Abstract
The analysis of the airborne particulate matter—PM—incorporated to plant leaves may be informative of the air pollution in the surroundings, allowing their use as biomonitoring tools. Regarding metals, their accumulation in leaves can be the result of both atmospheric incorporation of metallic PM on aboveground plant organs and root uptake of soluble metals. In this study, the use of Nerium oleander leaves as a biomonitoring tool for metallic airborne pollution has been assessed. The metal uptake in N. oleander was assessed as follows: (a) for radicular uptake by irrigation with airborne metals as Pb, Cd, Cr, Ni, As, Ce and Zn (alone and in mixture) and (b) for direct leave exposure to urban PM. Plants showed a high resistance against the toxicity of metals under both single and multiple metal exposures. Except for Zn, the low values of translocation and bioaccumulation factors confirmed the excluder behaviour of N. oleander with respect to the metals provided by the irrigation. For metal uptake from airborne pollution, young plants grown under controlled conditions were deployed during 42 days in locations of the city of Zaragoza (700,000 h, NE Spain), differing in their level of traffic density. Samples of PM2.5 particles and the leaves of N. oleander were simultaneously collected weekly. High correlations in Pb concentrations were found between leaves and PM2.5; in a lesser extent, correlations were also found for Fe, Zn and Ti. Scanning electron microscopy showed the capture of airborne pollution particles in the large and abundant substomatal chambers of N. oleander leaves. Altogether, results indicate that N. Oleander, as a metal resistant plant by metal exclusion, is a suitable candidate as a biomonitoring tool for airborne metal pollution in urban areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban air pollution is a serious concern for human health; as example, airborne particulate matter is related to the increase of respiratory and cardiac diseases (Künzli et al. 2000; Pope et al. 1995; Seaton et al. 1995). The breathable fraction of that particulate matter presenting diameters under 2.5 μm (PM2.5) is especially worrying because it is more strongly associated than inhalable fraction (PM10) with acute respiratory health effects, as it has been reported in children (Schwartz and Neas 2000). Metals are one of the main components of PM2.5 (USEPA 1996). Moreover, it has been described that certain toxic metals such as Pb, Cd, Ni and Cu are better associated with PM2.5 than PM10 fraction of the airborne particulate matter (Samara and Voutsa 2005).
Although we have spatial and quantitative detailed information of metal emission in different cities (Hjortenkrans and Bergbäck 2005; Kakareka et al. 2004), there is still a lack of immission data for these pollutants. The need of a large amount of sampling stations, whose operations are quite expensive, is an insuperable drawback for implementing the monitoring programs at high spatial resolution that prevent an adequate environmental risk assessment for metal exposure in urban environments. An alternative method that may help in tackling this obstacle could be the use of widespread organisms, as biomonitoring tools.
Large number of higher plants, such as Ligustrum japonicum, Olea europea, Pinus brutia, Platanus orientalis, Populus alba, Populus nigra, Robinia pseudoacacia, Capsella bursa-pastoris, Tillandsia capillaries and Phoenix dactylifera, have been used as biomonitoring tools in urban environments (Aksoy et al. 1999; Al-Alawi and Mandiwana 2007; Al-Shayeb et al. 1995; Çelik et al. 2005; Pignata et al. 2002; Sawidis et al. 1995). These studies showed that particulate matter adheres to leaves and may be absorbed, being thus used to assess the air pollution in the plant surroundings.
Nerium oleander is evergreen xerophyte species widespread in Zaragoza which has been proposed as a biomonitor of heavy metals in Mediterranean areas by several authors (Aksoy and Ozturk 1997; Dongarra et al. 2003; Fernández Espinosa and Oliva 2006; Mingorance and Oliva 2006; Mingorance et al. 2007; Rossini Oliva and Mingorance 2006). In this way, significant correlations between metal concentrations in surface soil and leaf samples have been obtained (Aksoy and Ozturk 1997), as well as between N. oleander leaves and PM10 particles (Fernández Espinosa and Oliva 2006). In addition, N. oleander is able to survive in extremely heavy metal polluted soils due to its strong excluder behaviour, as it has been reported (Franco et al. 2012, 2013).
In biomonitoring studies of metal pollution, it is difficult to distinguish between atmospheric and soil contribution. For this reason, both sources of metals should be considered. By means of washing N. oleander leaves, a maximum contribution of the airborne pollution of 56, 46, 37 and 39 % for Pb, Cd, Zn and Cu, respectively, has been reported by Aksoy and Ozturk (1997). There are only a few studies of N. oleander plants grown in metal polluted soils under controlled conditions. These studies showed synergistic uptake of Cu/Zn and Pb/Cd in N. oleander, being Pb and Cd mainly accumulated in roots (Drakatos et al. 2000; Kadukova et al. 2006; Kalavrouziotis and Drakatos 2002). Anyway, important aspects as the metal uptake and translocation ratios or the simultaneous presence of different metals in the soil have not been yet taken into account.
Thus, the aim of this study is to assess the use of N. oleander as a biomonitor of the immission of the airborne metal pollution related to inhalable particles (PM2.5) in the urban area of Zaragoza (700,000 h, NE Spain). To achieve this goal, two experimental approaches were designed: (a) greenhouse pot experiments in which N. oleander plants were treated with metals related to urban pollution (Pb, Cd, Cr, Ni, As, Ce and Zn), with the objective of both to assess the ability of resistance and to characterize the metal uptake behaviour of N. Oleander; and (b) a screening experiment in which samples of PM2.5 particles and samples of N. oleander leaves were collected simultaneously in seven locations within the urban area of Zaragoza city in order to study their correlation. This work was complemented with low temperature-scanning electron microscopy and energy dispersive X-ray analyses (LT-SEM-EDX) of N. oleander leaves from both experiments, in order to study the differences in the ultrastructure of leaf tissues and in the micro-localization of metals.
Materials and methods
Greenhouse pot experiment
Eight month-aged plants of N. Oleander L. cv. “Rosa Bartolini” in a height range of 15–20 cm were selected for this experiment. Plant material was provided by Garden Center Aznar (Zaragoza, Spain). Plants were transferred into 20 L plastic pots filled with organic substrate (Universal Substrate Blumenerde, Capriflor®) and silica sand in a 3:1 ratio as growth substrate. Plants were irrigated with tap water (pH 7.0; 644 μS/cm at 25 °C; trace elements were under detection limits) up to water holding capacity twice per week during 20 days as acclimatization period and kept into a greenhouse (20 ± 2 °C; 40–60 % humidity). A randomized block design was carried out as follows: eight treatments (Pb, Cd, Cr, Ni, As, Ce, Zn and mixture) ×3 doses (0, 10 and 100 μM) ×3 replicates (pots) plus 1 control × 4 replicates (each experimental unit consisted of one pot with one plant).
Metal solutions were prepared using CdCl2.2, 5H2O; CrCl3.6H2O; NiCl2.6H2O; Ce(SO4)2.4H2O; Na2AsO4.7H2O; PbCl2; and ZnSO4.H2O (all PA-ACS grade from PANREAC©, Barcelona, Spain) dissolved in deionized water. Separate solutions were prepared for Pb, Cd Cr, Ni, As and Zn treatments and all metals together for mixture treatment. Metal solutions for treatments and tap water for control were used to irrigate plants up to water holding capacity twice per week during 42 days. After irrigation, pH and EC were measured in the substrate of treated and control pots (pH 5.34–6.09; EC 195–409 μS/cm at 25 °C). Heights of plants were measured every 7 days using a measuring tape. After 42 days of treatment, substrate and plant samples were collected.
Screening of airborne metals using air sampler and N. oleander leaves
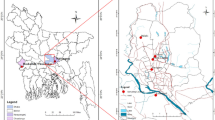
Samples of PM2.5 particles and samples of N. oleander leaves were collected simultaneously in seven different sites (COR, STA, JES, NAV, TFL, MAD and DEL, details in Fig. 1). These locations were selected because of their differences in the road traffic intensity as follows: 7552, 13,101, 306, 8107, 20,859, 20,646 and 14,734 vehicles per day (2010 yearly data provided by the City Council of Zaragoza). The sampling period ranged from September 2011 to August 2012. At each sampling point, four plants were placed, together with an air sampler MicroVol 1100 (Ecotech ©, Knoxfield, Australia), equipped with a PM2.5 inlet (collecting particles under 2.5 μm Ø). The sampler worked in continuous with a flow rate of 3 l/min. Fiberquartz filters of 47-mm diameter and 0.45-μm pore size were used as collection media. Soils of pots were covered with plastic to avoid the deposition of atmospheric pollution.
Samples of airborne particles and leaves of N. oleander were collected every 7 days during 42–47 days, depending on the location. Accordingly, between 6 and 7 samplings were done at every location, each one consisting in one filter containing PM2.5 and plant leaves. Healthy leaves (2–4) from a younger and completely developed whorl (with size similar to the older ones) were selected and cut off. Samples were dried 48 h at 60 °C and then ground to a fine powder and analyzed for metals. Concentrations of metals in both leaves and filters from each sampling were normalized per day of exposure to urban pollution and per dry weight, in order to plot and statistic calculations. Therefore, metal results were expressed as nmol g−1 dry weight day−1 for leaves and as nmol g−1 m−3 air day−1 for the PM2.5.
Analytical determinations
Substrate samples were air-dried and sieved at the minor than 2 mm prior to the analyses. Quantitative paper-filtered (Filter-Lab, S A) 1:2.5 and 1:5 substrate/water extracts made by mixing air-dried soil and deionised water, shaking at 180 rpm for 1 h and waiting for 30 min prior to using a digital pH meter and a conductivity meter (WTW Multiline P4 and Hach-Lange HQ) were performed respectively (Cabezas et al. 2008). The EDTA fraction was extracted from 10 g of substrate with 100 mL of 0.1 M ethylene-diamine tetra-acetic acid (EDTA) shaken in plastic flasks at 180 rpm for 4 h at room temperature (Chojnacka et al. 2005). For the total concentration of metals (pseudo-total), 100 mg of substrate and 5 mL mixture of hydrochloric-nitric acid (4:1) were digested in an open-vessel microwave digestion system (Ultraclave Microwave Milestone) for 20 min at 220 °C (Mingorance and Oliva 2006).
Plant material was thoroughly washed with tap water and was given a final rinse with deionised water. Leaves, stems and roots were dried at 60 °C for 48 h to determine dry weight. Dried matter was ground and digested as described above (Mingorance and Oliva 2006). Whole filters were also digested by the same protocol (Mingorance and Oliva 2006). Filtered EDTA extracts and acid digests were analysed for metal concentrations by simultaneous inductively coupled plasma (ICP/OES, Mod. ICAP 6500 DUO THERMO©). Calibration curves were performed using standard stock solution 1.0 mg/L diluted in 10 % nitric acid. Recovery for metals ranged from 80 % (Cr, Pb) to 92 % (Zn). The detection limit for all metals was established at 0.5 mg Kg−1 dry weight.
Low temperature-scanning electron microscopy and microanalysis
The N. oleander leaf pieces were mounted on aluminium stubs with adhesive (Gurr®, optimum cutting temperature control; BDH, Poole, UK), cryo-fixed in slush nitrogen (−196 °C), cryo-transferred to a vacuum chamber at −180 °C and then fractured using a stainless steel spike. Once inside the microscope, the samples underwent superficial etching under vacuum (−90 °C, 120 s, 2 kV) and then overlaid with Au for observation and microanalysis with a Zeiss digital scanning microscope (DSM 960). Fractured samples were observed at low temperature with a digital scanning electron microscope (Zeiss©, model DSM 960, Oberkochen, Germany) using secondary and back-scattered electrons. Secondary electron images (1024 × 960 pixels) were obtained at 133 eV operating at a 35° take-off angle, an accelerating voltage of 15 kV, a working distance of 25 mm and a specimen current of 1–5 nA.
Microanalysis was carried out in specific leaf areas (approximately 15 × 15 μm) using an energy dispersive X-ray microanalysis (EDX) Pentaflet apparatus (Pentaflet©, Oxford, UK). Areas tested were adaxial epidermis, palisade parenchyma, xylem vessels, spongy parenchyma and abaxial epidermis. Whenever possible, a single cell area was targeted. Semiquantitative element analysis was obtained using standard ZAF (atomic number, absorption and fluorescence) correction procedures, using Link Isis v3.2 Software (Link Isis©, Oxford, UK). Eight points of analysis per leaf tissue and three leaves per treatment were analyzed. Due to the limitations of the technique (LT-SEM-EDX) and according to the purpose to compare airborne vs root via, one representative sample (three leaves) from NAV location was selected as airborne polluted leaves. The thickness of the different leaf layers (adaxial epidermis, palisade parenchyma, spongy parenchyma and abaxial epidermis) was measured manually with image analysis software (Photoshop CS3; Adobe, San Jose, CA, USA) using images similar to those shown in Fig. 8 (total, 18 images = 3 images × 3 leaves × 2 treatments—airborne polluted and 100 μM mixture).
Statistical analyses
Values in the tables indicate mean values ± standard error (SE). The obtained data have normal distribution; therefore, differences among treatments were analysed by one-way analysis of variance (we focused on increasing the power of the analysis to detect differences between metals in a clearer way), followed by a post hoc multiple comparisons of means using the Duncan test (P < 0.05). Simple linear regressions between the concentration of each metal in leaves vs PM2.5 particles, plant organs (shoot and root) vs substrate (total and EDTA extractable) were calculated. The Pearson coefficients (R) were used to assess how well the regression line represents the data. Statistical analyses were performed using SPSS PC 10.0 package software.
Results
Impact of metals on plant growth
Exposure to metals inhibited plant growth impacting both heights and weights. Figure 2 showed the height of N. oleander shoots after 7, 14, 21, 28, 35 and 42 days of treatment. In general, higher decreases in height of treated plants in comparison to the control were observed with increasing metal dose and exposure time. Among metals, Pb, Cd, Cr and Ni showed a notable reduction of the weights even at 1 μM concentration (Fig. 2a–d). Significant decreases in heights in comparison to the control were only observed at 10 and 100 μM for As and Ce (Fig. 2e, f). Likewise, only the 100 μM concentration of Zn and mixture treatments reduced significantly the height of N. oleander plants (Fig. 2g, h). In the case of Zn, a higher weight in 1 and 10 μM treated plants than control was obtained (Fig. 2g).
The percentage of reduction in weight of treated plants in comparison to control at the end of the pot experiment (42 days) was shown in Fig. 3. A reduction in the biomass of treated plants was observed in all cases but 1 μM As and 1 μM Ce. A higher reduction of the biomass with increasing doses was observed though only significantly for the Cd, As, Zn and mixture treatment. For the 100 μM dose, the highest percentage of weight reduction was observed as followed: Cd (58.6 %), Pb (44.9 %), Cr (40.7 %), As (37.2 %), Zn (33 %), Ni (30.2 %), Ce (29.5 %) and mixture (28.8 %).
Percentage of biomass reduction of N. oleander shoots (g f.wt.) after 42-day-treatments with Cd, Pb, Cr, As, Zn, Ni, Ce and mixture treatments vs. control at 1 (dark grey), 10 (light grey) and 100 μM (medium grey) doses. Data are expressed as mean ± SE. Different non-italic letters indicate significant differences among treatments (P < 0.05; n = 3). Different italic letters in the same treatment indicate significant differences among doses (P < 0.05; n = 3)
Metals in the growth medium
The total concentration of metals in the growth substrate was higher with increasing doses (Fig. 4). The highest metal concentrations in the substrate were observed for 100 μM treatments. Regarding the presence of other pollutants in the medium, lower substrate concentrations in the mixture treatment than those supplied alone were obtained for Pb, Cd and Zn at 10 and 100 μM (Fig. 4a, b and g) and for Cr and Ni at 10 μM (Fig. 4c, d). However, higher substrate concentrations for As and Ce in the mixture than in the alone supply at all doses were observed (Fig. 4e, f). Thus, the maximum levels of total substrate concentration (nmol g−1) were as follows: Pb (587), Cd (499), Ni (461), Cr (392), Zn (352), As (229), and Ce (183). These values were notably lower than those established as the threshold for non-contaminated soils in the region (BOA 2008), in all cases except for As (still lower but close to the limit value) and Cd (four- to -fivefolds higher than the limit value). The average percentage of EDTA-extractable metal with respect to the total concentration in substrate was shown in Fig. 4h. The highest percentage of EDTA-extractable metal was observed for Cd (84 %) followed by As, Ni, Pb (55–57 %), Ce (27 %), Zn (14 %) and Cr (9 %).
Concentration of metals in the substrate (nmol g−1) after 42-day-treatments with Pb (a), Cd (b), Cr (c), Ni (d), As (e), Ce (f) and Zn (g), in both alone (dark grey) and mixture (medium grey) supply, at 1, 10 and 100 μM doses. Red line indicates the allowable limits in urban soils in the Aragon region. Percentage of EDTA-extractable metal with respect to the total concentration of metal in the substrate (light grey) (h). Data are expressed as mean ± SE. Different non-italics letters indicate significant differences among doses (P < 0.05; n = 3). Different italics letters in the same treatment indicate significant differences among alone and mixture supply (P < 0.05; n = 3). Zn treatment included control concentrations
Uptake and translocation of metals
Metal concentrations in root and shoot of N. oleander were shown in Fig. 5. Plant concentrations of Ce were under the limit of detection (<0.5 mg Kg−1). Root and shoot concentrations were higher with increasing doses for all pollutants but Zn. In all cases except for root Zn concentrations at 1 and 10 μM, lower plant concentrations in the mixture treatment than those in the corresponding separated ones were observed. Thus, the maximum concentration (nmol g−1 d.wt.) of metals in plants under one-metal treatments (alone) at 100 μM doses were the following: As (1539), Zn (683), Cr-Cd-Ni (85–107), Pb (29) and Ce (n.d.) for root and Zn (683), As (163), Cr, Cd, Ni (19-20), Pb (12) and Ce (n.d.) for shoot (Fig. 5). High positive correlation coefficients between substrate and plant concentrations were obtained in all cases but Zn (Table 1). Table 2 showed the translocation factor (TF) and the bioaccumulation factors (Total-BF and EDTA-BF) for N. oleander plants defined as follows:
Root and shoot concentration of metals (nmol g−1 d.wt.) after 42-day-treatments with Pb (a, b), Cd (c, d), Cr (e, f), Ni (g, h), As (i, j) and Zn (k, l), in both alone (dark grey) and mixture (light grey) supply, at 1, 10 and 100 μM doses. Data are expressed as mean ± SE. Different non-italics letters indicate significant differences among doses (P < 0.05; n = 3). Different italics letters in the same treatment indicate significant differences among alone and mixture supply (P < 0.05; n = 3). Zn treatment included control concentrations
The N. oleander plants showed low translocation factors (TF < 1) for all pollutants but Zn. Regarding BFs, levels ranged between 0.01 and 0.09 for Pb, Cd and Ni in all cases, 10-fold higher for As and EDTA-Cr and between 2 and 31 in the case of Zn (Table 2). For Cr, As and Zn, higher values in EDTA-BF than in Total-BF were found.
Airborne metal pollution accumulated in leaves
Concentrations of Cd and As in PM2.5 particles were below the limit of detection in all cases, as well as those of Ce and Ni for most of the collected samples (data not shown). Taking together all samples collected from the different sites, a significant linear correlation in the average Pb concentration between N. oleander leaves and PM2.5 particles was found (Fig. 6). Considering the metals separately, significant linear correlations between the concentration of metals of N. oleander leaves and PM2.5 were found for Fe and Pb (in NAV), Zn (in TFL) and Ti (in JES). As shown in Fig. 7, leaves accumulate metals as a function of the metals accumulated as PM2.5 in the air samplers.
Correlation in the concentration of Pb along the sampling period (42 days) between N. oleander leaves (nmol g−1 d.wt. day−1) and PM2.5 particles (nmol g−1 m−3 day−1) collected in each sampling site (R = Pearson coefficient, *significant at probability level P < 0.05). Error bars are standard deviations
The increase of metals accumulated in N. oleander leaves (normalized vs day 0) as a function of the accumulated exposure to the metals present in the surrounding PM2.5 (R = Pearson coefficient, **significant at probability level P < 0.01 and *P < 0.05). The number below each point indicates the days of exposure to PM2.5
Structural and chemical composition of leaves using LT-SEM-EDX
Leaf tissue structural information of cryo-fractured N. oleander leaves of airborne polluted (blue) and 100 μM mixture treated (red) plants was obtained using LT SEM (Fig. 8a–d). Leaves exposed to airborne pollution were thinner than 100 μM mixture treated leaves, with a 16.6 % reduction in total leaf thickness (Fig. 8a, b). The 100 μM treated plants show also a more compact mesophyll tissue than the airborne polluted ones (Fig. 8a, b). Figure 8c, d shows a notable number of particles trapped in substomatal chambers of airborne polluted leaves which were not presented in 100 μM mixture treated plants. The size and the abundance of the substomatal chambers in the abaxial surface of N. oleander leaves were shown in Fig 9.
Freeze–fracture low-temperature scanning electron micrographs of leaf transversal sections from airborne polluted (blue) and 100 μM mixture treated (red) N. oleander leaves. Leaf transversal sections on the left give three-dimensional information on the internal structure of the leaf, confirming the disorganisation caused by 100 μM mixture (b) in comparison to airborne pollution (a). Detailed zoom of the substomatal chambers showed the presence of particulate matter in to airborne polluted (c) in comparison to 100 μM mixture treated (d) leaves. In the middle part, a scheme of the N. oleander plant as a biomonitor of airborne metal pollution where metals from air pollution (blue points) are deposited on its leaves, and metals from soil pollution (red points) are retained mainly in root and only a few amount of those reach shoot. Scale bars—upper left corners—represent 200, 200, 20 and 50 μm (a, b, c, d)
EDX analysis (semiquantitative analysis) in the different layers of leaf cross, including adaxial epidermis, palisade parenchyma, xylem vessels, spongy parenchyma, and abaxial epidermis, showed no differences in the relative metal signals across the leaf section (details in Tab. S1 at Electronic Supplementary Information). Likewise, no differences in the EDX results between airborne polluted and 100 μM mixture treated leaves were found.
Discussion
The plants exposed to metals via root uptake were able to survive in all cases. These results are in agreement with the extremely high ability of resistance against the toxicity of metals reported by (Franco et al. 2012, 2013) for this plant under field conditions. Height measurements indicated that doses and exposure time were the main factors involved in the growth inhibition by metals (Figs. 2 and 3). The same behaviour has been widely reported in the literature (Kabata-Pendias and Pendias 1992). The exposure to metal mixture resulted in a lower biomass reduction than those found in single treatments (alone) (Fig. 3). This fact is in accordance to the lower concentration of metals in the mixture than in the single treatments found in the substrate, except for As and Ce, (Fig. 4), and also in N. oleander plants in all cases (Fig. 5). The lower substrate concentrations of Pb, Cd, Cr, Ni and Zn in the mixture than in the single treatments could be due to the competition of metals for the binding sites in the substrate.
The mixture of metals supplied to the substrate could lead to a prompt saturation of the binding sites and subsequent lixiviation of the remained metals in solution (Alloway 1995). However, when only one metal was added, a higher fraction of the metal was retained by the substrate. In the case of Ce and As, tetravalent and metalloid, respectively, their different physico-chemical characteristics in comparison to the Pb, Cd, Cr, Ni and Zn, all of them are bivalents except Cr (trivalent), could involve a different behaviour with respect to the binding sites of the substrate. An opposite behaviour between As and heavy metals with respect to their interaction with the soil fractions has been reported in a previous study (Fitz and Wenzel 2002).
An indirect approach to assess the relative affinity of each metal to the binding sites of the substrate is the determination of the bioavailable fraction of the metal in the substrate. The estimated bioavailability of metals is strongly dependent on the components of the growth media, concentration of the metal, the plant species and the selected extractant solution (Vázquez et al. 2008). Taking into account the high cation-exchange capacity (CEC) of the substrate, due to its high content in organic matter, a stronger extractant as EDTA than inorganic salts was selected. The order of bioavailability was Cd > As ∼ Ni ∼ Pb > Ce > Zn > Cr (Fig. 4h). A low bioavailability is a characteristic trail of Pb that iswidely reported in the literature (Adriano 2001). Thus, in this study, the bioavailability of Pb (55 %) could be overestimated by the EDTA-extractable metal because of the high stability of the Pb-EDTA complex (Niinae et al. 2008). This is in agreement to the high concentration of Pb found in the substrate (Fig. 4), which indicated an important retention of Pb by the substrate. According to our data, Cd was the most bioavailable metal (84 %). Likewise, a high bioavailability of Cd has also been reported in soils (Kabata-Pendias 2004). In the case of As, its estimated EDTA bioavailability could be shift by its opposite behaviour with respect to the biding sites in comparison to the metals and its close relationship to phosphorous (Fitz and Wenzel 2002). However, it is worth to mention that the most bioavailable metals, Cd (84 %) and As (57 %), are also those whose substrate concentrations were higher and closer to the reference levels reported in soils (BOA 2008), respectively (Fig 4b and e). This fact pointed out at Cd and As as the most potential hazardous pollutants in the growth media.
The order of toxicity of the metals with respect to N. oleander assessed by the maximum percentage of biomass reduction was Cd > Pb ∼ Cr > As > Zn ∼ Ni ∼ Ce ∼ mixture (Fig. 3). The toxicity of these metals in N. oleander is the product of several factors related to the substrate (concentration and bioavailability), mentioned above, and factors related to the plant. Regarding the latter, Zn has a clear different pattern with respect to the other metals: higher biomass under 1 and 10 μM Zn treatments (Fig. 3), high values in root and shoot concentrations of Zn and no increase of them with increasing doses (Fig. 5k, l). No significant correlations were found between substrate concentration and plant (Table 1), but higher TF and BFs than for the other metals were found (Table 2). The different pattern of Zn is due to the fact that Zn is a nutrient unlike the other metals considered as pollutants. This fact involved the existence of specific uptake and translocation mechanisms of Zn as well as a higher toxicity threshold of Zn in plants (Marschner 1995).
In the case of As, high root and shoot concentrations (Fig. 5j, k) and 10-fold higher BFs (Table 2) were observed. These results could be explained by the antagonism between As and P. Arsenic and phosphorous are intimately linked in plants due to the chemical similarity between them. It is thought that As to be taken up by plants via P uptake system and the subsequent toxicity in plant is due to the disturbance of many physiological phosphate functions (Fitz and Wenzel 2002; Meharg and Hartley-Whitaker 2002). Thus, a high correlation in shoot concentrations between As and P was observed (Tab. S2 Electronic Supplementary Information). Regarding the rest of the metals, the low values of TF and BFs indicated an excluder behaviour of N. oleander (Baker 1981). The same excluder behaviour of N. oleander with respect to heavy metals has been reported by (Franco et al. 2012, 2013) under field conditions.
LT-SEM pictures (Fig. 8a, b) showed a more disrupted leaf tissue structure in N. oleander plants polluted via root uptake than those via airborne deposition. These findings confirmed the inhibition of plant growth, both in height (Fig. 2h) and in weight (Fig. 3), induced by the 100 μM mixture treatment. The higher concentration of metals in the substrate in the pot experiment (Fig. 4) than those in the leaf surface in the screening experiment (Figs. 6 and 7) would explain the different grades of alteration in the leaf anatomy. Similarly, less thickness and more disorganized tissues in Beta vulgaris leaves with increasing doses of Zn have been reported by Sagardoy et al. (2010). In biomonitoring studies, the nature of the leaf surface is an important factor that affects the deposition process of airborne particulate matter on the leaf. Thus, the capture of particles in the substomatal chambers from the airborne pollution observed in this study (Fig. 8c, d) could result in an important contribution to the total amount of metals in N. oleander leaves, especially in this species due to the large size and number of these chambers in its abaxial side (Fig. 9). Similar findings in the ability to trap airborne particulate matter based on the leaf surface characteristics between Lamana camara and N. oleander were suggested by Fernández Espinosa and Oliva (2006).
The experiments exposing plants to urban pollution showed that Pb was incorporated in leaves as a function of the Pb present in the PM during the sampling period (Fig. 6). This significant relationship was also found for certain sites as NAV (see Fig. 7b), showing a clear exposure-related increase of the Pb in leaves. Other metals with significantly high correlations between N. oleander leaves and PM2.5 were Fe, Zn and Ti (Fig. 7a, c, d). Similar high correlations for Fe and Zn in N. oleander plants expose to air pollution were found by Fernández Espinosa and Oliva (2006) and Aksoy and Ozturk (1997), respectively. The % of Pb bioaccumulated from roots during the 42 days exposure to urban pollution has been also calculated. The value, 18 ± 6 %, strongly indicates that the rest of Pb found in leaves is the result of the exposure to urban pollution (calculations are shown at Tab. S3 Electronic Supplementary Information).
Lead has been reported as the main metal supplied by aerial deposition in urban areas in proportion to the density of traffic and distance from the roadside (Aksoy and Ozturk 1997; Dongarra et al. 2003). But in our case, it should be noticed an apparent lack of accordance between traffic intensity (JES < < COR < NAV < < STA < DEL < < MAD ≈ TFL, see “Screening of airborne metals using air sampler and N. oleander leaves” section) and Pb airborne pollution (COR < STA < JES ≈ NAV < TFL < MAD ≈ DEL). But this discordance is explained by the use of traffic intensity values that have been calculated in a different period, having thus only a qualitative value to characterize the potential level of air pollution at each site. Even though, as shown in Fig. 6, N. oleander leaves accumulate Pb as a function of the metals present as PM2.5, being thus a good indicator of the air pollution in their surroundings.
The lack of pattern and differences in the relative metal signals across the leaf section between 100 μM mixture treated and air polluted leaves indicated a non-significant penetration of the metals through N. oleander leaf tissues exposed to polluted air. This result supported the importance of the airborne particle matter trapped in the substomatal chambers of N. oleander leaves when they are used as biomonitoring tool. Regarding the 100 μM mixture treated leaves, these EDX results would also confirm the notably excluder behaviour of N. oleander. In this sense, preferential accumulation of metals in leaf tissues was only reported in N. oleander plants grown under extremely high levels of heavy metals (Franco et al. 2012, 2013).
Conclusion
Screening data indicated high correlations in the concentration of Pb and in lesser extent of Fe, Zn and Ti between N. oleander leaves and PM2.5 particles. N. oleander showed a high ability of resistance against the toxicity of trace metals, particularly for Cd and As that were considered the most potential hazardous metals according to their bioavailability and reference levels in soil. In comparison to single treatments, metal mixture treatments showed a lower retention of Pb, Cd, Cr, Ni and Zn in the substrate, lower plant concentrations of metals and subsequently a lower inhibition of plant growth. Except for Zn, N. oleander showed an excluder behaviour with respect to the metal pollutants present in the growth media.
LT-SEM analyses showed the capture of particle matter from the airborne pollution in the large and abundant substomatal chambers of N. oleander leaves. The leaves also showed the ability of accumulating metals (Pb and others) as a function of the metals presents as PM2.5 in their surroundings. All these findings indicated N. oleander plants as a suitable tool to assess the immission of the airborne metal pollution, especially for Pb, related to inhalable particles (PM2.5) in urban areas (see Fig. 6).
References
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer
Aksoy A, Ozturk MA (1997) Nerium oleander L. as a biomonitor of lead and other heavy metal pollution in Mediterranean environments. Sci Total Environ 205:145–150
Aksoy A, Hale WHG, Dixon JM (1999) Capsella bursa-pastoris (L.) Medic. as a biomonitor of heavy metals. Sci Total Environ 226:177–186. doi:10.1016/S0048-9697(98)00391-X
Al-Alawi MM, Mandiwana KL (2007) The use of Aleppo pine needles as a bio-monitor of heavy metals in the atmosphere. J Hazard Mater 148:43–46. doi:10.1016/j.jhazmat.2007.02.001
Alloway BJ (1995) Heavy metals in soils. Blackie Academic & Professional
Al-Shayeb SM, Al-Rajhi MA, Seaward MRD (1995) The date palm (Phoenix dactylifera L.) as a biomonitor of lead and other elements in arid environments. Sci Total Environ 168:1–10. doi:10.1016/0048-9697(95)04556-G
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654. doi:10.1080/01904168109362867
BOA (2008) Boletín Oficial de Aragón Orden del 05 de Mayo del 2008, del Departamento de Medio Ambiente, por la que se procede al establecimiento de los niveles genéricos de referencia para la protección de la salud humana de metales pesados y otros elementos traza en suelos de la Comunidad Autónoma de Aragón. Govierno de Aragón, Zaragoza, Spain
Cabezas A, González E, Gallardo B, García M, González M, Comín F (2008) Effects of hydrological connectivity on the substrate and understory structure of riparian wetlands in the Middle Ebro River (NE Spain): implications for restoration and management. Aquat Sc 70:361–376
Çelik A, Kartal AA, Akdoğan A, Kaska Y (2005) Determining the heavy metal pollution in Denizli (Turkey) by using Robinio pseudo-acacia L Environ Int 31:105-112 doi:10.1016/j.envint.2004.07.004
Chojnacka K, Chojnacki A, Górecka H, Górecki H (2005) Bioavailability of heavy metals from polluted soils to plants. Sci Total Environ 337:175–182
Dongarra G, Sabatino G, Triscari M, Varrica D (2003) The effects of anthropogenic particulate emissions on roadway dust and Nerium oleander leaves in Messina (Sicily, Italy). J Environ Monit 5:766–773. doi:10.1039/b304461k
Drakatos PA, Kalavrouziotis JK, Drakatos SP (2000) Synergism of Cu and Zn in plants irrigated with processed wastewater. Land Contamination & Reclamation 8:201–207
Fernández Espinosa AJ, Oliva SR (2006) The composition and relationships between trace element levels in inhalable atmospheric particles (PM10) and in leaves of Nerium oleander L. and Lantana camara L. Chemosphere 62:1665–1672
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil–rhizosphere–plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278. doi:10.1016/S0168-1656(02)00218-3
Franco A, Rufo L, de la Fuente V (2012) Metal concentration and distribution in plant tissues of Nerium oleander (Apocynaceae, Plantae) from extremely acidic and less extremely acidic water courses in the Río Tinto area (Huelva, Spain). Ecol Eng 47:87–91. doi:10.1016/j.ecoleng.2012.06.024
Franco A, Rufo L, Zuluaga J, Fuente V (2013) Metal uptake and distribution in cultured seedlings of Nerium oleander L. (Apocynaceae) from the Río Tinto (Huelva, Spain). Biol Trace Elem Res 155:82–92. doi:10.1007/s12011-013-9761-1
Hjortenkrans DST, Bergbäck BG, Häggerud AV (2007) Metal emissions from brake linings and tires: case studies of Stockholm, Sweden 1995/1998 and 2005 Environ Sci Technol 41:5224-5230 doi:10.1021/es070198o
Kabata-Pendias A (2004) Soil–plant transfer of trace elements—an environmental issue. Geoderma 122:143–149. doi:10.1016/j.geoderma.2004.01.004
Kabata-Pendias A, Pendias H (1992) Trace elements in soils and plants. vol v. 1991. CRC Press
Kadukova J, Manousaki E, Kalogerakis N (2006) Lead and cadmium accumulation from contaminated soils by Nerium Oleander. Acta Metallurgica Slovaca 12:181–187
Kakareka S, Gromov S, Pacyna J, Kukharchyk T (2004) Estimation of heavy metal emission fluxes on the territory of the NIS. Atmos Environ 38:7101–7109. doi:10.1016/j.atmosenv.2004.03.079
Kalavrouziotis IK, Drakatos PA (2002) Irrigation of certain Mediterranean plants with heavy metals. Int J Environ Pollut 18:294–300
Künzli N et al (2000) Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet 356:795–801
Marschner H (1995) Mineral nutrition of higher plants. Academic Press
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43. doi:10.1046/j.1469-8137.2002.00363.x
Mingorance MD, Oliva SR (2006) Heavy metals content in N. Oleander leaves as urban pollution assessment. Environ Monit Assess 119:57–68. doi:10.1007/s10661-005-9004-9
Mingorance MD, Valdés B, Oliva SR (2007) Strategies of heavy metal uptake by plants growing under industrial emissions. Environ Int 33:514–520. doi:10.1016/j.envint.2007.01.005
Niinae M, Nishigaki K, Aoki K (2008) Removal of lead from contaminated soils with chelating agents. Mater Trans 49:2377–2382. doi:10.2320/matertrans.M-MRA2008825
Pignata ML, Gudiño GL, Wannaz ED, Plá RR, González CM, Carreras HA, Orellana L (2002) Atmospheric quality and distribution of heavy metals in Argentina employing Tillandsia capillaris as a biomonitor. Environ Pollut 120:59–68. doi:10.1016/S0269-7491(02)00128-8
Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW (1995) Particulate air pollution as a predictor of mortality in a prospective study of US adults Am J Respir Crit Care Med 151:669-674 doi:10.1164/ajrccm/151.3_Pt_1.669
Rossini Oliva S, Mingorance MD (2006) Assessment of airborne heavy metal pollution by aboveground plant parts. Chemosphere 65:177–182. doi:10.1016/j.chemosphere.2006.03.003
Sagardoy R et al (2010) Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol 187:145–158. doi:10.1111/j.1469-8137.2010.03241.x
Samara C, Voutsa D (2005) Size distribution of airborne particulate matter and associated heavy metals in the roadside environment. Chemosphere 59:1197–1206. doi:10.1016/j.chemosphere.2004.11.061
Sawidis T, Marnasidis A, Zachariadis G, Stratis J (1995) A study of air pollution with heavy metals in Thessaloniki city (Greece) using trees as biological indicators. Arch Environ Contam Toxicol 28:118–124. doi:10.1007/bf00213976
Schwartz J, Neas LM (2000) Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology 11:6–10
Seaton A, Godden D, MacNee W, Donaldson K (1995) Particulate air pollution and acute health effects. Lancet 345:176–178. doi:10.1016/S0140-6736(95)90173-6
USEPA (1996) Air quality criteria for particulate matter. Volume 1 of 3. Environmental Protection Agency, Research Triangle Park, NC. National Center for Environmental Assessment.
Vázquez S, Moreno E, Carpena R (2008) Bioavailability of metals and As from acidified multicontaminated soils: use of white lupin to validate several extraction methods. Environ Geochem Health 30:193–198. doi:10.1007/s10653-008-9143-3
Acknowledgments
This work has been supported by the regional Aragon Government (Consolidated Applied Research Group ref. E61 and the Research Project ref. PI067/09 of the Call for Research Funding 2009 of the Aragon Government) and by the Spanish Ministry of Economy and Competitiveness (National Research Plan, ref. BFU2010-22053).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 89 kb)
Rights and permissions
About this article
Cite this article
Vázquez, S., Martín, A., García, M. et al. Metal uptake of Nerium oleander from aerial and underground organs and its use as a biomonitoring tool for airborne metallic pollution in cities. Environ Sci Pollut Res 23, 7582–7594 (2016). https://doi.org/10.1007/s11356-015-6002-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-6002-5