Abstract
The effects of foliar application of proline (20 mM) on growth, physio-biochemical, and yield parameters were assessed in two Brassica juncea (L.) Czern & Coss cultivars, namely, Varuna and RH-30, at different levels (2.8, 4.2, or 5.6 dsm−1) of NaCl in soil. At 29 days after sowing (DAS), plants were sprayed with either 20 mM proline or water in the presence or absence of NaCl stress. The NaCl negatively affected parameters related to growth, photosynthesis, and yield in both varieties but more in RH-30 than in Varuna. Exogenous application of proline counteracted the effects of salt stress in Varuna only, by increasing the antioxidative capacity of the plants. Moreover, proline was not effective in alleviating the detrimental effects of higher salt concentrations on the studied parameters. Proline application to unstressed plants increased growth, photosynthesis, and yield parameters in both varieties; however, the effects were more prominent in Varuna than in RH-30.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In arid and semi-arid regions, salt stress adversely affects physiological and biochemical processes such as photosynthesis, ion regulation, and water relations that ultimately lead to the reduction in yields, posing serious threats to agriculture (Ashraf 2004; Ashraf et al. 2008). Moreover, salinity stress decreases the activity of carbonic anhydrase (CA) enzyme which is important in plant carbon acquisition (Badger 2003). This enzyme catalyses the dehydration of HCO3− and provides a constant supply of CO2 to Rubisco in the chloroplast (Majeau and Coleman 1994). Plants respond to salt stress in two phases, a rapid osmotic phase that inhibits growth of young leaves and a slower ionic phase that accelerates senescence of mature leaves.
Temperature increase due to global climate change will intensify the effects of soil salinity in the near future (Huang et al. 2013). Therefore, it is imperative to identify and cultivate salt-tolerant plants. Many plants have evolved mechanisms to adapt to saline environments; one such mechanism involves osmotic adjustment for plant cellular homeostasis in saline conditions (Chen and Jiang 2009). Plants undergo osmotic adjustments under stress by excluding Na+ and Cl− ions which cause ion toxicity and accumulating K+ and Ca2+ that enable plants to withstand salt stress (Sahu et al. 2010). Moreover, plants also accumulate several compatible solutes in the cytosol such as polyols, betaine, trehalsose, ectoine, and proline (Hasegawa et al. 2000). Among these, proline has been extensively researched for osmoprotection against stress. Proline is a critical osmoprotectant that safeguards plants against oxidative stress (Ashraf and Foolad 2007). Proline is an essential amino acid which is ubiquitous in all plants, where it serves multiple metabolic roles (Szabados and Savoure 2009). It acts as a plant growth regulator by activating various signaling processes (Yang et al. 2009).
It is known that plants increase their proline levels with increase in salinity stress. When plants are relieved from the stress, the accumulated proline is metabolized to produce reducing agents which support mitochondrial oxidative phosphorylation and generation of ATP, for recovery and repair of stress-induced changes (Hare et al. 1998). Exogenous proline application as a foliar spray has been demonstrated to decrease the detrimental effects of various abiotic stresses in plants (Ali et al. 2007). Exogenous application of proline mitigated the negative effects of salt stress on plant growth (Nounjan et al. 2012), improved gas exchange parameters (Wani et al. 2012), up-regulated stress protective protein expression (Khedr et al. 2003), reduced lipid peroxidation (Okuma et al. 2004), and increased activities of antioxidant enzymes (Wani et al. 2012). With the above facts in mind, the present work was designed to determine whether foliar application of proline mitigates the toxicity generated by salt stress in mustard plants.
Materials and methods
Seeds of Brassica juncea (L.) Czern & Coss cv. Varuna and RH-30 were surface-sterilized by 0.01 % mercuric chloride solution and washed two to three times with double-distilled water (DDW) prior to sowing. The seeds were sown in earthen pots (25 × 25 cm) containing soil amended with different levels (2.8, 4.2, or 5.6 dsm−1) of NaCl. Pots were kept in the net house under natural conditions. Three plants were maintained per pot, and each treatment had five replicates. The experiment used a simple randomized block design.
Foliage was sprayed with DDW or proline (20 mM) at 29 days after sowing (DAS). The sprayer was adjusted such that it pumped out 1 mL of either liquid in one spray. The required number of plants was sampled at 60 DAS to assess growth and physiology. The remaining plants were harvested at maturity (about 120 DAS) to study yield characteristics.
Growth parameters
At 60 DAS, plants were removed along with soil and dipped in water to dislodge adhering soil particles without injuring the roots. The lengths of the root and shoot were measured on a meter scale. The roots and shoots were weighed separately to record fresh mass and were then oven-dried at 80 °C for 72 h. The samples were weighed again to record respective dry mass. Leaf area was determined by tracing the outline of the leaf on graph paper and counting the squares covered.
Electrolyte leakage and leaf water potential
The method described by Sullivan and Ross (1979) was followed to measure electrolyte leakage in leaves. The leaf water potential (LWP) was measured using a Psypro® water potential system (Wescor Inc., USA).
CA activity
CA activity was measured by the method of Dwivedi and Randhawa (1974).
SPAD chlorophyll value and photosynthetic attributes
An SPAD chlorophyll meter (Minolta 502) was used to assess the SPAD values of chlorophyll in intact leaves. The photosynthetic attributes [net photosynthetic rate (P N ), stomatal conductance (g s ), internal CO2 concentration (C i ), and transpiration rate (E)] were measured using a portable photosynthetic system (LICOR-6400, Lincoln, NE, USA). These measurements were recorded on the uppermost fully expanded leaf of the main branch between 11:00 and 13:00 h under bright sunlight. Atmospheric conditions during measurements were photosynthetically active radiation 1016 ± 61 mol m−2 s−1, relative humidity 60 ± 3 %, atmospheric temperature 22 ± 1 °C, and atmospheric CO2 360 μmol mol−1.
Maximum quantum yield of PSII
The maximum quantum yield of PSII (Fv/Fm) was measured on the adaxial surface of the intact leaf using the LICOR-6400 photosynthesis system. Prior to measurements, plants were left for 30 min in the dark at room temperature. The chlorophyll molecules were excited for 10 s by actinic light with a photon flux density of 40 μmol m−2 s−1.
Activity of antioxidant enzymes
Fresh leaves (0.5 g) were homogenized with 5 mL of 50 mM phosphate buffer (pH 7.0) containing 1 % polyvinylpyrrolidone (PVP). The homogenate was centrifuged at 10,000×g for 10 min. The supernatant was collected and used as a source for enzyme assays. The entire extraction process was carried out at 4 °C.
The activities of peroxidase (POX) and catalase (CAT) were assayed by the method of Chance and Maehly (1956), while the activity of superoxide dismutase (SOD) was assayed by the method of Beauchamp and Fridovich (1971).
Proline content
Proline content in fresh leaves was determined by the method of Bates et al. (1973).
Statistical analysis
Treatment means were compared by analysis of variance using SPSS® (SPSS version 17; Chicago, USA). Least significant difference (LSD) was calculated at the 5 % level of probability. Standard error between the replicates was calculated. Duncan’s multiple range test was used to the data to separate the means.
Experimental results
Growth parameters
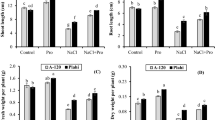
Foliar application of proline significantly (p < 0.05) increased growth parameters (length, fresh mass and dry mass of shoot and root, and leaf area) both in Varuna and RH-30 (Figs. 1 and 2a). Shoot length increased by 45 and 38 %, root length by 33 and 28 %, shoot fresh mass by 43 and 35 %, root fresh mass by 34 and 27 %, shoot dry mass by 49 and 40 %, root dry mass by 41 and 32 %, and leaf area by 23 and 18 % in Varuna and RH-30, respectively, over the control plants. However, plants grown in the NaCl-amended soil exhibited a sharp decrease in growth; variety RH-30 was more vulnerable than Varuna. The application of proline as a follow-up treatment to the stressed plants partially neutralized the damaging effects of the salt (Fig. 1).
Effect of proline (20 mM) as foliar spray and/or soil-applied sodium chloride (NaCl; 2.8, 4.2, or 5.6 dsm−1) on length (a, b), fresh mass (c, d), and dry mass (e, f) of shoot and root in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern & Coss. The letters A–H and A / –H / above the bars denote the statistical significance (Duncan’s multiple range test) of the differences between individual treatments (only those marked with different letters differ significantly at p < 0.05)
Effect of proline (20 mM) as foliar spray and/or soil-applied sodium chloride (NaCl; 2.8, 4.2, or 5.6 dsm−1) on leaf area (a), electrolyte leakage (b), leaf water potential (c), and carbonic anhydrase (CA) activity (d) in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern & Coss. The letters A–H and A / –G / above the bars denote the statistical significance (Duncan’s multiple range test) of the differences between individual treatments (only those marked with different letters differ significantly at p < 0.05)
Electrolyte leakage and leaf water potential
The applied NaCl significantly (p < 0.05) increased electrolyte leakage in both varieties. The 5.6 dsm−1 rate was most damaging; i.e., electrolyte leakage increased by 20 and 25 % in Varuna and RH-30, respectively, over the control plants at 60 DAS (Fig. 2b). However, the proline spray significantly decreased electrolyte leakage at 60 DAS as compared to the unsprayed control plants. Moreover, the follow-up treatment with proline spray to stressed plants completely neutralized the impact of the lowest concentration (2.8 dsm−1) of NaCl on leakage; this effect was more pronounced in Varuna than in RH-30.
Foliar application of proline increased LWP in both varieties at 60 DAS (Fig. 2c). The proline treatment increased LWP by 17 and 11 % in Varuna and RH-30, respectively, over the control. Soil-applied NaCl caused significant reduction in LWP, which was more pronounced in RH-30 than Varuna. However, proline applied as a follow-up treatment completely neutralized the LWP reduction caused by the lowest concentration (2.8 dsm−1) of NaCl.
CA activity
The different levels of NaCl significantly (p < 0.05) decreased CA activity in leaves of Varuna and RH-30 in a concentration-dependent manner at 60 DAS (Fig. 2d). However, proline application to non-stressed plants enhanced CA activity significantly by 17 and 10 % in Varuna and RH-30, respectively, over the control. Moreover, the damaging effects of the lowest concentration (2.8 dsm−1) of NaCl were completely neutralized by the follow-up action of proline in Varuna and partially in RH-30.
SPAD chlorophyll
Application of proline enhanced SPAD chlorophyll values in both varieties (Fig. 3a). However, the presence of NaCl in the soil significantly (p < 0.05) decreased SPAD chlorophyll values in a concentration-dependent manner. The 5.6-dsm−1 NaCl rate decreased SPAD chlorophyll by 24 and 31 % in Varuna and RH-30, respectively, compared with the control. However, proline as a follow-up treatment to NaCl-stressed plants neutralized the toxic effects of the lowest NaCl concentration (2.8 dsm−1).
Effect of proline (20 mM) as foliar spray and/or soil-applied sodium chloride (NaCl; 2.8, 4.2, or 5.6 dsm−1) on SPAD chlorophyll (a), net photosynthetic rate, P N (b); stomatal conductance, g s (c); internal CO2 concentration, C i (d); transpiration rate, E (e); and maximum quantum yield of PSII, Fv/Fm (f) in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern & Coss. The letters A–H and A / –H / above the bars denote the statistical significance (Duncan’s multiple range test) of the differences between individual treatments (only those marked with different letters differ significantly at p < 0.05)
Photosynthetic attributes
Proline foliar spray significantly (p < 0.05) increased the P N and related attributes, i.e., g s , C i, and E in both varieties (Varuna and RH-30) at 60 DAS (Fig. 3b–e). The P N increased by 25 and 18 %, g s increased by 40 and 36 %, C i increased by 17 and 10 %, and E increased by 24 and 14 % in Varuna and RH-30, respectively, over the control. NaCl in the soil resulted in a significant (p < 0.05) decrease in P N and its related attributes in both varieties. These effects were more pronounced in RH-30 than Varuna. Proline application to the stressed plants completely neutralized the damaging effects of the lowest NaCl concentration (2.8 dsm−1) in Varuna at 60 DAS.
Maximum quantum yield of PSII (Fv/Fm)
Proline application increased Fv/Fm values in both varieties (Varuna and RH-30) at 60 DAS (Fig. 3f). The increases in Fv/Fm values were 9 and 6 % in Varuna and RH-30, respectively, over the control. Soil-applied NaCl significantly (p < 0.05) decreased Fv/Fm values. Proline application to the salt-stressed plants completely overcame the toxic effects generated by the lowest NaCl concentration (2.8 dsm−1) in Varuna at 60 DAS. The variety RH-30 was less responsive to the proline application than was the Varuna.
Activities of antioxidant enzymes
Proline application and/or different concentrations of soil NaCl resulted in a significant (p < 0.05) increase in activities of the antioxidant enzymes (CAT, POX, and SOD) over the control (Fig. 4a–c). The highest NaCl concentration (5.6 dsm−1) with proline application generated maximal increases in CAT (41 and 23 %), POX (67 and 54 %), and SOD (74 and 50 %) in Varuna and RH-30, respectively, over the control. Of the two varieties, RH-30 expressed lower activity of antioxidant enzymes than did Varuna.
Effect of proline (20 mM) as foliar spray and/or soil-applied sodium chloride (NaCl; 2.8, 4.2, or 5.6 dsm−1) on activities of catalase, CAT (a); peroxidase, POX (b); superoxide dismutase, SOD (c); and proline content (d) in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern & Coss. The letters A–H and A / –H / above the bars denote the statistical significance (Duncan’s multiple range test) of the differences between individual treatments (only those marked with different letters differ significantly at p < 0.05)
Proline content
Foliar proline application significantly (p < 0.05) increased proline content by 17 and 13 % in Varuna and RH-30, respectively, compared with the control at 60 DAS (Fig. 4d). Moreover, soil-applied NaCl increased proline content and the increase was proportional to soil NaCl concentration. Furthermore, exogenous proline application to the stressed plants imparted an additive effect. The maximum increase in proline content was recorded in plants treated with the highest NaCl concentration and sprayed with proline; this effect occurred for both varieties.
Yield characteristics per plant
At the time of harvest, plants grown in the NaCl-amended soil experienced a sharp decrease in yield characteristics (number of pods per plant, number of seeds per pod, 100 seed mass, and seed yield) in both Varuna and RH-30 (Fig. 5a–d). The decrease was in proportion to degree of NaCl stress. However, the foliar proline application to stress-free plants significantly (p < 0.05) increased number of pods per plant (24 and 21 %) and seed yield (30 and 25 %) in Varuna and RH-30, respectively, over the control plants. Moreover, proline application to stressed plants partially neutralized the harmful effects of NaCl (2.8 dsm−1) in Varuna. The negative effects of the other NaCl concentrations (4.2 or 5.6 dsm−1) were not overcome by the proline spray.
Effect of proline (20 mM) as foliar spray and/or soil-applied sodium chloride (NaCl; 2.8, 4.2, or 5.6 dsm−1) on pods plant−1 (a), seeds pod−1 (b), 100 seed mass (c), and seed yield plant−1 (d) in two varieties (Varuna and RH-30) of Brassica juncea (L.) Czern & Coss at harvest (120 DAS). The letters A–G and A / –G / above the bars denote the statistical significance (Duncan’s multiple range test) of the differences between individual treatments (only those marked with different letters differ significantly at p < 0.05)
Discussion
Salt stress in soil decreased growth and production of mustard plants by impacting specific physio-biochemical characteristics (Wani et al. 2013). The excess salt present in the soil decreased the activity of carbonic anhydrase in both varieties in a concentration-dependent manner (Fig. 2d) due to the negative impact on gene expression of CA (Liu et al. 2012). Similar results have been reported by others (Wani et al. 2013; Liu et al. 2012; Hayat et al. 2011). As discussed earlier, CA plays a key role in enabling a constant supply of CO2 to Rubisco. The assimilation of carbon dioxide and the Calvin cycle are among the first processes adversely affected by the stress, and a decrease in CA activity will therefore hinder carbon availability for photosynthesis. Moreover, the inhibition of photosynthetic oxygen evolution may contribute to CA activity that caused carbon availability decrease as inhibition of photosynthetic oxygen evolution inhibition indicates inhibition of photosynthetic carbon fixation (Liu et al. 2012). However, the exogenous application of proline to stressed or stress-free plants improved the activity of the enzyme. It is known that proline interferes with the side chains of constituent amino acids of the proteins (enzymes) which govern their 3D structure, thus playing a protective role (Paleg et al. 1981). This thereby increases the activity of enzymes. A similar type of interaction might be taking place between CA and proline to enhance the activity of this enzyme.
The plants under salt stress lost a significant level of leaf chlorophyll (SPAD value) (Hayat et al. 2011; Wani et al. 2013; Akbari Ghogdi et al. 2012; Heidari 2012; Fig. 3a) in a concentration- and variety-dependent manner. It is possible that salinity either inhibits its synthesis or accelerates the degradation of chlorophyll molecules (Iyengar and Reddy 1996). However, the harmful effects of NaCl were overcome in the plants sprayed with proline. Being membrane-bound, the stability of chlorophyll molecules depends on membrane integrity which has been possibly maintained in our study by proline application, as it acts as a membrane stabilizer (Ashraf and Foolad 2007). These studies are in conformity with those for other crops (Ahmed et al. 2010; Ahmed et al. 2011b; Aggarwal et al. 2011).
Accumulation of ABA during salt stress causes closure of stomata (Yang and Lu 2005), which results in a decrease in partial pressure of CO2 in the stroma (Iyengar and Reddy 1996) that becomes the direct cause for the decrease in the g s , C i , and E as observed in the present study (Fig. 3c–e). These factors, in combination, lead to the observed decrease in the P N (Fig. 3b), as it has already been shown that P N is positively correlated with g s and C i (Lu et al. 2009). The loss in SPAD chlorophyll values (Fig. 3a) and CA activity (Fig. 2d) are other reasons to justify the decline of P N in the stressed plants. These results get further support from Wu et al. (2012), Wang et al. (2010), Ahmad et al. (2012), and Wani et al. (2013). The recovery in photosynthetic attributes in the stressed plants could be attained to some extent by exposing them to proline as a follow-up treatment (Fig. 3b–e). Photosynthesis depends on stomatal movement and mesophyll cell metabolism (proteins associated with PSI, PSII, and chlorophyll) (Athar and Ashraf 2005). From the present work, it can be therefore inferred that proline application causes an increase in stomatal conductance by maintaining appropriate cellular turgor (Kamran et al. 2009), thereby facilitating sub-stomatal accumulation and assimilation of CO2 at a higher rate. These observations suggest that photosynthetic enhancement primarily corresponds to increased stomatal conductance with a higher CO2 diffusion rate within the leaves to activate P N . Ahmed et al. (2010), working with young Olea europaea and Wani et al. (2012) with B. juncea plants proposed similar inferences. Moreover, higher chlorophyll contents (Fig. 3a) and CA activity (Fig. 2d) under exogenous proline application would be expected to result in higher P N . Beyond these findings, proline also improved cell water relations, i.e., leaf water potential (Fig. 2c) and membrane structure and stability (Slathia et al. 2012; Yan et al. 2011) in order to decrease electrolyte leakage (Fig. 2b), which would have been helpful in maintaining normal cellular metabolism.
The suppression of PSII activity resulted in decrease of photochemical efficiency under salt stress (Fig. 3f; Mehta et al. 2010). NaCl damages the PSII electron transport chain (Megdiche et al. 2008) where it blocks the transfer of electrons from the primary acceptor plastoquinone (QA) to the secondary acceptor plastoquinone (QB) at the acceptor side of PSII, which leads to the decrease in Fv/Fm values (Mehta et al. 2010; Shu et al. 2012). Our results receive further support from Kanwal et al. (2011), Wu et al. (2012), and Wani et al. (2013). The spray of proline to the stressed/stress-free plants improved the values of Fv/Fm (Fig. 3f). The similar results were also reported by Oukarroum et al. (2012), Moustakas et al. (2011), and Yan et al. (2011), who cultured plants under various types of stresses.
Under normal conditions, the reactive oxygen species (ROS) are generated at a very slow rate and an appropriate balance is maintained between their production and quenching. However, various environmental stresses disturb this balance and give rise to rapid increases in intra- and inter-cellular ROS levels (Sharma et al. 2010), which may induce oxidative damage to lipids, proteins, and nucleic acids (Sharma et al. 2012). In order to avoid this oxidative damage, plants raise the level of enzymatic (such as CAT, POX, and SOD) and non-enzymatic (such as proline) antioxidative components (Fig. 4a–c; Sharma et al. 2010) to scavenge the ROS. SOD, a metalloenzyme, is important in plant stress tolerance and provides the first line of defense against the toxic effects of ROS generated by the stress. These SODs remove the superoxide (O2 −) radical by catalyzing its dismutation, one O2 − being reduced to hydrogen peroxide (H2O2) and another oxidized to an O2 molecule. Removal of the O2 − radical decreases the risk of hydroxyl (OH•) radical formation via the Haber-Weiss reaction.
Catalase is important in the removal of H2O2 generated in peroxisomes by oxidases involved in β-oxidation of fatty acids, photorespiration, and purine catabolism. The increase in the antioxidant system in our study is further corroborated by other authors (Noreen et al. 2009; Ahmad et al. 2012; Wani et al. 2013). Moreover, salt-induced increase in proline content (Fig. 4d) could have been due to increased rate of protein hydrolysis (Irigoyen et al. 1992), as protein synthetic machinery is diverted toward proline accumulation (Claussen 2005). Secondly, an enhanced level of proline could be due to its slower rate of degradation (Kiyosue et al. 1996). Of the two cultivars tested, Varuna possessed both higher proline content and activities of CAT, POX, and SOD enzymes than did RH-30. Such responses as a function of salt tolerance have been reported earlier, in which salt-tolerant varieties possessed better antioxidative defense systems (both enzymatic and non-enzymatic components) than did salt-sensitive varieties (Sabir et al. 2011; Hayat et al. 2011). In the present study, we noted that treatment of stress-free and stressed plants with proline improved antioxidant enzyme activity and increased proline content (Fig. 4a–d). It is known that proline acts as a compatible solute, osmoprotectant, and hydroxyl radical scavenger (Ashraf and Foolad 2007; Hayat et al. 2012); therefore, the increases in activities of SOD, CAT, and POX under salinity stress in the presence of exogenous proline are expected. These results receive further support from Khedr et al (2003) who reported salinity-induced increases in CAT and POX activities in the presence of proline. Moreover, exogenous proline application suppresses H2O2 accumulation accompanied by an increase in CAT activity under salt stress (Islam et al. 2009). Being a membrane stabilizer, proline application results in its rapid uptake coupled with de novo synthesis (Zhu et al. 1990; Santos et al. 1996), thereby increasing the level of proline (Fig. 4d). Proline action is carried over through its involvement at transcription and/or translation level (Cuin and Shabala 2007; Ashraf and Foolad 2007). Furthermore, higher proline content improves water uptake (Jain et al. 2001), thereby maintaining leaf water potential (Fig. 2c). These findings suggest that the exogenous proline application improved salt tolerance in mustard plants by enhancing the activities of antioxidant enzymes under salt stress. Moreover, the increases in SOD, CAT, and POX activities and proline accumulation in leaves of saline-stressed plants strengthen the hypothesis of Khedr et al. (2003) that suggests a positive relationship among the antioxidant enzymes and proline accumulation under salt stress.
The mustard plants exposed to NaCl experienced reduction in growth characteristics including decreases in length, fresh and dry mass of shoots and roots, and leaf area (Figs. 1 and 2a). Salt stress results in decrease in division and elongation of cells (Pitann et al. 2009), mainly due to alterations in nutrient uptake, ROS accumulation (Ashraf 2009), inhibition of the activities of cytoplasmic enzymes, turgor loss (Pitann et al. 2009), and hormonal imbalance (Iqbal and Ashraf 2013), which impair plant growth and biomass production. Similar impacts of salt stress on the growth of B. juncea (Wani et al. 2013), Solanum lycopersicum (Hayat et al. 2010), Helianthus annuus (Akram and Ashraf 2011), Abelmoschus esculentus (Saleem et al. 2011), and Panicum miliaceum (Sabir et al. 2011) have been reported. However, the adverse effects generated by salt stress can, to some extent, be overcome by the application of proline as a follow-up treatment to salt-stressed plants. The increase in proline content (Fig. 4d) following its application to foliage protects enzymes (Khedr et al. 2003) and the 3-D structure of proteins (Paleg et al. 1981), cell organelles, and membranes by checking lipid peroxidation (Okuma et al. 2004) and facilitates the availability of energy for plant growth and survival, thereby helping to overcome stress (Ashraf and Foolad 2007). Therefore, a higher proline content acts as an osmoregulator to overcome the impact of salt stress and improves plant growth (Figs. 1 and 2a; Yancey 1994). Deivanai et al. (2011) and Shahbaz et al. (2013) reported higher proline content associated with improved growth in rice and eggplant.
During salt stress, the observed decrease in plant growth, slower rate of photosynthesis (Chen et al. 2009), and unfavorable nature of the conducting pathway where the leaves start behaving as sinks rather than source (Arbona et al. 2005) result in the loss of yield parameters at harvest (Fig. 5a–d). Varuna expressed slight resistance to salt stress as compared to RH-30. However, proline applied to foliage improved yield characteristics both in stressed and stress-free plants. The foliar spray of proline improved almost all growth parameters both in the presence or absence of the salt stress. However, more study is needed at the molecular level to disclose the cross talk between proline with other phytohormones in providing tolerance against stress. Of the two cultivars, Varuna was found more tolerant to salt stress. This varied growth response of the two mustard varieties could possibly be due to differential regulation of the processes related to growth at their genetic, biochemical, and physiological levels.
Conclusion
From the present study, it is concluded that salt stress occurring in soil adversely affected growth, physio-biochemical characteristics, and ultimately yield of both varieties of B. juncea (L.) Czern & Coss. Salinity stress induced the increase in antioxidant enzyme activity and proline content in both varieties. Variety RH-30 was more prone to salinity stress than was Varuna. In the absence of salt stress, foliar-applied proline improved growth and physio-biochemical characteristics which led to an increase in yield at harvest in both varieties. Moreover, in the Varuna variety, proline application mitigated the negative impact of the lowest concentration of NaCl. Overall, proline was not highly effective in alleviating the undesirable effects of higher degrees of salt stress in either variety.
References
Aggarwal M, Sharma S, Kaur N, Pathania D, Bhandhari K, Kaushal N, Kaur R, Singh K, Srivastava A, Nayyar H (2011) Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol Trace Elem Res 140:354–367
Ahmad P, Hakeem KUR, Kumar A, Ashraf M, Akram NA (2012) Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr J Biotechnol 11:2694–2703
Ahmed CB, Magdich S, Rouina BB, Sensoy S, Boukhris M, Abdullah FB (2011) Exogenous proline effects on water relations and ions contents in leaves and roots of young olive. Amino Acids 40:565–573
Ahmed CB, Rouina BB, Sensoy S, Boukhriss M, Abdullah FB (2010) Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J Agric Food Chem 58:4216–4222
Akbari Ghogdi E, Izadi-Darbandi A, Borzouei A (2012) Effects of salinity on some physiological traits in wheat (Triticum aestivum L.) cultivars. Ind J Sci Technol 5:1901–1906
Akram NA, Ashraf M (2011) Pattern of accumulation of inorganic elements in sunflower (Helianthus annuus L.) plants subjected to salt stress and exogenous application of 5-aminolevulinic acid. Pak J Bot 43:521–530
Ali Q, Ashraf M, Athar HUR (2007) Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak J Bot 39:1133–1144
Arbona V, Marco AJ, Ijlesias DJ, Lopez-Climent MF, Talon M, Gómez-Coudenas A (2005) Carbohydrate depletion in roots and leavers of salt stressed potted Citrus clemtina L. Plant Growth Regul 46:153–160
Ashraf M (2004) Some important physiological selection criteria for salt tolerance in plants. Flora 199:361–376
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93
Ashraf M, Athar HR, Harris PJC, Kwon TR (2008) Some prospective strategies for improving crop salt tolerance. Adv Agron 97:45–110
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Athar HR, Ashraf M (2005) Photosynthesis under drought stress. In: Pessarakli M (ed) Handbook of photosynthesis. CRC Press, Taylor and Francis Group, New York, pp 793–804
Badger M (2003) The roles of carbonic anhydrases in photosynthetic CO2 concentrating mechanisms. Photosynth Res 77:83–94
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Sci 39:205–207
Beauchamp LO, Fridovich I (1971) Superoxide dismutase improved assays and assay applicable to acrylamide gels. Ann Biochem 44:276–287
Chance B, Maehly AC (1956) Assay of catalase and peroxidase. Methods Enzymol 2:764–775
Chen C, Huang D, Liu J (2009) Functions and toxicity of nickel in plants: recent advances and future prospects. Clean-Soil Air Water 37:304–313
Chen H, Jiang JG (2009) Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol 219:251–258
Claussen W (2005) Proline as measure of stress in tomato plants. Plant Sci 168:241–248
Cuin TA, Shabala S (2007) Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ 30:875–885
Deivanai S, Xavier R, Vinod V, Timalata K, Lim OF (2011) Role of exogenous proline in ameliorating salt stress at early stage in two rice cultivars. J Stress Physiol Biochem 7:157–174
Dwivedi RS, Randhawa NS (1974) Evaluation of rapid test for the hidden hunger of zinc in plants. Plant Soil 40:445–451
Hare PD, Cress WA, van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pitchel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Hayat S, Mir BA, Wani AS, Hasan SA, Irfan M, Ahmad A (2011) Screening of salt tolerant genotypes of Brassica juncea based on photosynthetic attributes. J Plant Interact 6:53–60
Hayat S, Yadav S, Wani AS, Irfan M, Ahmad A (2010) Response of tomato to two possible modes of salinity stress—a comparative analysis. J Soil Salinity Water Qual 2:84–90
Heidari M (2012) Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. Afr J Biotechnol 11:379–384
Huang Z, Zhao L, Chen D, Liang M, Liu Z, Shao H, Long X (2013) Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS One 8(4):e62085. doi:10.1371/journal.Pone.0062085
Iqbal M, Ashraf M (2013) Gibberellic acid mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ Exp Bot 86:76–85
Irigoyen JJ, Emerich DW, Sanchez-Diaz M (1992) Water stress induced changes in concentration of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–64
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2009) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166:1587–1597
Iyengar ERR, Reddy MP (1996) Photosynthesis in high salt tolerant plants. In: Pesserkali M (ed) Hand Book of Photosynthesis. Marshal Deker. Baten Rose, USA, pp. 56–65
Jain M, Mathur G, Koul S, Sarin NB (2001) Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep 20:463–468
Kamran M, Shahbaz M, Ashraf M, Akram NA (2009) Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak J Bot 41:621–632
Kanwal H, Ashraf M, Shahbaz M (2011) Assessment of salt tolerance of some newly developed and candidate wheat (Triticum aestivum L.) cultivars using gas exchange and chlorophyll fluorescence attributes. Pak J Bot 43:2693–2699
Khedr AHA, Abbas MA, Wahid AAA, Quick WP, Abogadallah GM (2003) Proline induces the expression of salt-stress-responsive proteins and may improve the adaptation of Pancratium maritimum L. to salt-stress. J Exp Bot 54:2553–2562
Kiyosue T, Yoshiba Y, Yamaghuchi-Shinozaki K, Shinozaki K (1996) A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but down regulated by dehydration in Arabidopsis. Plant Cell 8:1323–1335
Liu W, Ming Y, Li P, Huang Z (2012) Inhibitory effects of hypo-osmotic stress on extracellular carbonic anhydrase and photosynthetic efficiency of green alga Dunaliella salina possibly through reactive oxygen species formation. Plant Physiol Biochem 54:43–48
Lu KX, Cao BH, Feng XP, He Y, Jiang DA (2009) Photosynthetic response of salt tolerant and sensitive soybean varieties. Photosynthetica 47:381–387
Majeau N, Coleman JR (1994) Correlation of carbonic anhydrase and ribulose-1,5-bisphosphate carboxylase/oxygenase expression in pea. Plant Physiol 104:1393–1399
Megdiche W, Hessini K, Gharbi F, Jaleel CA, Ksouri R, Abdelly C (2008) Photosynthesis and photosystem-2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica 46:410–419
Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll-a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. Plant Physiol Biochem 48:16–20
Moustakas M, Sperdouli I, Kouna T, Antonopoulou CI, Therios I (2011) Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul 65:315–325
Noreen S, Ashraf M, Hussain M, Jamil A (2009) Exogenous application of salicylic acid enhances antioxidative capacity in salt stressed sunflower (Helianthus annuus L.) plants. Pak J Bot 41:473–479
Nounjan N, Nghia PT, Theerakulpisut P (2012) Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol 169:596–604
Okuma E, Murakami Y, Shimoishi Y, Tada M, Murata Y (2004) Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci Plant Nutr 50:1301–1305
Oukarroum A, El Madidi S, Strasser RJ (2012) Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): a chlorophyll-a fluorescence study. Plant Biosyst 146:1037–1043
Paleg LG, Doughlas TJ, van Daal A, Keech DB (1981) Proline and betaine protect enzymes against heat inactivation. Aust J Plant Physiol 8:107–114
Pitann B, Schubert S, Mühling KH (2009) Decline in leaf growth under salt stress is due to an inhibition of H+ pumping activity and increase in apoplastic pH of maize leaves. J Plant Nutr Soil Sci 172:535–543
Sabir P, Ashraf M, Akram NA (2011) Accession variation for salt tolerance in proso millet (Panicum miliaceum L.) using leaf proline content and activities of some key antioxidant enzymes. J Agron Crop Sci 197:340–347
Sahu S, Das P, Ray M, Sabat SC (2010) Osmolyte modulated enhanced rice leaf catalase activity under salt-stress. Adv Biosci Biotechnol 1:39–46
Saleem M, Ashraf M, Akram NA (2011) Salt (NaCl) induced modulation in some key physio-biochemical attributes in okra (Abelmoschus esculentus L.). J Agron Crop Sci 197:202–213
Santos MA, Camara R, Rodriguez P, Glaparols I, Torne JM (1996) Influence of exogenous maize callus subjects to salt stress. Plant Cell Tiss Org Cult 47:59–65
Shahbaz M, Mushtaq Z, Andaz F, Masood A (2013) Does proline application ameliorate adverse effects of salt stress on growth, ions and photosynthetic ability of eggplant (Solanum melongena L.)? Sci Hortic 164:507–511
Sharma P, Jha AB, Dubey RS (2010) Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Pessarakli M (ed) Handbook of plant and crop stress, 3rd edn. CRC Press, Florida, pp 89–138
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Shu S, Guo SR, Sun J, Yuan LY (2012) Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol Plant 146:285–296
Slathia S, Sharma A, Choudhary SP (2012) Influence of exogenously applied epibrassinolide and putrescine on protein content, antioxidant enzymes and lipid peroxidation in Lycopersicon esculentum under salinity stress. Am J Plant Sci 3:714–720
Sullivan CY, Ross WM (1979) Selection for drought and heat tolerance in grain sorghum. In: Mussel H, Staples RC (eds) Stress physiology in crop plants. John Wiley & Sons, New York, pp 263–281
Szabados L, Savoure A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Wang M, Jiang W, Yu H (2010) Effects of exogenous epibrassinolide on photosynthetic characteristics in tomato (Lycopersicon esculentum Mill) seedlings under weak light stress. J Agri Food Chem 8:3642–3645
Wani AS, Ahmad A, Hayat S, Fariduddin Q (2013) Salt-induced modulation in growth, photosynthesis and antioxidant system in two varieties of Brassica juncea. Saudi J Biol Sci 20:183–193
Wani AS, Irfan M, Hayat S, Ahmad A (2012) Response of two mustard (Brassica juncea L.) cultivars differing in photosynthetic capacity subjected to proline. Protoplasma 249:75–87
Wu XX, Ding HD, Zhu ZW, Yang SJ, Zha DS (2012) Effects of 24-epibrassinolide on photosynthesis of eggplant (Solanum melongena L.) seedlings under salt stress. Afr J Biotechnol 11:8665–8671
Yan Z, Guo S, Shu S, Sun J, Tezuka T (2011) Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr J Biotechnol 10:18381–18390
Yancey PH (1994) Compatible and counteracting solutes. In: Strange K (ed) Cellular and molecular physiology of cell volume regulation. CRC Press, Boca Raton, pp 81–109
Yang SL, Lan SS, Gong M (2009) Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J Plant Physiol 166:1694–1699
Yang XH, Lu CM (2005) Photosynthesis is improved by exogenous glycine betaine in salt-stressed maize plants. Physiol Plant 124:343–352
Zhu M, Xu A, Yuan M, Huang CH, Yu Z, Wang L, Yu J (1990) Effects of amino acids on callus differentiation in barley anther culture. Plant Cell Tiss Org Cult 22:201–204
Acknowledgments
Dr. Arif Shafi Wani thanks SERB, DST, New Delhi, India, for providing a fellowship under the Young Scientist (SB/YS/LS-389/2013) Program. Authors are also thankful to the Prof. John Pichtel, Natural Resources and Environmental Management, West Quad, Room 110, Ball State University, Muncie, IN 47306, for the English corrections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wani, A.S., Ahmad, A., Hayat, S. et al. Is foliar spray of proline sufficient for mitigation of salt stress in Brassica juncea cultivars?. Environ Sci Pollut Res 23, 13413–13423 (2016). https://doi.org/10.1007/s11356-016-6533-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6533-4