Abstract
During many years of industrial development, soil system was contaminated with large amounts of toxic metals. In order to investigate the mobility and availability of metals from soil to mushrooms, the content of 13 elements (Al, Ba, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Sr, Ti, and Zn), in caps and stipes of wild-grown edible mushroom Macrolepiota procera and soil substrates, collected from five sites in Rasina region in central Serbia, was determined. Soil samples were subjected to the sequential extraction procedure proposed by the Community Bureau of Reference in order to fractionate acid-soluble/exchangeable, reducible, oxidizable, and residual fractions. Metal concentrations were determined by inductively coupled plasma optical emission spectrometer and inductively coupled plasma mass spectrometer and the results subjected to multivariate data analysis. A principal component analysis distinguished mushrooms samples from different geographical areas and revealed the influence of soil composition on metal content in mushrooms. Hierarchical cluster analyses confirmed that the first three phases of extraction were the most important for metal uptake by mushrooms from soil. The bioconcentration factors and translocation factors for each metal were also calculated. These results showed that M. procera could serve as a good dietary source of essential elements, especially Cu, Zn, Mn, and Fe but the consumption of mushrooms may pose a health risk for consumers during the “season of mushrooms,” due to the presence of cadmium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mushrooms, both wild growing and cultivated, are valuable healthy food, with great nutritional and pharmacological characteristics: low in calories, high in fibers, minerals, vitamins, specific β-glucans, and antioxidative and flavor constituents (Kalač 2009). Besides that, mushrooms have different concentrations of elements, which are important as essential trace elements or they can be potentially toxic, depending on their concentrations. Therefore, it is necessary to investigate the metal content in wild species. Metal content in mushrooms have been published in many papers (Sesli et al. 2008; Ouzouni et al. 2009; Chen et al. 2009; Sarikurkcu et al. 2011; Nnorom et al. 2013; Mleczek et al. 2013; Dimitrijević et al. 2015). Accumulation of elements is generally species-dependent, but it is interesting that contents of some trace elements in different samples of the same species differed considerably (Sesli and Tüzen 1999; Nikkarinen and Mertanen 2004; Busuioc et al. 2011; Giannaccini et al. 2012). Other factors that can be considered as important are as follows: substrate composition, distance from the source of pollution, age of fruiting bodies, and mycelium (Kalač et al. 1996; Işıloğlu et al. 2001). Generally, the main source of metals in mushrooms is their soil substrate. Numbers of studies have shown that increasing concentrations of metals in soil may increase concentrations in mushrooms (Blanuša et al. 2001; Cocchi et al. 2006; García et al. 2009; Aloupi et al. 2012; Kojta et al. 2012). Concentrations of metals in some species of wild edible mushrooms could be high, even if the degree of pollution in soil is low (Kalač et al. 2004). The study of total concentrations of metals in soil can give a good estimation of the degree of soil contamination, but it is not sufficient for better understanding of metals behavior in the sense of their potential availability and release of elements into mushrooms.
Since the mobility of metals is related to their chemical forms or type of the binding of the element, it is desirable to investigate the share of various metals in soil fractions. Many single or sequential extraction procedures have been applied to soils to fractionate metals by using different extractants or reagents in order to obtain more useful information about the bioavailability and mobility of metals (Alvarez et al. 2006; Kubová et al. 2008; Zimmerman and Weindorf 2010). One of the most widely applied extraction procedures is the original three-step (Davidson et al. 1998) or modified four-step Community Bureau of Reference (BCR) extraction procedure (Rauret et al. 2000; Mossop and Davidson 2003). The reagent used at each stage of original BCR procedure is intended to release metals associated with particular soil phases such as acid soluble/exchangeable, reducible, and oxidable. Modified four-step BCR procedure includes changes to the concentration of the reagent and pH of the second step and addition of a fourth step which includes extraction of solid residue with aqua regia or another acid mixture.
In Serbia, like in the most European countries, the consumption of cultivated mushrooms such as Agaricus bisporus and Pleurotus spp. has been preferred over wild ones, but several groups of people seasonally collect and eat wild growing mushrooms. One of the well known edible wild mushroom species is M. procera, formerly called Lepiota procera and known under the common name parasol mushroom. It grows in the woods or at the edges of woods or in the pastures. An edible part of the fruiting body is the cap, while the stipe is inedible. Investigations of metal content in it, both in rural and polluted areas, were described in several papers (Byrne et al. 1976; Kalač et al. 1996, 2004; Kalač and Svoboda 2000; Falandysz et al. 2001, 2003, 2007, 2008; Gucia and Falandysz 2003; Řanda and Kučera 2004; Benbrahim et al. 2006; Nováčková et al. 2007; Falandysz and Gucia 2008; Jarzyńska et al. 2011; Kojta et al. 2011; Giannaccini et al. 2012; Gucia et al. 2012a, b; Petkovšek and Pokorny 2013; Kuldo et al. 2014). Baptista et al. (2009) investigated the mechanisms response to nickel exposure in M. procera.
There has been no report, to our knowledge, on the element levels in wild growing mushroom species, including M. procera, in Rasina region in Central Serbia. The aims of this study were as follows: (i) to determine concentrations of 13 elements, essential and nonessential in caps and stipes of M. procera, and corresponding soil samples from five different sites, in order to estimate relationships between these elements in mushrooms and soil substrates, (ii) to evaluate differences between metal content of samples from different origins, (iii) calculation of translocation factors (TF) and bioconcentration factors (BCF) for each element in order to understand accumulation pattern and potential of M. procera to uptake elements from the substrate, and (iv) to estimate its nutrition value for dietary intake.
Materials and methods
Site characterization
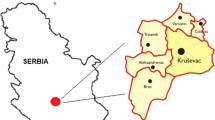
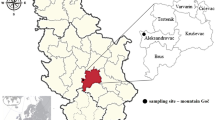
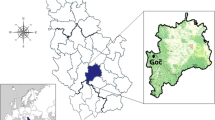
Forty-one fruiting bodies of M. procera and 30 underlaying soil substrate samples, taken in a depth of approximately 0–10 cm, were collected in autumn 2011 from five sampling sites (from S1 to S5) in Rasina region, Central Serbia, in the vicinity of Kruševac (S1), Trstenik (S2, S3 and S4), and at mountain Goč (S5) (Fig. 1). In this region, the forests are mixed, they consist of areas dominated by acacia (the forest near Kruševac), oak and beech (the forest near Trstenik), and pine and oak (mountain Goč) trees. Sampling site S1 was in the forest near Kruševac, approximately 2 km far from urban area, without potential, direct sources of pollution. Sampling sites S2–S4 were in the forest near Trstenik near the place of municipal landfill, which is potential source of pollution. These sites were at different distances from the landfill. Apart of it, there are no other direct sources of pollution. Urban area is about 3 km far from the forest. The sampling site S5 was from rural area, far away from populated places.
Mushroom and soil samples preparation
The mushroom samples were cleaned out of forest debris with a plastic knife, without washing (Gucia et al. 2012b). One complete fruiting body was used as a sample, after separation into cap and stipe. Fresh samples, without drying, were used for analysis. Samples were pulverized in a chopper, placed in polyethylene vessels, and kept in refrigerator. Mushroom samples (0.5 g) were transferred into PTFE cuvettes, and 7 ml of 65 % HNO3 and 1 ml 30 % H2O2 were added. Digestion was performed under following program: warm up for 10 min to 200 °C and held for 15 min at that temperature. After cooling, samples were quantitatively transferred into volumetric flask (50 ml) and diluted with distilled water.
In order to convert the measured concentrations to a dry weight basis, the moisture content of the caps and stipes was determined by drying approximately 3 g of fungal material in an oven at 105 °C to constant weight.
Soil samples, after removal of small stones, plants, and visible organisms were air dried at room temperature for 3 weeks and sieved. A modified four-step BCR sequential extraction procedure for soil samples was applied (Rauret et al. 2000; Mossop and Davidson 2003). The following solutions for extraction were used: for I phase (F1) 0.11 M acetic acid (HOAc extractable fraction), for II phase (F2) 0.5 mol/dm3 hydroxylamine hydrochloride adjusted to pH 1.5 (reducible fraction), for III phase (F3) 8.8 mol/dm3 hydrogen peroxide stabilized at pH 2 and 1 mol/dm3 ammonium acetate adjusted to pH 2 (oxidizable fraction), for IV phase (R) aqua regia 15 ml 37 % HCl and 5 ml 65 % HNO3, at 80 ° C during 5 h (residual fraction).
For determination of pseudo-total metal concentrations, 0.5 g of sample was digested in the same way as residual fraction in BCR sequential extraction procedure. After cooling, the digest was filtered through filter paper and finally diluted to 50 ml with distilled water. The metal recovery of sequential extraction analysis was determined by comparison of the sum of all four fractions with the pseudo-total metal concentrations.
In order to check the accuracy and precision of instruments condition, standard reference materials were applied: ERM-CD281 (Supplementary data—Tables A), BCR-100 (data not shown), TORT-2 (Lobster Hepatopancreas, data not shown). Only barium was missing in these reference materials, and accuracy of measurements of its concentrations was checked by spiking experiments. Recovery values were between 92 and 103 %. NRC Canada and CRM BCR-701 (lake sediment), IRMM (Institute for Reference Materials and Measurements) were applied for soil measurements (Supplementary data—Table B). The results of the analysis showed good agreement with the certified levels (±10 %).
Reagents and chemicals
Multi-element stock solution containing 1.000 g/L of elements was used to prepare standard solutions for ICP-OES measurements, and multi-element stock solution containing 10 mg/L of 22 elements was used to prepare standard solutions for ICP-MS measurements. 6Li, 45Sc, 115In, and 159Tb were used as internal standards (VHG standards, Manchester, UK). All other chemicals used for BCR sequential extraction were of analytical grade and were supplied by Merck (Darmstadt, Germany).
Instrumentation
The measurements of soil samples were carried out in an inductively coupled Atomic emission spectrometer, ICP-OES (Thermo Scientific, United Kingdom), model 6500 Duo. Instrument conditions and selected wavelengths are given in Table C (Supplementary data). The measurement of all elements in mushroom samples was carried out in a ICP-MS (iCAP Q, Thermo Scientific X series 2, UK). Each sample was analyzed in duplicate, and each analysis consisted of three replicates. Measured isotopes and instrument operating conditions for determination are given in Table D (Supplementary data). Microwave digestion was performed in microwave oven (Ethos 1, Advanced Microwave Digestion System, Milestone, Italy).
Statistical analysis
Descriptive statistics and Wilcoxon signed-rank test was performed by a demo version of NCSS statistical software (www.ncss.com). Principal component analysis (PCA) and hierarchical cluster analyses (HCA) were carried out by PLS ToolBox, v.6.2.1, for MATLAB 7.12.0 (R2011a). All data were autoscaled prior to any multivariate analysis to bring values to compatible units. PCA was carried out as an exploratory data analysis by using a singular value decomposition algorithm and a 0.95 confidence level for Q and T2 Hotelling limits for outliers. The analysis was based on correlation matrix and factors with eigenvalues greater than 1 were retained. The best results for HCA were obtained using the Ward method to calculate cluster distances and by applying Euclidean distance as a measure of distance between the samples.
Results and discussion
Water content
The average water content in caps and stipes of M. procera was 72 % (range 24–95 %) and 78 % (range 27–95 %), respectively. These values are very different from sample to sample (Table E, Supplementary data). Water contents, for most of the analyzed samples, are not in agreement with a consensus that the mean water content of mushrooms is 90 % (Kalač 2010). This is probably the consequence of the fact that the amount of dry matter, besides species and age of mushrooms, also depends on meteorological condition.
Profile of metals in Macrolepiota procera
Thirteen elements were identified and quantified, including Al, Ba, Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, Sr, Ti, and Zn, in caps and stipes of M. procera as well as in the soil substrates.
The summarized parameters of descriptive statistics (mean, standard deviation, median, and range) obtained for metal content in caps and stipes were calculated to dry weight (dw). The results for different regions are presented in Table 1.
In sites S2 to S5, the content of Cu was up to 168 mg/kg dw for caps and 116 mg/kg dw for stipes. The highest Cu content was found in M. procera from S1, near Kruševac, in cap and stipe (243 ± 102 mg/kg dw and 234 ± 63 mg/kg dw, respectively). Similar high content of Cu was found in caps of M. procera in rural area in Slovenia, 225 mg/kg dw (Byrne et al. 1976) and in polluted area in Czech Republic 236 mg/kg dw (Kalač et al. 1996). Content of zinc was in range of 64–216 mg/kg dw and 48–105 mg/kg dw in caps and stipes, respectively. Obtained zinc values were in agreement with reported values of Zn in unpolluted regions (Kalač and Svoboda 2000; Falandysz et al. 2001; Giannaccini et al. 2012). Results obtained in this study for manganese were in accordance with values reported in the literature (Řanda and Kučera 2004; Gucia et al. 2012a, b; Kuldo et al. 2014). In analyzed mushroom samples from sites S2–S5, the Ni content was observed to be in the range of 0.29 to 0.96 mg/kg dw and 1.5 to 2.4 mg/kg dw for caps and stipes, respectively. The highest content of Ni was found from site near Kruševac (6.6 ± 4.5 for caps and 6.0 ± 2.3 mg/kg dw for stipes). These results are slightly higher than literature data (Řanda and Kučera 2004; Yamaç et al. 2007). In our research, only the concentration of Co from S5 was in agreement with literature data (0.29 ± 0.26 mg/kg dw) (Gucia et al. 2012a, b; Kuldo et al. 2014). In other sites, the content of Co was higher than in data found in literature.
The Cd content in mushrooms ranged from 0.70 to 6.0 mg/kg dw in stipe samples and in range of 1.2 to 9.0 mg/kg dw in caps, except 17 mg/kg which was found in caps from site S1. These high concentrations could be explained by high availability of cadmium in soil substrate (Table G, Supplementary data and Fig. 4) and also the high potential of M. procera for uptake of Cd from the substrate (Gucia et al. 2012b).
In this study, results for Pb ranged from 0.24 to 1.22 mg/kg in caps for sites S2, S3, S4, and S5. These values are comparable with results of other researchers (Yamaç et al. 2007; Kuldo et al. 2014). The content of lead from S1 is much higher (8.8 ± 4.5 mg/kg in caps and 5.4 ± 1.7 mg/kg for stipes), but also comparable with the results obtained for unpolluted areas in Poland both for caps (8.5 ± 2.4 mg/kg dw) and stipes (5.2 ± 2.1 mg/kg dw) (Gucia et al. 2012b).
Concentrations of Ba and Sr in caps and stipes are similar with literature data (Gucia et al. 2012b; Kuldo et al. 2014). Contents of Al in some samples are very high, especially at site S1 (for caps and stipes), following S4 and S5 for caps and S5, S3, and S4 for stipes. Also, very high concentrations were obtained for Fe in samples from site S1 for both caps and stipes, followed by S5 and S4 for caps and S5, S3, and S4 for stipes. The contents of Cr in analyzed samples were higher than literature data (Řanda and Kučera 2004; Falandysz et al. 2008; Jarzyńska et al. 2011; Gucia et al. 2012a; Kuldo et al. 2014), for samples from site S1 (6.9 ± 4.6 mg/kg dw and 5.6 ± 3.2 mg/kg dw for caps and stipes, respectively). There is lack of available reference data from scientific literature on titanium in fruiting body of M. procera. In this study, the content for titanium from S1, both for caps and stipes (80.4 ± 55.3 mg/kg dw and 55.7 ± 39.4 mg/kg dw, respectively), is much higher than from other sites (4.2 to 6.5 mg/kg dw and 6.2 to 21.0 mg/kg dw for caps and stipes, respectively) and higher than literature data for some other mushrooms species (5.76 to 19.94 mg/kg dw, Elekes and Busuioc 2010).
For statistical evaluation of differences between samples of different origin, Kruskal-Wallis test was employed for each variable and two parts of M. procera separately. Namely, Kruskal-Wallis test was used to compare the medians and variances of all analyzed parameters for five habitats of mushroom (sites S1–S5). Based on the results presented in Table F (Supplementary data), in the case of caps all parameters differentiate mushrooms origin, while in the case of stipes, Ba, Cr, Mn, and Sr were not discriminating factors. In the cases where the Kruskal-Wallis test has indicated a statistically significant difference between the medians, Kruskal-Wallis Multiple-Comparison Z-value test was performed. Sites with different content of a given metal are denoted in parentheses (Table F, Supplementary data). It could be observed a statistically significant difference between metal content of samples from different origins in both parts of mushrooms.
Discrimination of Macrolepiota procera by geographical origin
A multivariate statistical method, PCA, was applied to establish criteria for classification and differentiation of mushrooms fruiting body, caps, and stipes, on one side and of mushrooms from diverse geographical sites, on the other side. The PCA provides the insight in the very structure of data. It is usually carried out at the exploratory (introductory) level; therefore, it is not used as a classification model, but rather as a hint what could be expected from the current data and to check if there is some logical patterns in the data that might be explained.
A PCA model applied on the results of metal content in mushrooms of different geographical origins for two parts of mushrooms separately revealed that approximately 90 % of variability among the data for both parts could be explained by three factors. The first two principal components (PCs) accounted for approximately 85 % of the overall data variance. The addition of more PCs did not significantly change the classification of the samples described below. Mutual projections of factor scores and their loadings for the first two PCs are presented in Fig. 2. Score plots (Fig. 2a, c) of models suggested the existence of three distinctive groups of objects belonging to different geographical sites. Namely, samples from the forest near Kruševac (site S1) formed distinguished cluster dissipated in a broader range, samples from mountain Goč (site S5) made the other compact cluster, while all samples from the forest near Trstenik (sites S2–S4), regardless of the proximity of the landfill formed third group. Generally, the site S1 was separated from other sites alongside the PC1 direction, while site S5 in the case of stipes alongside the PC2 direction. Loading plot (Fig. 2b, d) revealed that all parameters had positive influence on PC1 with the highest influence of variables Ba, Ni, Fe, Al, Cr, and Sr. High positive influence of elements Cd, Cu, and Co on PC2 suggested its domination in samples from area surrounding Kruševac and Trstenik (sites S1–S4).
Dissimilarity between groups of samples separated according to elements content in two parts of M. procera was evaluated by Wilcoxon signed-rank test (Table 2). The content of Cd, Cu, Pb, and Zn was significantly different in stipes compared to caps and govern a separation of two observed parts of mushrooms.
A PCA applied on the results of metal content in different parts of mushroom body resulted in a five-component model which explains 91.89 % of total variance. The first principal component, PC1, accounted for 60.76 % of the overall data variance, the second one, PC2, for 14.50 % and the third principal component, PC3, for 7.30 %. Mutual projections of factor scores and their loadings for the two PCs have been presented in Fig. 3. Taking into account PC2 and PC4 score values (Fig. 3a), two groups of samples belonging to two different parts of mushroom are obtained. There is some overlapping of Hotelling T2 ellipses among samples suggesting no clear translocation of elements within a mushroom. The loading plot (Fig. 3b) indicates correlations between the variables and the principal components. Namely, variables Co, Pb, Ti, Ni, Zn, Cd, and Cu have positive loadings on PC1, and denote a group of stipes objects, wherein Ni and Co vectors, on one side and Ti and Pb, on the other, were close together in the plot and therefore closely and positively related. Copper was not related (vectors making a 90 angle) with Ti and Pb vectors, while Cd with Ni and Co. Variables Sr, Mn, Ba, Fe, Al, and Cr have negative loadings on PC1 and pointed out a group of caps samples. Elements Sr, Mn, and Ba were positively correlated, but closely and negatively correlated with Ni, Ti, and Pb and not related with Fe.
The distribution of the metals studied into the four fractions of BCR sequential extraction varied among the samples from different sites. The results obtained for each elements by the modified BCR sequential extraction procedure, pseudototal obtained from aqua regia digestion, and recovery values are given in Table G (Supplementary data).
The sequential extraction patterns for each soil site (from S1 to S5) are shown in Fig 4 a, b, c, d, e. It can be noted great similarity in total content and pattern distribution of all metals between sites S2–S4, near Trstenik, regardless of the presence of the landfill. The fractionation profile of Cd shows that great amounts in all samples were found in the first two fractions (greater than 80 % of total). It indicates that cadmium could have been remobilized becoming readily available following a slight lowering of pH (Jain 2004). In the case of Pb, it was distributed mostly between the reducible and residual fraction in S1 and S5 samples (Fig. 4a, e) but in other samples (sites S2 to S4), lead was distributed in reducible, oxidizable, and residual fraction (Fig. 4 b–d). Distribution of Ba and Al is similar between all samples. The highest content of Ba was found in reducible fraction followed by the HOAc extractable, and the smallest amount was associated with oxidizable fraction. The dominating chemical form of Zn was associated with the residual fraction, followed by oxidizable fraction in S1 and S5 samples. But, in samples S2–S4, residual fraction was followed by the reducible fraction (Fig. 4 b–d). In the case of Mn, it was extracted in reducible fraction (59.9 %) and followed by oxidizable (23.2 %) in samples from Goč (S5), while in other samples, the most amounts were extracted in reducible fraction and followed by HOAc extractable fraction. It can be noted that the greatest proportion of Sr was associated with the HOAc extractable fraction in all samples (43.3–48.7 %), followed by the reducible (46.1–54.9 %). The metal bound to the residual fraction more than 80 % was Fe, which indicates that this metal is the least available to mushrooms. Cd, Ba, Co, Mn, and Sr are metals with approximately more then 80 % of total content in the sum of the first three fractions, so they can be considered as the most easily mobilized metals. The available amount (F1 + F2 + F3) of all elements, except of Cr, between all sites, did not vary in a greater extent. It indicated that there were no anthropogenic inputs of these metals in investigated sites in Rasina region. Titanium in soil is poorly available for mushrooms, mostly extracted (more than 98 %) in oxidizable and residual fractions from all sites.
To assess cluster of elements contents in relationships to soils and mushrooms at different collection sites, an agglomerative HCA was performed. HCA considers all the data variability contrary to univariate linear regression that concerns only two parameters separately. The distribution of the metals in four fractions of BCR sequential extraction as well as pseudo total together with its distribution in caps and stipes was included. Cluster analysis revealed two clusters at 1.0 variance weighted distance unit (Fig. 5). One cluster comprised metal content in IV phase of extraction and pseudo total, while the other accounts its distribution in caps, stipes, I, II, and III phase of extraction grouped in three subclusters. This dendogram confirms that the first three phases of extraction are the most important for metal uptake by mushrooms from soil.
Bioconcentration factor (BCF) and translocation factor (TF)
The bioconcentration factor expresses the potential of species for bioaccumulation and bioexclusion of mineral constituent from soils. For better understanding, relationship between available concentrations of metals in soil and metal content in mushrooms, BCF was calculated, using pseudo-total concentration in soil (BCF-pseudotot) and sum of the first three phases (BCF-I + II + III) regarding that these fractions are bioavailable for mushrooms (Krgović et al. 2015). Calculated BCF and TF are presented in Table H (Supplementary data).
Comparison of obtained values for BCF-pseudotot and BCF-I + II + III, indicated that there was no significant differences in the sense of conclusion which metal is accumulated. It could mean that potential of mushrooms to uptake an element from substrate is more important factor then availability of elements in soil substrate.
BCF values higher than 1 were found for Cd, Cu, and Zn, while the rest of the analyzed metals had BCF < 1. The median value of BCF for cadmium was the highest from site S1 (57.7 for caps and 24.2 for stipes), while for the rest of the sites were 20.2, 12.2, 5.6, and 3.3 for caps at sites S3, S4, S2, S5, and 11.4, 6.6, 2.0 and 1.6 for stipes at sites S3, S4, S5, and S2, respectively (Table H, Supplementary data). M. procera collected from the site S1 accumulate Cd in wide range (BCF ranged from 5.2 to 137 and 1.2 to 42 for caps and stipes, respectively). The BCF values of Cd were reported to be in total range from 1.7 to 140 for the caps and from 0.09 to 71 for the stipes (Gucia et al. 2012b). The BCF median values for Cu are different from site to site, and they are ranged from 2.4 to 11 for caps and stipes. Zn is moderately bioconcentrated in fruiting bodies (for site S1 median values are 2.7 and 1.8 for caps and stipes, respectively, total range 2.0–9.2 for caps and 1.2–3.0 for stipes and for other sites 1 < BCF < 2). For all studied sites in terms of bioconcentration and bioexclusion concept, Al, Ba, Co, Cr, Fe, Mn, Ni, Pb, Sr, and Ti are bioexcluded in M. procera.
The mobility of metals between two parts of the mushrooms was estimated by translocation factor (TF), presented in Table H (Supplementary data). M. procera showed efficient translocation of metal ions Zn, Cd, Cu, and Pb from stipes to caps (TF higher than 1), which was confirmed by W test (Table 2). Caps were more abundant in Cd than stipes, with median TF values from 1.7 to 4.0. Concentrations of Zn in caps are higher than in stipes, and the median TF values are between 1.2 and 2.2, which is in agreement with literature data (Gucia et al. 2012a). Median TF values for Cu are 0.68–1.7, which indicated that translocation of Cu depending on site. Also, M. procera translocated Pb from stipe to caps, except for site S4 where the TF is < 1 (Table H, Supplementary data).
Dietary intake
An average consumer with body mass of 70 kg (European Food Safety Authority (EFSA 2012a) and 300-g fresh weight portion of caps per meal were used to calculate element intakes from M. procera. Calculated contents of investigated elements for daily intake are presented in Table 3.
-
(a)
Essential elements Cu, Zn, Fe, Mn and Co
The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) decided to derive adequate intakes (AIs) for copper based on observed intakes in several EU countries. AIs for copper of 1.6 mg/day for men and 1.3 mg/day for women (AI for pregnant women to 1.5 mg/day) are proposed (EFSA 2015a). The tolerable upper intake level (UL) for adults is 5 mg/day and is not applicable during pregnancy or lactation (EFSA 2006). The European Population Reference Intake (PRI) for zinc for adult males and females is 9.5 and 7.0 mg/day, respectively. UL for adults is 25 mg/day and applies also to pregnant and lactating women (EFSA 2006). The average requirements (AR) and PRI for iron in all age groups of men and postmenopausal women is 6 and 11 mg/day, respectively, and for premenopausal women is 7 and 16 mg/day, respectively (EFSA 2015b). No UL has been set for iron by the Scientific Committee for Food (SCF) or EFSA (EFSA 2006). An AI for manganese of 3 mg/day is proposed for adults, including pregnant and lactating women (EFSA 2013). An upper level for this element has not been set (EFSA 2006).
A 300-g portion of analyzed mushroom species will provide 4.41–9.84 mg of Cu, 3.45–11.6 mg of Zn, 3.84– 23.1 mg of Fe, and 0.75–1.85 mg of Mn, (Table 3) rendering them as a good source of these metals.
Cobalt is an essential trace element as a part of vitamin B12, which is necessary for folate and fatty acid metabolism. There is no clear recommended amount of cobalt because there are just recommendations for vitamin B12 (EFSA 2015c).
-
(b)
Toxic elements Cd and Pb
Concentrations of Cd and Pb in some species of edible mushrooms are regulated in European Union (EU 2008). Maximum levels (ML) for cadmium are 0.2 μg/g wet weight for cultivated fungi (Champignon Mushroom, Oyster Mushroom and Shi-take) and 1.0 μg/g wet weight for other fungi. Maximum level for lead is 0.3 μg/g for cultivated fungi.
In our study, mean value for Cd in caps exceeded a ML at sites S3 and S4 (1.59 and 2.0 μg/g wet weight, respectively). In the case of Pb, only caps collected from the site S1 (0.54 μg/g wet weight) contain elevated concentration of this metal.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) established a provisional tolerable monthly intake (PTMI) for cadmium of 25 μg/kg body weight (b.w.), (World Health Organization (WHO) 2011) which corresponds to a weekly intake of 5.8 μg/kg b.w. This value differs from the tolerable weekly intake (TWI) of 2.5 μg/kg b.w. established by the EFSA’s Panel on Contaminants in the Food Chain (CONTAM Panel) in 2009 and subsequently confirmed in 2011 (EFSA 2009, 2011). This is equivalent to 0.36 μg/kg b.w. per day, which corresponds 0.025 mg for an adult of 70 kg. A 300-g portion of fresh mushrooms will provide 0.10 to 0.60 mg of Cd per day (Table 3), which could result in exceeding TWI, as well as PTMI. Thus, the consumption of M. procera at all investigated sites, may pose a health risk for consumers during the “season of mushrooms,” when intake is high.
The CONTAM panel concluded that provisional tolerable weekly intake (PTWI) of 25 μg/kg b.w. set by JECFA and endorsed by the SCF is no longer appropriate (EFSA 2010, 2012b). This conclusion was confirmed by JECFA (World Health Organization WHO 2011). The CONTAM panel identified developmental neurotoxicity in young children and cardiovascular effects as well as nephrotoxicity in adults as the critical effects for the risk assessment. The respective BMDL (Benchmark Dose Lower Confidence Limit) derived from blood lead levels in μg/L (corresponding dietary intake values in μg/kg b.w. per day) was: developmental neurotoxicity BMDL0112 μg/L (0.50 corresponds 0.035 mg for an average consumer), effects on systolic blood pressure BMDL01 36 μg/L (1.50 corresponds 0.105 mg for an average consumer), effects on kidney in adults BMDL10 15 μg/L (0.63 corresponds 0.044 mg for an average consumer) (EFSA 2010).
Estimated daily intake of Pb from consumption of 300-g caps of M. procera from S2 and S3 sites was 0.015–0.032 mg and hence poses no toxicological risk from lead content point of view, but from other sites may pose a health risk for consumers.
-
(c)
Other elements Cr, Ni, Al
The CONTAM Panel derived a tolerable daily intake (TDI) of 300 μg Cr(III)/kg b.w. per day. Evaluation is limited to trivalent chromium because it is the form of chromium naturally occurring in food (EFSA 2014). Owing to limited data, the SCF was unable to set a UL. It was stated that, in a number of limited studies, there was no evidence of adverse effects associated with supplemental intake of chromium up to a dose of 1 mg/day (EFSA 2014).
Portions of mushrooms picked from all five sites do not contain chromium above this value. A portion of investigated mushrooms has smaller amount of Ni then TDI of 2.8 μg/kg b. w. (EFSA 2015d), equivalent 196 μg for average consumer and, hence, there is no risk for health. As far as aluminum is concerned, TWI of 1 mg Al/kg bw/week is more appropriate than a TDI (EFSA 2008). That gives 10 mg per day for an average consumer. The estimated daily dietary exposure to aluminum in the general population, varied from 0.2 up to 2.3 mg/kg bw/week in highly exposed consumers. The TWI of 1 mg aluminum/kg bw/week is likely to be exceeded in a significant part of population in Europe (EFSA 2008). From our results, one portion of M. procera from the sites S1, S4, and S5 contain aluminum approximately 2–3 times more than recommended value.
Conclusions
The obtained results of element composition of M. procera confirmed that this wild growing mushroom could contain high concentrations of both essential and toxic elements, even in unpolluted areas. The principal component analysis and cluster analysis were carried out in order to provide better understanding of the nature of associations of trace elements in mushrooms and soil substrates. A PCA model applied on the results of metal content in mushrooms of different geographical origin showed three groups of clusters, belonging to the forest near Kruševac, mountain Goč, and forest near Trstenik, regardless of the proximity of the landfill. Applied modified BCR sequential extraction procedure showed that Cd, Ba, Co, Mn, and Sr were metals that could be considered as the most easily mobilized. Performed hierarchical cluster analyses confirmed that the first three phases of extraction had been the most important for metal uptake by mushrooms from soil. M. procera has high potential for accumulation of Cd, Cu and Zn (BCF values higher than 1) from soil substrate, while Al, Ba, Co, Cr, Fe, Mn, Ni, Pb, Sr, and Ti are bioexcluded. Also, there is efficient translocation of Zn, Cd, Cu, and Pb from stipes to caps (TF higher than 1). Contents of Cd, and to a lesser extent Pb, in some caps of analyzed samples exceeded the maximum levels set in the EU for cultivated mushrooms, which indicates that there may be high level of these toxic elements in M. procera, even in unpolluted areas.
Calculated possible element dietary intakes showed that these species could serve as a good dietary source of essential elements, especially Cu, Zn, Mn, and Fe. However, the consumption of these mushrooms can be considered as a toxicological risk due to presence of cadmium and lead.
References
Aloupi M, Koutrotsios G, Koulousaris M, Kalogeropoulos N (2012) Trace metal contents in wild edible mushrooms growing on serpentine and volcanic soils on the island of Lesvos, Greece. Ecotox Environ Safe 78:184–194
Alvarez JM, Lopez-Valdivia LM, Novillo J, Obrador A, Rico MI (2006) Comparison of EDTE and sequential extraction tests for phytoavailability prediction of manganese and zinc in agricultural alkaline soils. Geoderma 132:450–463
Baptista P, Ferreira S, Soares E, Coelho V, Bastos MDL (2009) Tolerance and stress response of Macrolepiota procera to nickel. J Agric Food Chem 57:7145–7152
Benbrahim M, Denaix L, Thomas AL, Balet J, Carnus JM (2006) Metal concentrations in edible mushrooms following municipal sludge application on forest land. Environ Pollut 144:847–854
Blanuša M, Kučak A, Vernai VM, Sarić MM (2001) Uptake of cadmium, copper, iron, manganese and zink in mushrooms (Boletaceae) from Croatian forest soil. J AOAC Int 84(6):1964–1971
Busuioc G, Elekes CC, Stihi C, Iordache S, Ciulei SC (2011) The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ Sci Pollut Res 18(6):890–896
Byrne AR, Ravnik V, Kosta L (1976) Trace element concentrations in higher fungi. Sci Total Environ 6:65–78
Chen XH, Zhou HB, Qiu GZ (2009) Analysis of several heavy metals in wild edible mushrooms from regions of China. Bull Environ Contam Toxicol 83:280–285
Cocchi L, Vescovi L, Petrini LE, Petrini O (2006) Heavy metals in edible mushrooms in Italy. Food Chem 98:277–284
Davidson CM, Duncan AL, Littlejohn D, Ure AM, Garden LM (1998) A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal Chim Acta 363:45–55
Dimitrijević MV, Mitić VD, Cvetković JS, Stankov Jovanović VP, Mutic JJ, Nikolić Mandić SD (2015) Update on element content profiles in eleven wild edible mushrooms from family Boletaceae. Eur Food Res Technol 242:1–10
EFSA (2006) Tolerable upper intake levels for vitamins and minerals; Scientific Committee on Food, Scientific Panel on Dietetic Products, Nutrition and Allergies. http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf
EFSA (2008) Safety of aluminium from dietary intake; Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J 754:1–34
EFSA (2009) Cadmium in food scientific opinion of the panel on contaminants in the Food Chain. EFSA J 980:1–139
EFSA (2010) Scientific opinion on lead in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 8(4):1570
EFSA (2011) Statement on tolerable weekly intake for cadmium. EFSA J 9(2):1975
EFSA (2012a) Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA J 10(3):2579
EFSA (2012b) Lead dietary exposure in the European population. EFSA J 10(7):2831
EFSA (2013) Scientific opinion on dietary reference values for manganese. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J 11(11):3419
EFSA (2014) Scientific opinion on dietary reference values for chromium; EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J 12(10):3845
EFSA (2015a) Scientific opinion on dietary reference values for copper. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J 13(10):4253
EFSA (2015b) Scientific opinion on dietary reference values for iron. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J 13(10):4254
EFSA (2015c) Scientific opinion on dietary reference values for cobalamin (vitamin B12). EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). EFSA J 13(7):4150
EFSA (2015d) Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 13(2):4002
Elekes CC, Busuioc G (2010) The mycoremediation of metals polluted soila using wild growing species of mushrooms. Proceedings of the 7th WSEAS international conference on Latest trands on Eng Educ, Corfu Island Greece, 2010, pp. 36–39.
EU (2008) Commision Regulation (EC) No 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Office J European Union 3.7.2008 L 173/6-9.
Falandysz J, Gucia M (2008) Bioconcentration factors of mercury by Parasol Mushroom (Macrolepiota procera). Environ Geochem Health 30:121–125
Falandysz J, Szymczyk K, Ichihashi H, Bielawski L, Gucia M, Frankowska A, Yamasaki SI (2001) ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit Contam 18:503–513
Falandysz J, Kubota R, Kunito T, Bielawski L, Brzostowski A, Gucia M, Jedrusiak A, Lipka K, Tanabe S (2003) Relationships between selenium and mercury in the fruiting bodies of some mushrooms growing in Poland. J Phys IV France 107:443–446
Falandysz J, Gucia M, Mazur A (2007) Content and bioconcentration factors of mercury by Parasol Mushroom Macrolepiota procera. J Environ Sci Health B 42:735–740
Falandysz J, Kunito T, Kubota R, Gucia M, Mazur A, Falandysz JJ, Tanabe S (2008) Some mineral constituents of Parasol Mushroom (Macrolepiota procera). J Environ Sci Health B 43:187–192
García MA, Alonso J, Melgar MJ (2009) Lead in edible mushrooms levels and bioaccumulation factors. J Hazard Mater 167(1–3):777–783
Giannaccini G, Betti L, Palego L, Mascia G, Schmid L, Lanza M, Mela A, Fabbrini L, Biondi L, Lucacchini A (2012) The trace element content of top-soil and wild edible mushroom samples collected in Tuscany, Italy. Environ Monit Assess 184:7579–7595
Gucia M, Falandysz J (2003) Total mercury content in Parasol Mushroom Macrolepiota procera from various sites in Poland. J Phys IV France 107:581–584
Gucia M, Jarzyńska G, Kojta AK, Falandysz J (2012a) Temporal variability in 20 chemical elements content of Parasol Mushroom (Macrolepiota procera) collected from two sites over a few years. J Environ Sci Health B 47:81–88
Gucia M, Jarzyńska G, Rafal E, Roszak M, Kojta AK, Osiej I, Falandysz J (2012b) Multivariate analysis of mineral constituents of edible Parasol Mushroom (Macrolepiota procera) and soils beneath fruiting bodies collected from Northern Poland. Environ Sci Pollut Res 19:416–431
Işıloğlu M, Yılmaz F, Merdivan M (2001) Concentrations of trace elements in wild edible mushrooms. Food Chem 73:169–175
Jain CK (2004) Metal fractionation study on bed sediments of River Yamuna, India. Water Res 38:569–578
Jarzyńska G, Gucia M, Kojta AK, Rezulak K, Falandysz J (2011) Profile of trace elements in Parasol Mushroom (Macrolepiota procera) from Tucholskie Forest. J Environ Sci Health B 46:741–751
Kalač P (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113:9–16
Kalač P (2010) Trace element contents in European species of wild growing edible mushrooms: a review for the period 2000–2009. Food Chem 122:2–15
Kalač P, Svoboda L (2000) A review of trace element concentrations in edible mushrooms. Food Chem 69:273–281
Kalač P, Nižnanská M, Bevilaqua D, Stašková I (1996) Concentrations of mercury, copper, cadmium and lead in fruiting bodies of edible mushrooms in the vicinity of a mercury smelter and a copper smelter. Sci Total Environ 177:251–258
Kalač P, Svoboda L, Havličková B (2004) Contents of cadmium and mercury in edible mushrooms, review. J Appl Biomed 2:15–20
Kojta AK, Gucia M, Jarzyńska G, Lewandowska M, Zakrzewska A, Falandysz J, Zhang D (2011) Phosphorous and certain metals in Parasol mushrooms (Macrolepiota procera) and soils from the Augustowska forest and ELK region in north-eastern Poland. Fresen Environ Bull 20(11A):3044–3052
Kojta AK, Jarzyńska G, Falandysz J (2012) Mineral composition and heavy metal accumulation capacity of Bay Bolete (Xerocomus badius) fruiting bodies collected near a former gold and copper mining area. J Geochem Explor 121:76–82
Krgović R, Trifković J, Milojković-Opsenica D, Manojlović D, Marković M, Mutić J (2015) Phytoextraction of metals by Erigeron Canadensis L. from fly ash landfill of power plant “Kolubara”. Environ Sci Pollut Res 22:10506–10515
Kubová J, Matúš P, Bujdoš M, Hagarová I, Medved J (2008) Utilization of optimized BCR three-step sequential and dilute HCl single extraction procedures for soil-plant metal transfer predictions in contaminated lands. Talanta 75:1110–1122
Kuldo E, Jarzyńska G, Gucia M, Falandysz J (2014) Mineral constituents of edible parasol mushroom Macrolepiota procera (Scop. Ex Fr.) Sing and soils beneath its fruiting bodies collected from a rural forest area. Chem Pap 68:484–492
Mleczek M, Magdziak Z, Goliński P, Siwulski M, Stuper-Szablewska K (2013) Concentrations of minerals in selected edible mushroom species growing in Poland and their effect on human health. Acta Sci Pol Technol Aliment 12(2):203–214
Mossop KF, Davidson CM (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta 478:111–118
Nikkarinen M, Mertanen E (2004) Impact of geological origin on trace element composition of edible mushrooms. J Food Compos Anal 17:301–310
Nnorom IC, Jarzyńska G, Drewnowska M, Dryżalowska A, Kojta A, Pankavec S, Falandysz J (2013) Major and trace elements in sclerotium of Pleurotus tuber-regium (Ósū) mushroom—dietary intake and risk in southeastern Nigeria. J Food Compos Anal 29:73–81
Nováčková J, Fiala P, Chrastný V, Svoboda L, Kalač P (2007) Contents of mercury, cadmium and lead in edible mushrooms and in underlying substrates from a rural area with an occurrence of serpentines and amphiboles. Ekol Bratislava 26:322–329
Ouzouni PK, Veltsistas PG, Paleologos EK, Riganakos KA (2009) Determination of metal content in wild edible mushroom species from regions of Greece. J Food Compos Anal 20:480–486
Petkovšek SAS, Pokorny B (2013) Lead and cadmium in mushrooms from the vicinity of two large emission sources in Slovenia. Sci Total Environ 443:944–954
Řanda Z, Kučera J (2004) Trace elements in higher fungi (mushrooms) determined by activation analysis. J Radioanal Nucl Chem 259:99–107
Rauret G, López-Sánchez JF, Sahuquillo A, Barahona E, Lachica M, Ure AM, Davidson CM, Gomez A, Lück D, Bacon J, Yli-Halla M, Muntau H, Quevauviller P (2000) Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J Environ Monit 2:228–233
Sarikurkcu C, Copur M, Yildiz D, Akata I (2011) Metal concentration of wild edible mushrooms in Soguksu National Park in Turkey. Food Chem 128:731–734
Sesli E, Tüzen M (1999) Levels of trace elements in the fruiting bodies of macrofungi growing in the East Black Sea region of Turkey. Food Chem 65:453–460
Sesli E, Tüzen M, Soylak M (2008) Evaluation of trace metal contents of some wild edible mushrooms from Black sea region, Turkey. J Hazard Mater 160:462–467
World Health Organization (WHO) (2011) Evaluation on certain food additives and contaminants 73rd Report of the Joint FAO/WHO Expert Committee on Food Additives. Technical Report Series No 960 Geneva (Switzerland)
Yamaç M, Yıldız D, Sarıkürkcü C, Çelikkollu M, Solak MH (2007) Heavy metals in some edible mushrooms from the Central Anatolia, Turkey. Food Chem 103:263–267
Zimmerman AJ, Weindorf DC (2010) Heavy metal analysis in soil by sequential extraction: a review of procedures. Int J Analyt Chem 2010:1–7
Acknowledgments
This research was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, project nos. 172017 and 172030.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 223 kb)
Rights and permissions
About this article
Cite this article
Stefanović, V., Trifković, J., Mutić, J. et al. Metal accumulation capacity of parasol mushroom (Macrolepiota procera) from Rasina region (Serbia). Environ Sci Pollut Res 23, 13178–13190 (2016). https://doi.org/10.1007/s11356-016-6486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6486-7