Abstract
Many trace heavy elements are carcinogenic and increase the incidence of cancer. However, a comprehensive study of the correlation between multiple trace elements and DNA oxidative damage is still lacking. The aim of this study is to investigate the relationships between the body burden of multiple trace elements and DNA oxidative stress in college students in Guangzhou, China. Seventeen trace elements in urine samples were determined by inductively coupled plasma-mass spectrometry (ICP-MS). Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG), a biomarker of DNA oxidative stress, was also measured using liquid chromatography tandem mass spectrometer (LC-MS/MS). The concentrations of six essential elements including manganese (Mn), copper (Cu), nickel (Ni), selenium (Se), strontium (Sr), and molybdenum (Mo), and five non-essential elements including arsenic (As), cadmium (Cd), aluminum (Al), stibium (Sb), and thallium (Tl), were found to be significantly correlated with urinary 8-OHdG levels. Moreover, urinary levels of Ni, Se, Mo, As, Sr, and Tl were strongly significantly correlated with 8-OHdG (P < 0.01) concentration. Environmental exposure and dietary intake of these trace elements may play important roles in DNA oxidative damage in the population of Guangzhou, China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many trace elements exist in the environment, in foodstuff and in human body fluids with levels from nanograms per gram to micrograms per gram (Lu et al. 2015; Jiang et al. 2015; Esteban-Vasallo et al. 2012). Humans are exposed to these trace elements mainly through air inhalation, food intake, and water consumption in their daily lives. According to their various functions in the human body, trace elements can be divided into essential and non-essential (Fraga, 2005). Many studies have focused on non-essential trace elements, even at low concentrations, can lead to adverse health effects in humans, such as arsenic (As), cadmium (Cd), lead (Pb), mercury (Hg), and stibium (Sb) as these toxic elements (Lu et al. 2015; Lin et al. 2015; Yu et al. 2012; Görür et al. 2012). Although essential trace elements, such as iron (Fe), zinc (Zn), copper (Cu), manganese (Mn), and molybdenum (Mo) are necessary to maintain normal physiological functions, excessive intake can damage various organ systems and lead to adverse health effects (Magge et al. 2013; Zoni and Lucchini 2013). For example, it has been reported that high concentrations of Mo can negatively affect semen quality (Meeker et al. 2008). Therefore, both essential and non-essential trace elements should be controlled at safe concentrations.
Occupational and environmental exposure to trace heavy elements can generate reactive oxygen species (ROS) in the human body. When heavy metals enter the body, they can react with DNA leading to permanent mutations (Stohs and Bagchi 1995). Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG, also named 8-oxo-7, 8- dihydro-2′- deoxyguanosine), a residue of DNA damage, is mainly excreted through urine without further metabolism and is widely used as a biomarker for assessing the extent of DNA oxidative damage and stress due to its high measurement sensitivity in easily collected urine samples (Chen et al. 2005). Many studies have shown that oxidative DNA lesions play an important role in the incidence of cancer (Shi et al. 2004).
The Pearl River Delta (PRD), located in south China, is one of the most commercialized and industrialized regions in China. Guangzhou, the central city of the PRD, had more than 20 million residents and two million motor vehicles in 2013. Rapid economic growth and high motor vehicle quantity led to serious environmental pollution, especially air pollution caused by heavy traffic (Duzgoren-Aydin et al. 2006; Zhang et al. 1999; Li et al. 2013). Several studies have focused on environmental pollutants and their effects on the human body in Guangzhou (Li et al. 2015a, b; Tan and Duan 2013). Some reports indicated that the people in Guangzhou had high exposure levels to polycyclic aromatic hydrocarbons, benzene, and toluene, and there were significant dose-effects between the exposure levels and 8-OHdG concentrations (Li et al. 2015a, b). A previous study suggested that young individuals were more susceptible to chemicals and absorbed and accumulated more heavy metals than adults (Szymańska-Chabowska et al. 2009). However, to the best of our knowledge, no comprehensive study on the correlation between the human body burden of trace elements and oxidative DNA damage in this region has been reported. Thus, it is necessary to determine the burden of trace elements and its health effects in young individuals in Guangzhou, China.
In this study, 53 college students from Guangzhou, China were recruited and we aimed (1) to investigate the levels of the ten essential trace elements: chromium (Cr), Mn, Fe, Cu, stannum (Sn), cobalt (Co), nickel (Ni), selenium (Se), strontium (Sr), and Mo, and seven non-essential trace elements: As, Cd, Hg, Pb, aluminum (Al), Sb, and thallium (Tl) in their urine; (2) to investigate the associations between urinary levels of trace elements and oxidative DNA damage by measuring 8-OHdG.
Materials and methods
Chemicals and reagents
Ultra-pure analytical grade concentrated nitric acid (68 %) and methanol were purchased from Merck (Merck Chemicals, Co., Ltd., USA). Standard solutions of ten essential trace elements (Cr, Mn, Fe, Cu, Sn, Co, Ni, Se, Sr, and Mo) and seven non-essential trace elements (As, Cd, Hg, Pb, Al, Sb, and Tl) were obtained from the National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials (NCATN) (Beijing, China). A mixed tuning solution containing Co, Tl, ytterbium (Yb), cerium (Ce), and lithium (Li) at a concentration of 1.0 μg/L was purchased from Agilent (Agilent Co., Ltd., USA). An internal standard solution containing scandium (Sc), germanium (Ge), rhodium (Rh), and rhenium (Re) was also obtained from NCATN (Beijing, China). 8-OHdG was purchased from Sigma (St. Louis, MO, USA). 15N5-8-hydroxy-2′-deoxyguanosine (15N5-8-OHdG, 15N5 purity 98 % and 95 % chemical purity) was from Cambridge Isotope Laboratory (Andover, MA, USA). Glacial acetic acid (HAC), sodium acetate (NaAC), and KH2PO4 (HPLC grade) were purchased from Fisher Scientific (Houston, TX, USA). Solid phase extraction (SPE) cartridges (Bond Elut C18, 500 mg/6 mL) were obtained from Agilent, Santa Clara, CA, USA. An Xpera-C18 column (5 μm, 4.6 × 250 mm, Waters, Milford, MA,) was used to separate urinary 8-OHdG. The water used in this study was generated from a Millipore pure water system (Millipore Co., Ltd., Billerica, MA, USA). Ultra-pure grade argon (99.999 %) was used as the carrier gas in the trace element analysis.
Study subjects and sample collection
Sample collection was carried out in November, 2011. Fifty-three college students aged 20 to 26 years from a university in Guangzhou, China were recruited. Of these subjects, 37 were male and 16 were female. Before sample collection, each participant was asked to complete a questionnaire which included personal information regarding age, gender, weight, stature, dietary habits, smoking habits, and alcohol consumption. Detailed demographic data of the subjects are presented in Table 1.
Spot urine samples were collected in polyethylene bottles which had been cleaned with de-ionized water and 0.1 M hydrochloric acid. After collection, samples were taken to the laboratory within 2 h and urinary creatinine concentration in each sample was determined by the Jaffée method (Taussky 1954). The samples were then stored at −20 °C until sample pretreatment and instrumental analysis.
Sample pretreatment and instrumental analysis
For trace element analysis, 1.0 mL of urine sample was transferred into a Teflon vessel and acidified with 0.5 mL of nitric acid. The vessel was then capped and placed in a thermostatic oven at 100 °C for 3 h. After digestion, the solution was transferred to a 10-mL polyethylene flask and diluted to the final volume with ultra-pure water. The final solution was analyzed for trace elements using an Agilent 7700× inductively coupled plasma mass spectrometer (ICP-MS). The optimized instrumental parameters are listed in Table 2.
For 8-OHdG analysis, urine samples were prepared and analyzed as detailed in our previous reports with slight modifications (Li et al. 2015a). In brief, 2 mL of urine sample was spiked with 15N5-8-OHdG to the level of 10 μg/L, and then 3 mL of sodium acetate buffer was added. Then 8-OHdG was extracted using SPE cartridges. The extracts were concentrated to 100 μL in methanol and analyzed using a 20A HPLC (Shimadzu, Japan) coupled with an API Q-Trap 5500 mass spectrometer (AB, SCIEX, USA). 8-OHdG was quantified by its own isotope labeled internal standard.
Quality assurance (QA) and quality control (QC)
Instead of glassware, Teflon or plastic containers were used in the experiments to avoid potential contamination of trace elements. Calibration standard solutions of the trace elements were prepared with 1 % of nitric acid and ranged from 0.002–0.500 μg/L for Co, Se, Sn, Hg, and Tl, 0.005–3.000 μg/L for Cd, 0.010–50.00 μg/L for Mn, 0.030–3.500 μg/L for Cr, Sb, and Pb, 0.100–4.000 μg/L for Ni and Cu, and 0.100–50.00 μg/L for Al, Fe, Sr, Mo, and As, respectively. The calibration standard solution of 8-OHdG ranged from 0.100 to 10.00 μg/L and was prepared in methanol. The regression coefficients (r 2) of the calibration standard solutions for the 17 trace elements and 8-OHdG were all greater than 0.999. Internal standard solutions were used to check the signal response and drift. Moderate standard solutions of trace elements and 8-OHdG were analyzed during each batch of ten samples to investigate the stability of detector response and confirm that the relative standard deviation was less than 10 %. Urine samples were spiked with standard solution at two levels (2 and 10 μg/L) for trace elements and 8-OHdG and recoveries in the present study were between 85 and 105 %. A solvent blank was analyzed per ten samples to check for potential contamination.
Statistical analysis
Concentrations of trace elements and 8-OHdG were expressed as micrograms per liter urine or adjusted for creatinine and expressed as micrograms per gram creatinine. Statistical analysis was performed using SPSS (IBM, version 20.0, NY, USA). A concentration below the limit of quantification (LOQ) was given a value of half the LOQ instead of zero. Mann–Whitney U was carried out to detect significant differences in geometric means for different variables. The Spearman non-parametric method (two tailed) was used to test the associations between urinary trace elements and 8-OHdG concentrations. The level of significance was set at P < 0.05.
Results and discussion
Concentrations of urinary trace elements and 8-OHdG
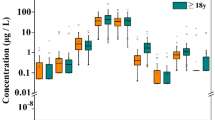
The concentrations of trace elements and 8-OHdG in urine samples are listed in Table 3. Large variations in the concentrations of trace elements were observed. The essential trace elements, Mn, Fe, Cu, Co, Se, Sr, and Mo, were detected in all urine samples, and their median concentrations were 1.421, 25.92, 12.90, 0.1800, 16.79, 181.1, and 76.10 μg/L, respectively. Cr, Sn, and Ni were detected only in some of the urine samples and their median concentrations were 1.031, 0.2960, and 6.353 μg/L, respectively.
The non-essential trace elements were detected in all urine samples with the exception of Cd. The median concentrations were 35.69, 0.464, 3.737, 48.43, 0.194, and 0.439 μg/L for As, Hg, Pb, Al, Sb, and Tl, respectively. Cd was found in some urine samples and the median concentration was 0.554 μg/L. Of these elements, Al was the most abundant, followed by As.
The concentration of urinary 8-OHdG in all studied subjects ranged from 1.280 to 26.40 μg/L, with a median concentration of 9.195 μg/L. No significant difference in urinary 8-OHdG level was observed between male and female subjects (P > 0.1).
Profile concentrations of urinary trace elements and potential sources
Of the essential trace elements, Sr was the most abundant, with concentration 2 to 3 orders of magnitude higher than those of Mn, Co, Cr, Sn, and Ni. In addition, the levels of Fe, Cu, and Se were also high, indicating a relatively higher intake dose in the studied subjects. As most of these were essential trace elements, dietary intake may be the main route for meeting the necessary requirements in humans (Jiang et al. 2015).
The concentrations of Al and As were significantly higher than those of other non-essential trace elements. It is well known that Al is a low-toxicity element and aluminum additives are commonly used in the processing of flour products, puffed food, and seafood (Saiyed and Yokel 2005; Yang et al. 2014). Many Chinese people like to eat twisted cruller (Chinese style) and jellyfish, which are prepared using aluminum additives (Yang et al. 2014). Intake of these foods may result in a relatively high level of urinary Al. Although Al is thought to have low toxicity, excessive intake can lead to adverse effects in the nervous system, immune system, and reproductive system (French et al. 1989; Nayak and Chatterjee 2001; Krewski et al. 2007). Many studies have suggested that excessive intake of Al is associated with Alzheimer’s disease (Campbell 2002; Gupta et al. 2005). Arsenic is one of the most toxic metalloid metals, which occurs in both inorganic and organic species (Lin et al. 2015; Jomova et al. 2011), and has attracted increased attention in recent years due to its carcinogenic and other toxic properties (Gilbert-Diamond et al. 2011). Thus, the risk of Al and As intake in college students in Guangzhou should be investigated further.
Comparison of urinary trace elements with published data
As urinary trace element levels reflect exposure to the corresponding trace elements from all possible routes, biomonitoring of these levels is a useful tool to assess their health risk in humans. In order to further investigate the urinary levels of trace elements in college students in Guangzhou, a comparison of the concentrations of trace elements in the present study with those published in the literature was conducted, and the results are documented in Table 4. As shown in Table 4, the concentrations of most essential trace elements in college students in Guangzhou in the present study were comparable to those reported in other studies with the exception of Cr, Sr, Ni, and Mo.
Several reports showed that levels of urinary heavy elements varied according to age and gender besides environments and life habits (Lee et al. 2012; Roca et al. 2016). Due to the specific behavior and undeveloped metabolism system, children are always more sensitive to pollutants than adults, including heavy metals and PAHs (Roca et al. 2016; Li et al. 2015b). Furthermore, the difference levels of urinary heavy metals between man and women might come from working spots and life habits in most cases, such as smoking (Lee et al. 2012). In our study, because all the recruited subjects are college students and the limitation of sample number, the samples were not divided into groups according to gender and ages.
In the present study, the median concentrations of Cr and Ni were 1.031 and 6.353 μg/L, respectively, which were much higher than those in the USA and Europe. As industrial materials, Cr and Ni can be released into the environment during the use of products containing these elements. Humans can be exposed to these trace elements by consuming contaminated water and foodstuff. The high levels of Cr and Ni in urine samples in college students in Guangzhou may be attributed to industrial processes. A previous study suggested that sediments collected from the Pearl River were significantly contaminated by Cr and Ni (Chen et al. 2012).
Sr is essential for the human body. However, excessive intake of Sr can lead to disease. Recently, a study investigated the urinary levels of Sr in breast cancer patients in Guangzhou, China, and a strong positive correlation was found between urinary Sr and the incidence of breast cancer in women. The urinary levels of Sr were 230.7 μg/L and 163.8 μ/L in breast cancer patients and controls, respectively (Tan and Duan 2013). These levels were comparable to those in our study (median 218.2 μg/L) but were much higher than those observed in the inhabitants of Germany (range 10–77 μg/L) (Heitland and Köster 2006). As only urinary levels of Sr were investigated and no intake data regarding different routes were available, more researches on dietary intake and environmental exposure routes to Sr should be carried out to comprehensively assess the exposure risk of Sr for the inhabitants in this area.
Pb is one of the most toxic heavy metals. Generally, Pb enters the human body via air inhalation, food intake, and water consumption. It can accumulate in bone and is released into the blood and other tissues when the capacity of bone is exceeded. In the present study, the urinary concentration of Pb was approximately two times higher than that in the USA, indicating a relatively higher exposure burden in our study subjects. It was reported that the level of atmospheric Pb in Guangzhou was high and was mainly due to the exhaust from heavy traffic (Halliwell and Gutteridge 2007). Although the Chinese government introduced the use of unleaded petrol instead of leaded petrol in 2000, there are still large numbers of vehicles using diesel in Guangzhou, particularly trucks. As the central city of the PRD, Guangzhou is also the largest logistics center and port city in south China. Emission from vehicles with diesel engines, especially container trucks, may contribute to high Pb exposure in Guangzhou.
Relationship between urinary trace elements and 8-OHdG
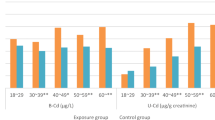
The correlations between individual urinary trace elements and 8-OHdG were analyzed using the Spearman test (two tailed) (shown in Table 5). For the essential trace elements, the levels of Mn, Cu, Ni, Se, Sr, and Mo were positively correlated with 8-OHdG concentration in urine (P < 0.05), and the correlation coefficients were 0.278, 0.323, 0.354, 0.415, 0.432, and 0.474, respectively. Of these elements, Se, Sr, and Mo correlated significantly with the urinary 8-OHdG level (P < 0.01). However, no significant correlations were found between urinary 8-OHdG and Cr, Fe, Sn, or Co concentrations (P > 0.05). The levels of non-essential trace elements, i.e., As, Cd, Al, Se, and Tl correlated significantly with the urinary 8-OHdG level (P < 0.05). However, Hg and Pb did not correlate significantly with the 8-OHdG level (P > 0.05). These results indicated that the elements which showed a good correlation with 8-OHdG may play an important role in the formation of 8-OHdG in urine. Our results regarding the significant correlations between Cd, Ni, and 8-OHdG were in accordance with other studies (Valavanidis et al. 2009; Komatsu et al. 2009).
Many studies have confirmed that trace elements can interact with nuclear proteins or DNA in the human body to cause oxidative stress in biological macromolecules (Valko et al. 2005; Jomova and Valko 2011; Valko et al. 2007). Previous studies have suggested that Cu and Al can increase urinary 8-OHdG level due to the redox-cycling reactions (Jomova and Valko 2011; Valko et al. 2007) and these redox-active metals may undergo a series of cycling reactions to transfer electrons between metals and substrates and then produce deleterious free radicals causing DNA damage (Prousek 2007). Arsenic is thought to bind directly with critical thiols and is widely considered an important factor in increasing 8-OHdG level (Valko et al. 2007). The existence of this type of metal could produce more 8-OHdG (Kumar et al. 2009; Filipič and Hei 2004). Although the specific mechanisms in the toxicity and carcinogenicity of these metals are unknown, their toxicity and carcinogenicity have been widely accepted (Al-Saleh et al. 2014; Cavallo et al. 2002; Nowicka et al. 2014; Valko et al. 2006).
Conclusion
Various trace elements and 8-OHdG were determined in urine samples collected from college students in Guangzhou, China. Individual trace elements, such as Ni, Se, Mo, As, Sr, and Tl, were significantly correlated with urinary 8-OHdG (P < 0.01). Environmental and food exposure routes may be important factors in biomonitoring results and can lead to higher oxidative damage of DNA. More subjects should be recruited and other exposure routes should be controlled to consolidate these conclusions in future studies.
References
Al-Saleh I, Al-Rouqi R, Obsum CA, Shinwari N, Mashhour A, Billedo G et al (2014) Interaction between cadmium (Cd), selenium (Se) and oxidative stress biomarkers in healthy mothers and its impact on birth anthropometric measures. Int J Hyg Environ Health 218(1):66–90
Batista BL, Rodrigues JL, Tormen L, Curtius AJ, Barbosa JF (2009) Reference concentrations for trace elements in urine for the Brazilian population based on q-ICP-MS with a simple dilute-and-shoot procedure. J Braz Chem Soc 20(8):1406–1413
Campbell A (2002) The potential role of aluminium in Alzheimer’s disease. Nephrol Dial Transplant 17(2):17–20
Cavallo D, Iavicoli I, Setini A, Marinaccio A, Perniconi B, Carelli G et al (2002) Genotoxic risk and oxidative DNA damage in workers exposed to antimony trioxide. Environ Mol Mutagen 40(3):184–189
Chen CY, Qu LY, Li B, Li X, Jia G, Wang TC et al (2005) Increased oxidative DNA damage, as assessed by urinary 8-hydroxy-2′-deoxyguanosine concentrations, and serum redox status in persons exposed to mercury. Clini Chem 51(4):759–767
Chen LJ, Tang LY, He JR, Su Y, Cen YL, Yu DD et al (2012) Urinary strontium and the risk of breast cancer: a case-control study in Guangzhou, China. Environ Res 112:212–217
Duzgoren-Aydin NS, Wong CSC, Aydin A, Song Z, You M, Li XD (2006) Heavy metal contamination and distribution in the urban environment of Guangzhou, SE China. Environ Geochem Health 28(4):375–391
Esteban-Vasallo MD, Aragonés N, Pollan M, López-Abente G, Perez-Gomez B (2012) Mercury, cadmium, and lead levels in human placenta: a systematic review. Environ Health Perspect 120(10):1369–1377
Filipič M, Hei TK (2004) Mutagenicity of cadmium in mammalian cells: implication of oxidative DNA damage. Mutat Res Fundam Mol Mech Mutagen 546(1):81–91
Fraga CG (2005) Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med 26:235–244
French P, Gardner MJ, Gunn AM (1989) Dietary aluminium and Alzheimer’s disease. Food Chem Toxicol 27:495–498
Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ et al (2011) Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci USA 108(51):20656–20660
Görür FK, Keser R, Akçay N, Dizman S (2012) Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere 87:356–361
Gupta VB, Anitha S, Hegde ML, Zecca L, Garruto RM, Ravid R et al (2005) Aluminium in Alzheimer’s disease: are we still at a crossroad. Cell Mol Life Sci 62:143–158
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, vol 3. Oxford University Press, Oxford, pp 1–543
Heitland P, Köster HD (2006) Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP–MS. J Trace Elem Med Bio 20(4):253–262
Ivanenko NB, Ivanenko AA, Solovyev ND, Zeimal’ AE, Navolotskii DV, Drobyshev EJ (2013) Biomonitoring of 20 trace elements in blood and urine of occupationally exposed workers by sector field inductively coupled plasma mass spectrometry. Talanta 116:764–769
Jiang J, Lu S, Zhang H, Liu G, Lin K, Huang W (2015) Dietary intake of human essential elements from a total diet study in Shenzhen, Guangdong Province, China. J Food Compos Anal 39:1–7
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicol 283(2):65–87
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D et al (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31(2):95–107
Komatsu F, Kagawa Y, Ishiguro K, Kawabata T, Purvee B, Otgon J et al (2009) The association of very high hair manganese accumulation and high oxidative stress in Mongolian people. Curr aging Sci 2(1):28–42
Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J et al (2007) Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev 10(1):1–269
Kumar V, Bal A, Gill KD (2009) Aluminium-induced oxidative DNA damage recognition and cell-cycle disruption in different regions of rat brain. Toxicol 264(3):137–144
Lee JW, Lee CK, Moon CS, Choi IJ, Lee KJ, Yi S-M et al (2012) Korea National Survey for Environmental Pollutants in the Human Body 2008: heavy metals in the blood or urine of the Korean population. Int J Hyg Environ Health 215:449–457
Li F, Zeng XY, Wu CH, Duan ZP, Wen YM, Huang GR et al (2013) Ecological risks assessment and pollution source identification of trace elements in contaminated sediments from the Pearl River Delta, China. Biol Trace Elem Res 155(2):301–313
Li J, Fan R, Lu S, Zhou Y, Lv Y (2015a) Exposure to polycyclic aromatic hydrocarbon could cause their oxidative DNA damage: a case study for college students in Guangzhou, China. Environ Sci Pollut Res 22:1770–1777
Li J, Lu S, Liu G, Zhou Y, Lv YS, Fan R et al (2015b) Co-exposure to polycyclic aromatic hydrocarbons, benzene and toluene and their dose-effects on oxidative stress damage in kindergarten-aged children in Guangzhou, China. Sci Total Environ 524–525:74–80
Lin K, Lu S, Wang J, Yang Y (2015) The arsenic contamination of rice in Guangdong province, the most economically dynamic provinces of China: arsenic speciation and its potential health risk. Envir Geochem Health 37:353–361
Lu SY, Zhang HM, Sojinu SO, Liu GH, Zhang JQ, Ni HG (2015) Trace elements contamination and human health risk assessment in drinking water from Shenzhen, China. Environ Monit Assess 187(1):1–8
Magge H, Sprinz P, Adams WG, Drainoni ML, Meyers A (2013) Zinc protoporphyrin and iron deficiency screening trends and therapeutic response in an urban pediatric center. Jama Pediatr 167:361–367
Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D et al (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116(11):1473–1479
Nayak P, Chatterjee AK (2001) Differential responses of certain brain phosphoesterases to aluminium in dietary protein adequacy and inadequacy. Food Chem Toxicol 39:587–592
Nowicka AM, Krasnodebska-Ostrega B, Wrzosek B, Jastrzebska M, Sadowska M, Mackiewicz M et al (2014) Detection of oxidative damage of synthetic oligonucleotides caused by thallium (III) complexes. Electron Anal 26(2):340–350
Paschal DC, Ting BG, Morrow JC, Pirkle JL, Jackson RJ, Sampson EJ et al (1998) Trace metals in urine of United States residents: reference range concentrations. Environ Res 76:53–59
Prousek J (2007) Fenton chemistry in biology and medicine. Pure Appl Chem 79:2325–2338
Roca M, Sànchez A, Pèrez R, Pardo O, Yusà V (2016) Biomonitoring of 20 elements in urine of children. Levels and predictors of exposure. Chemosphere 144:1698–1705
Saiyed SM, Yokel RA (2005) Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Addit Contam 22:234–244
Shi H, Hudson LG, Liu KJ (2004) Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radical Biol Med 37(5):582–593
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–326
Sughis M, Nawrota TS, Riaz A, Ikram-Dar U, Mahmood A, Haufroid V et al (2014) Metal exposure in schoolchildren and working children. A urinary biomonitoring study from Lahore, Pakistan. Int J Hyg Environ Health 217:669–677
Szymańska-Chabowska A, Beck A, Poręba R, Andrzejak R, Antonowicz- Juchniewicz J (2009) Evaluation of DNA damage in people occupationally exposed to arsenic and some heavy metals. Pol. J. Environ Stud 18:1131–1138
Tan JH, Duan JC (2013) Heavy metals in aerosol in China: pollution, sources, and control strategies. J Graduate Univ Chi Acad Sci 30(2):145–155
Taussky HH (1954) A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem 208(2):853–862
Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci health Part C Environ Carcinog Rev 27(2):120–139
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Valko M, Rhodes CJ, Moncol J, Izakovic MM, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact 160(1):1–40
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
White MA, Sabbioni E (1998) Trace element reference values in tissues from inhabitants of the European Union. X. A study of 13 elements in blood and urine of a United Kingdom population. Sci Total Environ 216(3):253–270
Yang M, Jiang LX, Huang HP, Zeng SB, Qiu F, Yu M et al (2014) Dietary exposure to aluminium and health risk assessment in the residents of Shenzhen, China. PLoS One 9(3):1–8
Yu T, Zhang Y, Hu XN, Meng W (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake, China. Ecotoxicol Environ Saf 81:55–64
Zeiner M, Ovari M, Zaray G, Steffan L (2006) Selected urinary metal reference concentrations of the Viennese population-urinary metal reference values (Vienna). J Trace Elem Med Biol 20:240–244
Zhang YH, Xie SD, Zeng LM, Wang HX, Yu KH, Zhu CJ et al (1999) Traffic emission and its impact on air quality in Guangzhou area. J Environ Sci 11:355–360
Zoni S, Lucchini RG (2013) Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Curr Opin Pediatr 25:255–260
Acknowledgments
This research was financially supported by the National Nature Science Foundation of China (No. 41303094), the Natural Science Foundation of Guangdong Province, China (No. 2015A030313869), the Shenzhen Municipal Government Research Projects (No. JCYJ20140415091352816, JCYJ20120616154147373), and the Earmarked Fund of the State Key Laboratory of Organic Geochemistry (OGL-201405).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Lu, S., Ren, L., Fang, J. et al. Trace elements are associated with urinary 8-hydroxy-2′-deoxyguanosine level: a case study of college students in Guangzhou, China. Environ Sci Pollut Res 23, 8484–8491 (2016). https://doi.org/10.1007/s11356-016-6104-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6104-8