Abstract
The dissipation of trifloxystrobin and its metabolite trifloxystrobin acid in apples and soil was studied, and the half-life (DT50) was estimated in a field study carried out at three different locations for apples and four different locations for soil. Trifloxystrobin was sprayed on apples at 127 g a.i./ha for the dissipation study. Samples of apple and soil for the dissipation experiment were collected at time intervals of 0, 1, 3, 7, 14, 21, 30, and 45 days after treatment. The quantification of residues was done by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The DT50 of trifloxystrobin ranged from 0.54 to 8.8 and 4.8 to 9.5 days in soil and apples at different latitude sites. Photolysis may be the main dissipation pathway for trifloxystrobin, and the number of sunshine hours may be the main factor affecting the trifloxystrobin dissipation rate in the field. For trifloxystrobin acid residues in soil and apples, it first increased and then began decreasing. It was indicated that the risk of trifloxystrobin application in shorter sunshine hour area should be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
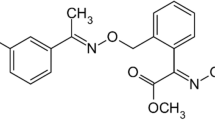
The strobilurins is an important class of agricultural fungicide, with a basic structure of (E)-β–methoxyacrylate moiety (Balba 2007; Bartlett et al. 2002). They are derivatives of natural substances isolated mainly from mushrooms (basidiomycetes) (Balba 2007; Anke et al. 1977). Trifloxystrobin (TFS) is one widely marketed strobilurin, as well as azoxystrobin, kresoxim-methyl, and pyraclostrobin (Tomlin 2006). It was discovered by Novartis (now Syngenta) under patent EP0460575 in 1998 (Isenring and Weiss 1991) and later sold to Bayer in 2000 (Balba 2007; Bartlett et al. 2002). Its development involved replacing the structure of (E)-β-methoxyacrylate moiety with methoxyiminoacetate moiety to be photo-stable (Fig. 1a). It was registered for use in various field and horticultural crops such as rice, potato, cucumber, tomato, apple, mandarin etc., in order to control a broad spectrum of plant pathogenic fungi through mainly preventive activity.
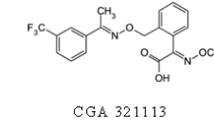
Trifloxystrobin acid (TFSA) (Fig. 1b), also known as CGA321113, is the major metabolite of TFS. TFSA is generated from the hydrolysis of the ester moiety to form the corresponding carboxylic acid. TFSA accounts for approximately 30 % of TFS terminal residue in some plants (Joint FAO/WHO Meeting on Pesticide Residues 2004). Similar characteristics were observed on other strobilurins. It was reported that the demethylated kresoxim-methyl was the only metabolite of kresoxim-methyl in wheat cell suspension cultures (Myung et al. 2013), and azoxystrobin acid was the major metabolite of azoxystrobin in soil and water (Singh et al. 2010; Ghosh and Singh 2009a). Since acid metabolites are more water-soluble and mobile than their parent esters, these compounds have more potential to contaminate the environment and cause harm to beneficial flora and fauna (Banerjee et al. 2007; Ghosh and Singh 2009b). MRLs for TFS in apples were established by several countries and organizations (0.7 mg/kg CODEX; 0.5 USA; 0.5 EU; 3 Japan; 0.5 Canada; 0.3 Australia), and residue definitions included TFS and TFSA, except for Japan (FAO and WHO 2013; US Government Printing Office 2014; DG SANCO 2014; Japan Food Chemical Research Foundation 2014; Health Canada 2012; Food Standards Australia New Zealand 2014). Hence, it is necessary to take the TFSA into account when studying the environmental behavior of TFS.
There are a few studies regarding the degradation of TFS. Patyal et al. (2013) reported the half-life of TFS on apples was between 19.375 and 28.860 days at four locations in the Himalayan region of India. Several researches indicated that TFS is sensitive to photolysis. TFS hydrolyzed very slowly in neutral or weak acid conditions, and the half-life can last several weeks or even several years (Joint FAO/WHO Meeting on Pesticide Residues 2004). However, while under the same conditions, the photolytic half-lives were 20.4 to 31.5 h, which is much shorter than the hydrolysis. Banerjee et al. (2006) studied the degradation of TFS in humic acid solutions at different sites. In the same sites, the half-lives in winter were longer than that in summer. In the same season, the half-lives showed no significant difference except for winter on different sites with the higher latitude sites having longer half-lives in winter. Similarly, another strobilurin azoxystrobin was unstable to light in water solutions and soil under laboratory and field conditions (Garau et al. 2002; Boudina et al. 2007). Also, azoxystrobin degradation was faster under alkaline conditions than in neutral or acidic conditions (Singh et al. 2010).
The purpose of this study is to investigate the dissipation principle of TFS and TFSA in soil and apples under field conditions and to analyze the main influencing factor and pathway for TFS.
Material and methods
Apparatuses
The 1602MP8-1 balance (readability 0.1 mg) was from Sartorius AG, Germany; JY2002 balance was from the Shanghai Precision & Scientific Instrument Co., Ltd., China; TDL-40B centrifuge was from the Shanghai Anting Scientific Instrument Factory, China; 3K15 centrifuge was from SIGMA Laborzentrifugen GmbH, Germany; QL-861 vortex mixer was purchased from the Haimen Qilinbeier Instrument Manufactural Co., Ltd., Jiangsu, China; HZQ-C shaker was purchased from the Harbin Donglian Electronic & Technology Development Co. Ltd., China; and 0.22-μm nylon membrane filter was purchased from the Beijing Ruifengtongchuang Analysis Instrument Co., Ltd., China.
Chemicals
The analytical standards for TFS and TFSA purchased from Dr. Ehrenstorfer were GmbH with 99.6 and 96.3 % purity, respectively. A series of standard solutions were prepared with MeCN according to the experiment’s needs. Trifloxystrobin-tebuconazole 75 % (trifloxystrobin 25 %, tebuconazole 50 %) water-dispersible granule (WG) was purchased from Jiangsu Agricultural Hormone Engineering Technology Research Center Co., Ltd. Acetonitrile (MeCN) was of high-performance liquid chromatography (HPLC) grade and purchased from Fisher Scientific, USA. Sodium chloride (analytical reagent) was purchased from Beijing Chemical Works, China. Stearyl bonded silica gel (C18, 40–60 μm) was purchased from Agela Technologies, Tianjin, China. Water was purified with an Aquapro ABW-6000-U water purifier (Chongqing, China).
Analytical method
Sample preparation, extraction, and cleanup
The apple samples were cut into small pieces and homogenized with a Philips HR2004 blender. The soil samples were sieved through a 1-mm sieve and then mixed well. All the samples were stored in a deep freezer at −20 °C within 2 months before analyzing.
Sub-samples of 10.0 g of apples and soil were extracted by a formic acid water solution of 5 mL (pH = 5), 10 mL acetonitrile, and 3 g NaCl in a 50-mL centrifuge tube and shaken for 30 min. The centrifuge tube was centrifuged at 3500 rpm for 5 min, and the supernatant MeCN layer was cleaned up as described below.
A dispersive solid-phase extraction (DSPE) was utilized for the sample cleanup. One milliliter of supernatant MeCN layer was transferred into a 2-mL centrifuge tube containing 50 mg of C18. The tube was centrifuged for 5 min at 10,000 rpm after 30 s of vortexing. The supernatant extract was filtered with a 0.22-μm PTFE membrane filter and then analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
HPLC-MS/MS condition
The determination was achieved using the Agilent 1200 HPLC series and 6410B triple-quadrupole mass spectrometer (Agilent Technologies, USA) equipped with an electrospray ionization interface and a 3.5-μm Eclipse Plus C18 (2.1 × 50 mm) column (Agilent Technologies, USA). The mobile phase was MeCN-0.1 % formic acid:water (85/15, v/v), and the injection volume was 5 μL. The column temperature was maintained at 30 °C with a flow rate of 0.15 mL/min. The instrument was operated in the positive ion mode. The source parameters were desolvation gas temperature of 350 °C, desolvation gas flow of 10.0 L/min, nebulizer gas (N2) pressure of 35.0 psi, and capillary voltage of 4000 V. The multiple reaction monitoring (MRM) was applied, and the parameters are listed in Table 1.
The external standard method was chosen for the determination of TFS and TFSA. All the samples were compared to the matrix standard solution to eliminate the matrix effect.
Field experiments
The field dissipation experiments of trifloxystrobin in apples were conducted in Zibo (Shandong Province, China, 36° 97′ N and 118° 8′E), Beijing (China, 39° 9′ N and 116° 3′ E), and Xingcheng (Liaoning Province, China, 40° 45′ N and 120° 51′ E) in 2010. The field dissipation experiments of trifloxystrobin in soil were also conducted at the same three sites, and one more site, Harbin (Heilongjiang Province, China, 45° 75′ N and 126° 63′ E), was added.
The dissipation trials on apples were conducted in three plots, and each plot contained two apple trees. Trifloxystrobin-tebuconazole 75 % WG was diluted 2667 times and sprayed evenly on the apple trees. The dissipation of trifloxystrobin in soil was carried out in one plot of 20 m2, and trifloxystrobin-tebuconazole 75 % WG were applied once with 254 g a.i./ha. Approximately 2 kg of apple samples and 2 kg of surface soil (0–10 cm deep) samples were collected randomly from several points in each plot at 2-h intervals (calculated as the original concentration), on the 1st, 3rd, 7th, 14th, 21st, 30th, and 45th days after spraying. The samples were placed in a deep freezer at −18 °C for analysis within 2 months.
Results and discussion
Method validation
In order to evaluate the linearity and sensibility of the analytical method for trifloxystrobin and trifloxystrobin acid, a series of matrix standard solutions were diluted by the apple and soil blank matrix extract. A linear calibration curve was obtained for trifloxystrobin and trifloxystrobin acid by plotting the average peak area against concentration. The range of the six-point calibration curve was from 0.001 to 0.2 mg/L in the apples and soil. The calibration curves showed good linearity with typical correlation coefficients (R 2) between 0.9904 and 0.9996. The calibration curve was used to calculate the concentration of trifloxystrobin and trifloxystrobin acid residues in the apples and soil. The LOD value was 5.0 × 10−3 ng, and the LOQ value was 0.01 mg/kg.
The accuracy and precision were evaluated by a fortified recovery experiment. There were three fortified levels for each matrix, with five duplicates for each fortified level (see Table 2). The fortified recoveries ranged from 89.9 to 105.3 %, and the relative standard deviation (RSD) ranged from 2.9 to 9.1 %.
Dissipation dynamics
The samples were analyzed and drawn in scatter plots with several outliers removed as shown in Figs. 2 and 3. The dissipation curve of TFS was fitted with first-order kinetics, and the half-lives (DT50) were calculated as shown in Table 3.
In the soil, the dissipation DT50 of TFS ranged from 0.54 to 8.8 days. The soil pHs of the four sites were close (7.23–8.18), which was unlikely to have caused the huge difference in DT50. However, as the latitude of the site increased, DT50 of TFS decreased. It was normally considered that high temperatures benefit pesticide degradation, and the low latitude areas had high temperatures, which was contradictory to the results. However, at the field trial period (from July to September), higher latitude areas had longer sunshine hours. This indicated that photolysis may be the main dissipation pathway of TFS in soil and that sunshine hours may be the main influencing factor of the TFS dissipation rate in soil.
In the apples, the dissipation DT50 of TFS ranged from 4.8 to 9.5 days and showed the same pattern as the soil. As the latitude of the site increased, the DT50 of TFS decreased. Without the differences of soil property, this supports the speculation that photolysis is the main dissipation pathway of TFS in apples, and that sunshine hours are the main influencing factor of TFS dissipation rate in apples.
The residue of TFSA first increased and then decreased, basically in both the soil and apples, which means that TFS degrading to TFSA was the main progress at first. The only exception is that for TFSA in the apples at Zibo, the residue kept decreasing. This is consistent with the dissipation DT50 of TFS, because the rate the TFSA degrading to other metabolites was always higher than the rate the TFS degrading to TFSA, due to the high DT50 of TFS (9.5 days).
At the same site, the dissipation DT50S of TFS in soil and apples were all similar. However, TFSA generated and dissipated more slowly in the soil than in the apples. The reason for this may be attributed to the high water solubility and mobility of the acid TFSA, causing TFSA to move from top soil to deep soil and surface water. Another possible reason could be that the degradation pathway of TFS or TFSA may differ in soil and apples.
Conclusions
Overall, DT50 of TFS ranged from 0.54 to 8.8 days in soil and 4.8 to 9.5 days in apples at different latitude sites. Photolysis may be the main dissipation pathway of TFS, and sunshine hours may be the main influencing factor of TFS dissipation rate. The residue of TFSA first increased and then decreased, basically in both the soil and apples. This indicates that there is a higher risk when the application of TFS is in a short sunshine hour area, which should be carefully evaluated.
References
Anke, T., Oberwinkler, F., Steglich, W., & Schramm, G. (1977). The strobilurins—new antifungal antibiotics from the basidiomycete Strobilurus tenacellus (Pers. ex Fr.) Sing. Journal of Antibiotics, 30, 806–810.
Balba, H. (2007). Review of strobilurin fungicide chemicals. Journal of Environmental Science and Health, Part B Pesticides, 42, 441–451.
Banerjee, K., Ligon, A. P., & Spiteller, M. (2006). Environmental fate of trifloxystrobin in soils of different geographical origins and photolytic degradation in water. Journal of Agricultural and Food Chemistry, 54, 9479–9487.
Banerjee, K., Ligon, A. P., & Spiteller, M. (2007). Spectral elucidation of the acid metabolites of the four geometric isomers of trifloxystrobin. Analytical and Bioanalytical Chemistry, 388, 1831–1838.
Bartlett, D. W., Clough, J. M., Godwin, J. R., Hall, A. A., Hamer, M., & Parr-Dobrzanski, B. (2002). The strobilurine fungicides. Pest Management Science, 58(7), 649–662.
Boudina, A., Emmelin, C., Baaliouamer, A., Paisse, O., & Chovelon, J. M. (2007). Photochemical transformation of azoxystrobin in aqueous solutions. Chemosphere, 68, 1280–1288.
DG SANCO. (2014). EU pesticides database. http://ec.europa.eu/sanco_pesticides/public/index.cfm. Accessed 15 Aug 2012.
FAO, & WHO. (2013). Codex pesticides residues in food online database. http://www.codexalimentarius.net/pestres/data/index.html. Accessed 15 Aug 2014.
Food Standards Australia New Zealand. (2014). Food standards code. http://www.foodstandards.gov.au/code/Pages/default.aspx. Accessed 15 Aug 2014.
Garau, V. L., Angioni, A., Del Real, A. A., Russo, M., & Cabras, P. (2002). Disappearance of azoxystrobin, pyrimethanil, cyprodinil, and fludioxonil on tomatoes in a greenhouse. Journal of Agricultural and Food Chemistry, 50, 1929–1932.
Ghosh, R. K., & Singh, N. (2009a). Effect of organic manure on sorption and degradation of azoxystrobin in soil. Journal of Agricultural and Food Chemistry, 57, 632–636.
Ghosh, R. K., & Singh, N. (2009b). Leaching behaviour of azoxystrobin and metabolites in soil columns. Pest Management Science, 65, 1009–1014.
Health Canada. (2012). Maximum residue limits for pesticides. http://pr-rp.hc-sc.gc.ca/mrl-lrm/index-eng.php. Accessed 15 Aug 2014.
Isenring, H. P., & Weiss, B. (1991). Preparation of [(iminooxy)tolyl]acrylates and analogs as fungicides. Europe Patent EP0460575.
Japan Food Chemical Research Foundation. (2014). The Japanese positive list system for agricultural chemical residues in foods. http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/MRLs-p. Accessed 15 Aug 2014.
Joint FAO/WHO Meeting On Pesticide Residues. (2004). JMPR evaluation: trifloxystrobin. http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation04/TRIFLOXYSTROBIN.pdf. Accessed 27 Dec 2013.
Myung, K., Williams, D. A., Xiong, Q. B., & Thornburgh, S. (2013). Metabolism of strobilurins by wheat cell suspension cultures. Journal of Agricultural and Food Chemistry, 61, 47–52.
Patyal, S. K., Sharma, I. D., Chandel, R. S., & Dubey, J. K. (2013). Dissipation kinetics of trifloxystrobin and tebuconazole on apple (Malus domestica) and soil—a multi location study from north western Himalayan region. Chemosphere, 92, 949–954.
Singh, N., Singh, S. B., Mukerjee, I., Gupta, S., Gajbhiye, V. T., Sharma, P. K., Goel, M., & Dureja, P. (2010). Metabolism of 14C-azoxystrobin in water at different pH. Journal of Environmental Science and Health, Part B Pesticides, 45, 123–127.
Tomlin, C.D.S. (2006). The pesticide manual: a world compendium. Alton, Hampshire, UK: British Crop Protection Council 1349
US Government Printing Office. (2014). Tolerances and exemptions for pesticide chemical residues in food. http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&sid=5d35d354cc838eb105a733f5dff13ab8&tpl=/ecfrbrowse/Title40/40cfr180_main_02.tpl. Accessed 15 Aug 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Wu, J., Zhang, Y. et al. Field dissipation of trifloxystrobin and its metabolite trifloxystrobin acid in soil and apples. Environ Monit Assess 187, 4100 (2015). https://doi.org/10.1007/s10661-014-4100-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4100-3