankovich, and J. Boyer. 2010. Distribution of diatoms and development of diatom-based models for inferring salinity and nutrient concentrations in Florida bay and adjacent coastal wetlands of South Florida (USA). Estuar. Coasts 33 (5): 1080–1098.
Walker, C.E., and Y. Pan. 2006. Using diatoms to assess urban stream condition. Hydrobiologia 561 (1): 179–189.
Wei, T., V. Simko, M. Levy, Y. Xie, Y. Jan, and J. Zemla. 2017. Package ‘corrplot. https://cran.r-project.org/web/packages/corrplot/corrplot.pdf
Weilhoefer, C.L., W.G. Nelson, and P. Clinton. 2015. Tidal channel diatom assemblages reflect within wetland environmental conditions and land use at multiple scales. Estuar. Coasts 38 (2): 534–545.
Wetzel, R.G., and G.E. Likens. 1991. Limnological analysis. New York: Springer-Verlag.
Whitfield, A.K., M. Elliott, A. Basset, S.J.M. Blaber, and R.J. West. 2012. Paradigms in estuarine ecology – a review of the Remane diagram with suggested revised models for estuaries. Estuar. Coast. Shelf Sci. 97: 78–90.
Whiting, M.C., and C.D. McIntire. 1985. An investigation of distributional patterns in the diatom flora of Netarts Bay, Oregon, by correspondence analysis. J. Phycol. 21: 655–661.
Wickham, H. 2009. ggplot2 elegant graphics for data analysis. New York: Springer. https://doi.org/10.1007/978-0-387-98141-3.
Worm, B., E.B. Barbier, N. Beaumont, J.E. Duffy, C. Folke, B.S. Halpern, J.B.C. Jackson, H.K. Lotze, F. Micheli, S.R. Palumbi, E. Sala, K.A. Selkoe, J.J. Stachowicz, and R. Watson. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science 314 (5800): 787–790.
Yamamoto, M., T. Chiba, and A. Tuji. 2017. Salinity responses of benthic diatoms inhabiting tidal flats. Diatom Res 32 (3): 243–250.
Funding
This research was funded in part by a grant from the M. J. Murdock Charitable Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Just Cebrian
Rights and permissions
About this article
Cite this article
Weilhoefer, C.L., Matteucci, C.N. & Turner, F. Multiple Estuarine Gradients Influencing Tidal Flat Benthic Algal Biomass and Community Structure in the Yaquina Estuary, OR, USA. Estuaries and Coasts 44, 1392–1407 (2021). https://doi.org/10.1007/s12237-020-00854-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00854-6