Abstract
A new combined difenoconazole and fluxapyroxad fungicide formulation, as an 11.7 % suspension concentrate (SC), has been introduced as part of a resistance management strategy. The dissipation of difenoconazole and fluxapyroxad applied to apples and the residues remaining in the apples were determined. The 11.7 % SC was sprayed onto apple trees and soil in Beijing, Shandong, and Anhui provinces, China, at an application rate of 118 g a.i. ha−1, then the dissipation of difenoconazole and fluxapyroxad was monitored. The residual difenoconazole and fluxapyroxad concentrations were determined by ultrahigh-performance liquid chromatography tandem mass spectrometry. The difenoconazole half-lives in apples and soil were 6.2–9.5 and 21.0–27.7 days, respectively. The fluxapyroxad half-lives in apples and soil were 9.4–12.6 and 10.3–36.5 days, respectively. Difenoconazole and fluxapyroxad residues in apples and soil after the 11.7 % SC had been sprayed twice and three times, with 10 days between applications, at 78 and 118 g a.i. ha−1 were measured. Representative apple and soil samples were collected after the last treatment, at preharvest intervals of 14, 21, and 28 days. The difenoconazole residue concentrations in apples and soil were 0.002–0.052 and 0.002–0.298 mg kg−1, respectively. The fluxapyroxad residue concentrations in apples and soil were 0.002–0.093 and 0.008–1.219 mg kg−1, respectively. The difenoconazole and fluxapyroxad residue concentrations in apples were lower than the maximum residue limits (0.5 and 0.8 mg kg−1, respectively). An application rate of 78 g a.i. ha−1 is therefore recommended to ensure that treated apples can be considered safe for humans to consume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Apples are a rich source of phytochemicals and have been found to have very strong antioxidant activities, allowing them to inhibit cancer cell proliferation, suppress lipid oxidation, and lower cholesterol concentrations when consumed (Soler et al. 2009). Apples are one of the main fruits that are consumed around the world, and the consumption of apples has increased in recent decades. China produces more apples than any other country. Farmers apply pesticides shortly before harvesting apples to prevent diseases that cause the fruit to be damaged. The use of fungicides has become considered essential over the last 20 years for ensuring that adequate agricultural yields of apples of acceptable quality are achieved (Ngugi et al. 2011). Commercial interest has recently grown in developing fungicide mixtures to improve disease control by achieving a broader spectrum than can be achieved using individual fungicides. Such mixtures are expected to more efficiently protect crops than individual fungicides and allow resistance to be effectively managed (Zhang et al. 2015).
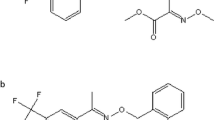
An 11.7 % suspension concentrate (SC) of difenoconazole and fluxapyroxad (containing 4.7 % difenoconazole and 7.0 % fluxapyroxad) has been registered in China for controlling alternaria leaf spot in apples because the mixture has a broad range of action and is more efficient than either fungicide alone. Difenoconazole (C19H17Cl2N3O3), shown in Fig. 1a, is a broad-spectrum triazole fungicide and a 14 α-demethylation inhibitor (Reuveni et al. 2002). The use of triazole fungicides is increasing more quickly than the use of other fungicides. Difenoconazole has been used widely in agriculture (Wang et al. 2012a; Zhang et al. 2015) and has typically been applied directly to plants to both protect from and cure disease (Mukhopadhyay et al. 2011; Wang et al. 2008). Fluxapyroxad (C18H12F5N3O), shown in Fig. 1b, is a new active ingredient developed by BASF Corporation (Strathmann et al. 2011). Fluxapyroxad is a pyrazole and carboxamide fungicide that inhibits succinate dehydrogenase in complex II of the mitochondrial respiratory chain, causing spore germination and germ tube and mycelia growth in target fungi to be inhibited (Veloukas et al. 2013).
The application of mixed pesticides directly to apples is currently increasing in China. It is becoming essential to measure multiple pesticide residues in apples to ensure food safety because pesticide residues in apples can persist until harvest, and a pesticide mixture may be able to persist longer than a single pesticide. Methods for determining difenoconazole (alone) in environmental matrices have previously been published. These methods have involved such techniques as gas chromatography mass spectrometry (Dedola et al. 2014), gas chromatography with a pulsed flame photometric detector (Guo et al. 2010), gas chromatography coupled with electron capture and nitrogen phosphorus detection (Stowik-Borowiec et al. 2015), and liquid chromatography tandem mass spectrometry (MS/MS) (Hingmire et al. 2015). Liquid chromatography MS/MS has been used to determine fluxapyroxad concentrations in water (Gulkowska et al. 2014) and in cereals, vegetables, and fruits (Dong et al. 2012). No method for simultaneously determining difenoconazole and fluxapyroxad in apple and soil samples is currently available. The aim of this study was to develop a simple and sensitive method for simultaneously determining difenoconazole and fluxapyroxad in apple and soil samples using a “quick, easy, cheap, effective, rugged, and safe” procedure and ultrahigh-performance liquid chromatography (UPLC) MS/MS.
Residues of difenoconazole and fluxapyroxad applied to apples could remain in the apples and cause adverse effects in humans consuming them. It is therefore essential for the behaviors of difenoconazole and fluxapyroxad residues in apples to be studied before commercial products containing both difenoconazole and fluxapyroxad are approved for sale (Liang et al. 2011). The dynamics of residues of difenoconazole applied in a number of formulations to a number of plants (including grape vines, apple trees, chili plants, rice plants, and banana plants) have previously been studied (Banerjee et al. 2008; Bhat et al. 2015; Guo et al. 2010; Huan et al. 2013; Mukhopadhyay et al. 2011; Wang et al. 2012a). However, no studies of the dissipation of fluxapyroxad applied to any type of plant and fluxapyroxad residues in any plant material have previously been published. The environmental fate of a mixture of pesticides will be complex, and there are many gaps in our understanding of the behaviors of difenoconazole and fluxapyroxad in the environment.
This study was performed to investigate the behaviors of difenoconazole and fluxapyroxad applied to apples and soil, and to quantify the difenoconazole and fluxapyroxad residues in apples and soil after treatment. The study was expected to provide useful information on the dissipation of difenoconazole and fluxapyroxad applied to apples and soil and basic information on how fungicides should be controlled to maintain apple quality without endangering humans. The study was also expected to help the Chinese government establish maximum residue limits (MRLs) for difenoconazole and fluxapyroxad in apples and develop guidance for the appropriate and safe use of difenoconazole and fluxapyroxad.
Materials and methods
Chemicals and reagents
Difenoconazole (purity 96.3 %) and fluxapyroxad (purity 99.7 %) were obtained from BASF Corporation (Shanghai, China). The molecular structures of difenoconazole and fluxapyroxad are shown in Fig. 1. A 1.0 mg mL−1 stock solution of each standard in pesticide-grade acetonitrile was prepared carefully, using gloves, under an extraction hood. The stock solutions were stored in sealed amber bottles at −20 °C. The standards (in pure solvent) required to produce a calibration curve over the concentration range 1–200 μg L−1 were prepared from the stock solutions by performing serial dilutions. Matrix-matched standard solutions were prepared by adding the calibration standards (at concentrations of 1, 2, 5, 10, 20, 50, 100, and 200 μg L−1) to blank (soil and apple) sample extracts. All of the solutions were stored in sealed amber bottles wrapped in aluminum foil (to ensure they were protected from light) and stored at −20 °C until use.
Pesticide-grade acetonitrile and HPLC-grade acetonitrile were purchased from Sigma-Aldrich (Steinheim, Germany), analytical-grade acetonitrile was purchased from Beihua Fine Chemical Co. (Beijing, China), and ultrapure water was produced using a Milli-Q system (EMD Millipore Corp., Billerica, MA, USA).
Primary and secondary amine sorbent (40 μm diameter) and octadecylsilane sorbent (40 μm diameter) were purchased from Yuexu Technologies Co. Ltd. (Shanghai, China). Analytical grade NaCl and MgSO4 were purchased from Beihua Fine Chemical Co. Each concentrated extract was passed through a 0.22-μm nylon syringe filter (Waters Corp., Milford, MA, USA). A commercial difenoconazole and fluxapyroxad formulation (11.7 % SC) was provided by BASF Corporation.
Apparatus
An ACQUITY UPLC H-class with a Xevo TQD MS/MS instrument (Waters Corp.) was used to analyze the samples. The UPLC system had a binary solvent manager, a built-in vacuum degasser, a 10-μL injection loop, a temperature-controlled autosampler, and a column oven. Separation was achieved using a stainless steel ACQUITY UPLC-bridged ethylene hybrid shield RP C18 column (100-mm long, 2.1-mm inner diameter, 1.7 μm particle size; Waters Corp.). The UPLC system was coupled to a Xevo TQD triple-stage quadrupole mass spectrometer, which was fitted with an electrospray ionization source.
The samples were extracted in solvent in vessels placed in an HZS-HA digital oscillating water bath (China Harbin Electronic Technologies Co. Ltd., Harbin, China). Before being extracted, each apple sample was homogenized using a Foss Tecator 2094 homogenizer (Foss, Hillerød, Denmark). An R-215 rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) was used to evaporate solutions in organic solvents.
Field trial
The experiment was designed following the NY/T 788–2004 guidelines for pesticide residue trials issued by the Institute for the Control of Agrochemicals, Ministry of Agriculture, China. A dissipation field trial and a final residue field trial were performed.
The field trials were performed in Beijing Province in northern China, which has a subhumid warm temperate continental monsoon climate, Shandong Province on the eastern coast of China, which has a warm temperate humid monsoon climate, and Anhui Province in central China, which has a warm temperate monsoon climate. The trials were performed between June and October 2014. Beijing, Shandong, and Anhui provinces have annual precipitation rates of 630, 640, and 800 mm, respectively, and average relative humidities of 40, 54, and 55 %, respectively. The field at the Beijing site had a loamy soil with an organic matter content of 2.12 g kg−1 and a pH of 7.1. The field at the Shandong site had a loamy soil with an organic matter content of 2.05 g kg−1 and a pH of 7.1. The field at the Anhui site had sandy clay loam soil with an organic matter content of 2.08 g kg−1 and a pH of 7.2.
Each experimental plot had an area of 30 m2 and contained three apple trees. Each treatment was performed in triplicate (i.e., on three plots). Each test plot was separated from the plots around it by a buffer area. A Jacto HD400 heavy-duty sprayer (Jacto Inc., Tualatin, OR, USA) with a nozzle diameter of 1.0 mm and an operating pressure of 4–5 kg cm−2 was used to apply the SC.
The 11.7 % SC application rate in the experiments on dissipation in apples was 118 g a.i. ha−1 (1.5 times the recommended application rate). The SC was sprayed on the apples once when the mean apple size was about 5 cm. The 11.7 % SC was sprayed directly onto the soil once, at an application rate of 118 g a.i. ha−1, in the experiments on dissipation in soil. Representative apple and soil samples were randomly collected 2 h and 1, 3, 7, 14, 21, 30, and 45 days after the SC had been sprayed. Control samples were collected from plots to which the SC was not applied. In total, 48 treated soil and apple samples and six control samples were collected at each site. The samples were stored in the dark at −20 °C until analysis.
The final difenoconazole and fluxapyroxad residue concentrations in apples and soil were determined by performing trials using application rates of 78 g a.i. ha−1 (the recommended dose) and 118 g a.i. ha−1 (1.5 times the recommended dose) on different plots. The SC was sprayed onto some plots twice and other plots three times, with 10 days between applications. Each plot was separated from the plots around it by a buffer area. Representative soil and apple samples were collected from each plot at preharvest intervals of 14, 21, and 28 days. In total, 108 treated soil and apple samples and six control samples were collected after the last SC dose had been applied. The samples were stored in the dark at −20 °C until analysis.
Analytical procedure
Each apple sample was thoroughly blended. Large stones were removed from each surface soil (0–15 cm deep) sample, then the sample was air-dried, crushed with a hammer, and passed through a 40 mesh (380 μm) sieve. A 20.00 g aliquot of a soil or apple sample was added to a 150 mL conical flask, then 50 mL acetonitrile and 7 g NaCl were added and the flask was sealed with a stopper. The flask was then placed in an oscillating water bath set at 30 °C and left for 30 min. A 10 mL aliquot of the acetonitrile was then removed and evaporated to dryness under vacuum using a rotary evaporator. The residue was dissolved in 2 mL of a 1:1 (v/v) mixture of acetonitrile and water, then transferred to a 2 mL centrifuge tube containing 150 mg anhydrous MgSO4, 50 mg of the primary and secondary amine sorbent, and 50 mg of the octadecylsilane sorbent. The mixture was shaken vigorously using a vortex machine for 30 s, then centrifuged at 18,000 rpm for 1 min. The supernatant was passed through a 0.22-μm nylon syringe filter before an aliquot was injected into the UPLC-MS/MS system.
The mobile phase flow rate was 0.3 mL min−1. Solvent A was acetonitrile and solvent B was 0.2 % (v/v) formic acid in water, and the gradient elution program was 80 % A decreasing to 20 % A between 0 and 2 min; 20 % A increasing to 40 % A between 2 and 4 min; 40 % A increasing to 80 % A between 4 and 5 min. The column was kept at 35 °C to keep the viscosity of the mobile phase appropriately low, and the sample manager temperature was 10 °C. A 3 μL aliquot of each sample was injected.
The nebulizer gas was 99.95 % nitrogen, the collision gas was 99.99 % argon, and the pressure in the T wave cell was 3.2 × 10−3 mbar. The MS/MS was operated in positive ionization switching mode, and the parameters were optimized for the target compounds. The typical conditions were 3.0 kV capillary voltage, source temperature 150 °C, desolvation temperature 500 °C, cone gas flow rate 50 L h−1, and desolvation gas flow rate 600 L h−1. The multiple reaction monitoring mode was used to detect all of the compounds of interest, and the MS/MS parameters are shown in Table 1.
Calculations
The data were collected and analyzed using MassLynx 4.1 software. The limit of detection was defined as the concentration required to give a quantitative ion transition peak area with a signal to noise (peak–peak) ratio of 3. The limit of quantification (LOQ) was defined as the lowest spike concentration for which a satisfactory recovery (70–120 %) and relative standard deviation (RSD; ≤20 %) were found (Cheng et al. 2014). The linearity of the instrument was evaluated by plotting the standard concentrations against the peak areas. The precision, repeatability, and reproducibility of the method were determined and expressed as the RSD. The repeatability was expressed as the intra-day precision (RSDa), which was defined as the RSD of the analyte concentrations in spiked samples analyzed on the same day. The reproducibility was expressed as the inter-day precision (RSDb), which was defined as the RSD of the analyte concentrations in spiked samples analyzed on five different days by five different operators.
Matrix effects were quantified using the equation F = (B − A)/A × 100, where F is the influence of matrix effects, B is the slope of the matrix-matched calibration standard, and A is the slope of the calibration standard in pure solvent. An F value of up to ±10 % was assumed to indicate that matrix effects could be ignored. An F value of ±10–20 % was assumed to indicate mild signal suppression or enhancement occurred through matrix effects. An F value of ±20–50 % was assumed to indicate medium matrix effects, and an F value of >±50 % was assumed to indicate strong matrix effects (Li et al. 2013). An F value of >±20 % was taken to indicate that the matrix-matched calibration standards should be used to accurately quantify the analyte.
The residual difenoconazole and fluxapyroxad concentrations were calculated using the first-order kinetics equation C t = C 0 e − kt, and the half-lives were calculated using the equation t 1/2 = ln(2)/k. In these equations, t is the time elapsed since the pesticide was applied, C t is the pesticide concentration at time t, C 0 is the initial pesticide concentration immediately after the pesticide was applied (i.e., at t = 0), k is the dissipation coefficient, and t 1/2 is the time required for the pesticide concentration to reach half of the initial concentration (Koch et al. 2005).
Results
Method validation
The linear regression equations, limits of detection, and LOQs for difenoconazole and fluxapyroxad in apples and soil are shown in Table 2. The linearity of the instrument was excellent for both analytes (R 2 > 0.99 in all cases), but matrix effects were found (the F values were between −15 and 66 %) for both analytes in both apples and soil. Electrospray ionization mass spectrometry was used, so these matrix effects could have been caused by competition between the compound of interest and other compounds for the charge available in the electrospray ionization chamber (Dong et al. 2010). The extent to which matrix effects affect the analysis of a sample can be influenced by the type of instrument used, the type and amount of matrix present, the sample pretreatment procedure used, and the concentration of the compound of interest in the sample (Cheng et al. 2014). Matrix-matched standard solutions were used to compensate for the matrix effects when identifying and quantifying the analytes in this study. The limits of detection for difenoconazole and fluxapyroxad were estimated to be between 0.01 and 0.14 μg kg−1. The LOQ for difenoconazole in apples was 2 μg kg−1, which is lower than the difenoconazole MRLs in China, the European Union, Japan, and the USA (0.5, 0.5, 1.0, and 4.5 mg kg−1, respectively). The LOQ for fluxapyroxad in apples was 2 μg kg−1, which is lower than the fluxapyroxad MRL in the European Union and the USA (0.7 and 0.8 mg kg−1, respectively) (www.mrldatabase.com).
The recoveries and RSDs for difenoconazole and fluxapyroxad in spiked apple and soil samples are shown in Table 3. The mean recoveries (85.0–100.0 %) and precision (all RSDs were <20 % at the three spike concentrations used) were satisfactory. The mean difenoconazole recoveries were 85.6–100 % for the apple samples and 86.4–97.8 % for the soil samples, and the fluxapyroxad recoveries were 86.6–97.2 % for the apple samples and 85.0–87.8 % for the soil samples. The RSDa (n = 5) and RSDb (n = 15) values were 4.2–14.8 % and 3.8–14.5 %, respectively. For the trace analysis of pesticide residues, recoveries of 70–120 % and an RSD of up to ±20 % are generally considered acceptable.
Dissipation of difenoconazole and fluxapyroxad in apples and soil
The difenoconazole dissipation curves for apples and soil under field conditions are shown in Fig. 2. The curves were based on the difenoconazole residues found in samples collected at different times after the difenoconazole had been applied. The initial difenoconazole concentrations in the apples collected from the Beijing, Shandong, and Anhui sites were 0.090, 0.049, and 0.052 mg kg−1, respectively. The dissipation regression equation for the Beijing trial was C = 0.0696 e−0.107 t (R 2 = 0.9047) and the half-life was 6.5 days, the equation for the Shandong trial was C = 0.0632 e−0.111 t (R 2 = 0.9496) and the half-life was 6.2 days, and the equation for the Anhui trial was C = 0.0385 e−0.073 t (R 2 = 0.7812) and the half-life was 9.5 days. The initial difenoconazole concentrations in the soil samples from the Beijing, Shandong, and Anhui sites were 0.145, 0.110, and 0.075 mg kg−1, respectively. The dissipation regression equation for the Beijing trial was C = 0.0898 e−0.033 t (R 2 = 0.697) and the half-life was 21.0 days, the equation for the Shandong trial was C = 0.0968 e−0.029 t (R 2 = 0.9726) and the half-life was 23.9 days, and the equation for the Anhui trial was C = 0.0596 e−0.025 t (R 2 = 0.6915) and the half-life was 27.7 days.
The fluxapyroxad dissipation curves for apples and soil under field conditions are shown in Fig. 3. The initial fluxapyroxad concentrations in the apples collected from the Beijing, Shandong, and Anhui sites were 0.070, 0.095, and 0.051 mg kg−1, respectively. The dissipation regression equation for the Beijing trial was C = 0.0648 e−0.065 t (R 2 = 0.9633) and the half-life was 10.7 days, the equation for the Shandong trial was C = 0.0672 e−0.055 t (R 2 = 0.9073) and the half-life was 12.6 days, and the equation for the Anhui trial was C = 0.0377 e−0.074 t (R 2 = 0.9454) and the half-life was 9.4 days. The initial fluxapyroxad concentrations in the soil samples from the Beijing, Shandong, and Anhui sites were 0.403, 0.179, and 0.243 mg kg−1, respectively. The dissipation regression equation for the Beijing trial was C = 0.2949 e−0.067 t (R 2 = 0.9126) and the half-life was 10.3 days, the equation for the Shandong trial was C = 0.1551 e−0.019 t (R 2 = 0.8935) and the half-life was 36.5 days, and the equation for the Anhui trial was C = 0.2041 e−0.037 t (R 2 = 0.7196) and the half-life was 18.7 days.
Final difenoconazole and fluxapyroxad residues in apples and soil
The final difenoconazole and fluxapyroxad residue concentrations in the apple and soil samples from the Beijing, Shandong, and Anhui sites are shown in Table 4. The difenoconazole concentrations were 0.002–0.052 mg kg−1 in the apples and 0.002–0.298 mg kg−1 in the soil samples (i.e., for all of the samples that had been sprayed at the recommended dose and at 1.5 times the recommended dose both twice and three times). The fluxapyroxad residue concentrations in the apple and soil samples were 0.002–0.052 mg kg−1 and 0.008–1.219 mg kg−1, respectively.
Discussion
The highest initial difenoconazole concentration in apples sprayed with the 11.7 % SC was 0.090 mg kg−1 (in the Beijing trial) and the highest initial fluxapyroxad concentration was 0.095 mg kg−1 (in the Shandong trial). The highest initial difenoconazole and fluxapyroxad concentrations, 0.145 and 0.403 mg kg−1, respectively, were both found in the Beijing trial. The highest initial difenoconazole and fluxapyroxad concentrations in apples were similar. However, the highest initial fluxapyroxad concentration in soil was more than twice the highest initial difenoconazole concentration. This suggested that the initial concentrations in the apples and soil were determined by a number of factors. Apart from the effects of the physical and chemical properties of the fungicides, environmental factors (such as exposure to light, heat, wind, and moisture) the plant parts exposed, the plant species, the characteristics of the site, and growth dilution might all play significant roles in the deposition of a pesticide (Guo et al. 2010; Liang et al. 2011). In our experiment, the initial difenoconazole and fluxapyroxad concentrations in the apples and soil did not correlate with the application rate, and no relationships were found between the initial concentrations and the characteristics of the experimental sites.
One of the most important parameters that must be taken into account when the fate of a pesticide in the environment is assessed is the degree to which the pesticide is dissipated. The degradation of fluxapyroxad in agricultural samples has not previously been reported. The fluxapyroxad was found to have a half-life of less than 12.6 days in apples and less than 36.5 days in soil in this experiment. This suggests that fluxapyroxad degrades very quickly in apples and soil.
Difenoconazole was found to have a half-life of 6.2–9.5 days in apples, which is somewhat lower than the half-life of 4.9–5.4 days found for difenoconazole in a 10 % wettable powder applied at a rate of 30 g a.i. ha−1 in a previous study (Guo et al. 2010), and also somewhat lower than the half-life of 5.3–6.7 days found for difenoconazole in a 25 % emulsifiable solution applied at a rate of 75 g a.i. ha−1 in another study (Bhat et al. 2015). The difenoconazole half-lives found in the Beijing, Shandong, and Anhui trials were similar. This could have been because the soil pH values and organic matter contents were similar in the different trials. However, difenoconazole in a 10 % wettable powder was previously found to have a half-life in soil of 9.6–68.4 days, which is rather different from the half-lives we found. This suggested that the difenoconazole half-life in soil is different for different formulations and application rates.
The behavior of a pesticide in the environment will be governed by a range of complex physical, chemical, and biological processes, including sorption and desorption, volatilization, chemical and biological degradation, uptake by plants, surface runoff, and leaching (Wang et al. 2012b). In our experiments, difenoconazole and fluxapyroxad dissipated more quickly in the apples than in the soil. This could have been because the initial concentrations in the soil were much higher than the initial concentrations in the apples. The fluxapyroxad half-lives were longer than the difenoconazole half-lives in both apples and soil. These results show that difenoconazole and fluxapyroxad in the 11.7 % SC have a synergistic relationship in controlling alternaria leaf spot in apple trees in the field.
The final difenoconazole and fluxapyroxad residue concentrations in the apples and soil in the different trials followed a trend in that a shorter harvest interval and higher application rate caused the residual concentrations in both apples and soil to be higher.
The Codex Alimentarius Commission has not specified a MRL for either difenoconazole or fluxapyroxad. The difenoconazole MRLs for apples in China, the European Union, Japan, and the USA are 0.5, 0.5, 1.0, and 4.5 mg kg−1, respectively. The maximum final residue concentration in apples before harvest in the field trials at the three experimental sites was less than 0.5 mg kg−1. No MRL has been set for fluxapyroxad in China. The MRLs of fluxapyroxad in the European Union and the USA are 0.7 and 0.8 mg kg−1, respectively. The fluxapyroxad residue concentrations on the apples at harvest time were lower than these MRLs. These results suggest that it is safe to harvest apples 10 day or more after the recommended doses of difenoconazole and fluxapyroxad have been applied. No clear differences were found between the final residue concentrations in the Beijing, Shandong, and Anhui trials.
Conclusions
A quick, easy, effective, rugged, reliable, and accurate method was developed for determining difenoconazole and fluxapyroxad concentrations in apples and soil using UPLC-MS/MS. Difenoconazole and fluxapyroxad eluted from the UPLC column in less than 5.0 min, and the specificity of the analytical method was good. The mean recoveries of both analytes in apples and soil were 78.4–107.2 %. The difenoconazole and fluxapyroxad dissipation kinetics and the final difenoconazole and fluxapyroxad residue concentrations in apples and soil were determined to allow the safety of using these pesticides to be assessed in terms of the health of people consuming apples that have been treated with difenoconazole and fluxapyroxad. The highest final difenoconazole and fluxapyroxad residue concentrations in the apples treated in our experiments were <0.01 mg kg−1. This suggests that it is safe to harvest apples 10 day or more after the recommended difenoconazole and fluxapyroxad doses have been applied. This work will be useful in developing MRLs for fluxapyroxad and in ensuring that difenoconazole and fluxapyroxad are used safely in the future.
References
Banerjee K, Oulkar DP, Patil SH, Dasgupta S, Adsule PG (2008) Degradation kinetics and safety evaluation of tetraconazole and difenoconazole residues in grape. Pest Manag Sci 64:283–289
Bhat M, Wani AA, Mukhtar M, Sherwani A, Bhat AH, Showkat A (2015) Dissipation patterns of the fungicide difenoconazole (25% EC) in apples grown in Kashmir. J Environ Sci Health B 187
Cheng YP, Dong FS, Liu XG, Xu J, Meng W, Liu N, Chen ZL, Tao Y, Zheng YQ (2014) Simultaneous determination of fipronil and its major metabolites in corn and soil by ultra-performance liquid chromatography-tandem mass spectrometry. Anal Methods 6:1788–1795
Dedola F, Cabizza M, Satta M (2014) Determination of 28 pesticides applied on two tomato cultivars with a different surface/weight ratio of the berries, using a multiresidue GC-MS/MS method. J Environ Sci Health, Part B 49:671–678
Dong FS, Liu XA, Li J, Cheng L, Zhang CP, Jing-Jingan, Zheng YQ (2010) Determination of 4-chloro-2-methylphenoxyacetic acid residues in wheat and soil by ultra-performance liquid chromatography/tandem mass spectrometry. J AOAC Int 93:1013–1019
Dong FS, Chen X, Liu XG, Xu J, Li YB, Shan WL, Zheng YQ (2012) Simultaneous determination of five pyrazole fungicides in cereals, vegetables and fruits using liquid chromatography/tandem mass spectrometry. J Chromatogr A 1262:98–106
Gulkowska A, Buerge IJ, Poiger T (2014) Online solid phase extraction LC-MS/MS method for the analysis of succinate dehydrogenase inhibitor fungicides and its applicability to surface water samples. Anal Bioanal Chem 406:6419–6427
Guo C, Li JZ, Guo BY, Wang HL (2010) Determination and safety evaluation of difenoconazole residues in apples and soils. Bull Environ Contam Toxicol 85:427–431
Hingmire S, Oulkar DP, Utture SC, Shabeer TPA, Banerjee K (2015) Residue analysis of fipronil and difenoconazole in okra by liquid chromatography tandem mass spectrometry and their food safety evaluation. Food Chem 176:145–151
Huan ZB, Xu Z, Lv DZ, Xie DF, Luo JH (2013) Dissipation and residues of difenoconazole and azoxystrobin in bananas and soil in two agro-climatic zones of china. Bull Environ Contam Toxicol 91:734–738
Koch RL, Burkness EC, Hutchison WD, Rabaey TL (2005) Efficacy of systemic insecticide seed treatments for protection of early-growth-stage snap beans from bean leaf beetle (Coleoptera: Chrysomelidae) foliar feeding. Crop Prot 24:734–742
Li MM, Liu XG, Dong FS, Xu J, Kong ZQ, Li YB, Zheng YQ (2013) Simultaneous determination of cyflumetofen and its main metabolite residues in samples of plant and animal origin using multi-walled carbon nanotubes in dispersive solid-phase extraction and ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1300:95–103
Liang HW, Li L, Li W, Wu YJ, Zhou ZQ, Liu FM (2011) Dissipation and residue of dimethomorph in pepper and soil under field conditions. Ecotoxicol Environ Saf 74:1331–1335
Mukhopadhyay S, Das S, Bhattacharyya A, Pal S (2011) Dissipation study of difenoconazole in/on chili fruit and soil in India. Bull Environ Contam Toxicol 87:54–57
Ngugi HK, Esker PD, Scherm H (2011) Meta-analysis to determine the effects of plant disease management measures: review and case studies on soybean and apple. Phytopathology 101:31–41
Reuveni M, Sheglov D, Sheglov N, Ben-Arie R, Prusky D (2002) Sensitivity of red delicious apple fruit at various phenologic stages to infection by Alternaria alternata and moldy-core control. Eur J Plant Pathol 108:421–427
Soler C, Soriano JM, Manes J (2009) Apple-products phytochemicals and processing: a review. Nat Prod Commun 4:659–670
Stowik-Borowiec M, Szpyrka E, Walorczyk S (2015) Gas chromatographic determination of pesticide residues in white mustard. Food Chem 173:997–1005
Strathmann S, Walker S, Barnes J (2011) Fluxapyroxad: a new broad-spectrum fungicide. Phytopathology 101:S172–S172
Veloukas T, Markoglou AN, Karaoglanidis GS (2013) Differential effect of SdhB gene mutations on the sensitivity to SDHI fungicides in botrytis cinerea. Plant Dis 97:118–122
Wang ZH, Yang T, Qin DM, Gong Y, Ji Y (2008) Determination and dynamics of difenoconazole residues in Chinese cabbage and soil. Chin Chem Lett 19:969–972
Wang K, Wu JX, Zhang HY (2012a) Dissipation of difenoconazole in rice, paddy soil, and paddy water under field conditions. Ecotoxicol Environ Saf 86:111–115
Wang L, Zhao PY, Zhang FZ, Li YJ, Du FP, Pan CP (2012b) Dissipation and residue behavior of emamectin benzoate on apple and cabbage field application. Ecotoxicol Environ Saf 78:260–264
Zhang ZY, Jiang W, Jian Q, Song WC, Zheng ZT, Wang DL, Liu XJ (2015) Residues and dissipation kinetics of triazole fungicides difenoconazole and propiconazole in wheat and soil in Chinese fields. Food Chem 168:396–403
Acknowledgments
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (No. 201303027) and the Youth Research Foundation of Beijing Academy of Agriculture and Forestry Science (No. QNJJ201210).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
He, M., Jia, C., Zhao, E. et al. Concentrations and dissipation of difenoconazole and fluxapyroxad residues in apples and soil, determined by ultrahigh-performance liquid chromatography electrospray ionization tandem mass spectrometry. Environ Sci Pollut Res 23, 5618–5626 (2016). https://doi.org/10.1007/s11356-015-5750-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5750-6