Abstract
The aim of the study is to evaluate the acute biochemical and histological changes in rat kidneys after treatment with grayanotoxin (GTX) of rhododendron honey (RH). A total of 60 Sprague–Dawley female rats were divided into five groups of 12 rats each, one being a control group (group 1) and group 2 was treated with 0.015 mg/kg/bw of GTX standard preparation via intraperitoneal injection. Groups 3, 4, and 5 were given RH at doses of 0.1, 0.5, and 2.5 g/kg/bw, respectively, via oral gavage. Compared to the control group, significant increases were observed in glucose, blood urea nitrogen (BUN), and creatinine levels of the GTX-injected groups after 1 h. However, in low dose RH group, such an increase was not observed and had a normal appearance histologically. Therefore, low dose (1 g/kg/bw) of RH produces no acute adverse effects on renal functions of rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhododendron is a genus in the Ericaceae family, characterized by shrubs and small to large trees, either evergreen or deciduous. Turkish Rhododendron species grow naturally from the sea level and consist of five species (Rhododendron luteum Sweet, Rhododendron caucasicum Pallas, Rhododendron ponticum L., Rhododendron ungernii Trautv., Rhododendron smirnovii Trautv.) and 12 taxa. Some species of Rhododendron are poisonous, and therefore, ingestion of their leaves, flowers, or secondary products as honey may result in intoxication. However, some beekeepers produce rhododendron honey which is known in the region as “deli bal,” literally meaning “mad honey,” purposely for its supposed therapeutic effects on some health problems such as gastrointestinal disorders, hypertension, and sexual dysfunction (Sogut et al. 2009).
Species of the Rhododendron genus have been shown to possess many bioactive compounds (Qiang et al. 2011). However, grayanotoxins which are also known as rhodotoxins are a group of closely related toxins found in rhododendrons, and consumption of grayanotoxin containing rhododendron honey may result in intoxication. Kim et al. (2010) reported that among the 18 isoforms of grayanotoxin (GTX) found in mad honey, GTX I–IV are unique classes of toxic diterpenoids and GTX III is the principle toxic isomer. Scott et al. (1971) detected that the intraperitoneal LD50 value for grayanotoxin III was 0.908 mg/kg in mice.
A number of mad honey cases have been documented in Turkey. Those patients ingested differing amounts (20–200 g) of grayanotoxin containing rhododendron honey (Gunduz et al. 2006). Symptoms of mad honey intoxication are dose-related. The most common symptoms are dizziness, hypotension, and bradycardia, while rare symptoms such as excess salivation and perspiration, impaired consciousness and seizures, and atrioventricular block (AVB) have been reported as well (Sogut et al. 2009; Gunduz et al. 2006; Ozhan et al. 2004; Gunduz et al. 2007; Durmus et al. 2007). It is suggested that some symptoms may be caused by an inability to inactivate neural sodium ion channels resulting in continuous increased vagal tone (Jansen et al. 2012) as M2-receptor subtypes are involved in grayanotoxin-induced cardiotoxicity as well (Okuyan et al. 2010).
While medicinal use of grayanotoxin is not well understood, rhododendron honey is often consumed traditionally for its perceived medicinal potential as an aphrodisiac or as a treatment for gastritis and peptic ulcers, weakness, arthritis, diabetes, or hypertension (Aliyev et al. 2009; Yarlioglues et al. 2011). Research on the medicinal use of the Rhododendron and rhododendron honey, while still in its early stages, has revealed initial results confirming to an extent, some of the claims of the indigenous medicine. Jing et al. (2009) have found that the root of the rhododendron plant may attenuate renal damages by downregulating the activation of nuclear factor kappa B (NF-κB) in glomerulonephritis model rats. Some other researches have also identified possible antioxidant, antimicrobial (Silici et al. 2010), and antiinflammatory (Erdemoglu et al. 2008) activities. The efficacy of grayanotoxin was shown in diabetic rats with a significant decrease in the mean blood glucose and lipid levels after its administration (Oztasan et al. 2005). Furthermore, mad honey intoxication cases demonstrated a clear effect of GTX on the heart with a significant decrease in blood pressure and heart rate (Yilmaz et al. 2006), and despite this evidence of powerful bradycardic and antihypertensive action, the potential medical use in cardiovascular disease has not yet been fully investigated. In this point, combined biochemical and histopathological analysis forms the database for most diagnostic investigations.
Rhododendron honey contains a number of chemical compounds that, depending on their concentration and application, can also be considered medicinal or poisonous. From that point of view, our research is the first study and aims to evaluate the biochemical and histopatalogical changes in rats following the administration of rhododendron honey and grayanotoxin depending on the concentration.
Materials and methods
Animal material
In the current study, a total of 60 Sprague–Dawley female rats (6–8 months old; 250–300 g; Laboratory Animal Unit, Erciyes University, Kayseri, Turkey) were used. The rats were divided into five groups of 12 rats each, one being the control group (group 1) and the remaining being the experimental groups (groups 2 to 5). The rats were housed at a fixed temperature of 22–24 °C in standard cages and maintained in a 12:12 light/dark photoperiod and were fed a standard pelleted diet and tap water ad libitum. The chemical analysis of the pellets showed that they contained 23 % crude protein, 7 % crude cellulose, and 2600 kcal/kg diet. The study protocol was approved by the Ethical Committee of the Veterinary Faculty of Erciyes University.
Granayanotoxin and honey samples
Grayanotoxin III hemi(ethyl acetate) was purchased from Sigma-Aldrich (Germany). Eleven fresh honey samples (extracted honey type) of Rhododendron in different parts of Black Sea Region of Turkey were collected from beekeepers in the period of June–July in 2012, when the Rhododendron species were flowery. The samples were stored at room temperature in jars for further analysis. Honey doses used in the study were calculated as 0.1, 0.5, and 2.5 g/kg/bw. The amount of honey that a person consumes an average of 70 kg in mind was calculated (7 g (one teaspoon), 35 g (one to two tablespoons), and overdose of 175 g (seven to eight tablespoons).
Analysis of sediment for the identification of honey samples
The honey samples were classified according to their botanical origin after the pollen spectrum was obtained, and melissopalynological analysis was carried out using the methods of Louveaux et al. (1978) and Von der Ohe et al. (2004). Pollen grains were microscopically observed and compared with the reference slides for identification. Pollen grains were identified under the microscope, and counts were expressed as percentages. Botanical classification was achieved when the pollen spectrum contained >45 % of the corresponding dominant pollen. Monofloral rhododendron honeys (>45 %) were used for the GTX analysis (Table 1).
GTX III analysis of rhododendron honey samples
Reagents
GTX III standard was supplied as Grayanotoxin III hemi(ethyl acetate) from Sigma-Aldrich (St. Louis, MO, USA). Hypergrade methanol for liquid chromatography–mass spectrometry (LC-MS) and glacial acetic acid were obtained from LiChrosolv® (Merck KGaA, Darmstadt, Germany). High-quality ultrapure water was supplied by Human Zeneer Navi Power I Integrate (Human Corporation, Korea). LC syringe filters (RC membrane, 0.2 μm) were from Sartorius Minisart RC 15, Sartorius (Darmstadt, Germany).
Preparation of honey and GTX III
Monofloral rhododendron honeys (n = 6) were dried at room temperature for experiments. About 5 g of honey was extracted with 30 mL methanol in a flask attached the condenser at 60 °C in 6 h. Extract was filtered to remove particles, and final volume was determined. The remaining extract was evaporated and dried with rotary evaporator (IKA, Werke, USA) at 40 °C. The residues were dissolved in 10 mL distilled water and transferred to C18 solid-phase extraction (SPE) cartridge, which was initially conditioned with 5 mL methanol followed by 5 mL water. The cartridge was washed with 5 mL water to remove unbound materials. GTX III was eluted from C18 SPE using 5 mL methanol. The organic solvents were evaporated in a rotary evaporator with reduced pressure at 40 °C. The residue was weighed and dissolved in methanol for LC/MS-MS analysis. One milligram of Grayanotoxin III hemi(ethyl acetate) was dissolved with 1 mL hypergrade methanol. For calibration graphs and method validation, different dilutions ranging from 0.03125 to 2.5 μg/mL of the standard were used.
LC-MS/MS analysis
A Thermo Scientific LC coupled with a TSQ Quantum Access Max triple-stage quadrupole mass spectrometer (San Jose, CA USA) was used for all analyses. The analytical column was a Phenomenex C-18 (15 cm × 3 mm × 5 μm) (Torrance, California, USA). GTX III was eluted under isocratic conditions using a mobile phase consisting 50:50 water/methanol solution containing 1 % acetic acid into 8 min. The flow rate is 0.3 mL/min. MS data was acquired by ESI in the negative-ion mode using selected reaction monitoring (SRM) after describing the real molecular weight of GTX III by full scan in the range of 200–500 m/z. The instrument was tuned on MS/MS mode by optimizing the response of m/z 369 as the negative-ion form of m/z 370 using 1 μg/mL GTX III into the mobile phase at 10 μL/min flow rate with flash syringe. Fragmentation pattern study was applied for m/z 279, m/z 297, and m/z 315 mass ions as product masses of m/z 369 (Fig. 1).
Experimental groups
The groups received the following bolus administrations:
-
Group 1
(control): 1 mL of normal saline, i.e., 0.9 % NaCl solution i.p. (n = 12)
-
Group 2
(GTX): 0.015 mg/kg/bw of grayanotoxin III was injected i.p. (n = 12)
-
Group 3
(rhododendron honey, RH1): 0.1 g/kg/bw of RH was given by oral gavage (n = 12)
-
Group 4
(RH2): 0.5 g/kg/bw of RH was given by oral gavage (n = 12)
-
Group 5
(RH3): 2.5 g/kg/bw of RH was given by oral gavage (n = 12)
Collection of blood samples
The animals were fasted for 8 h prior to the treatment, and collection of blood samples was performed 1 h after the treatment. Due to risk of change in biochemical parameters, the animals were not administered general anesthesia prior to taking the samples. The animals were maintained under light ether anesthesia, performed just before the collection of blood samples, and samples were slowly collected from each animal into tubes both with and without anticoagulants, by means of cannula inserted into the heart. Blood was taken from all 12 animals in each group, and totally, 60 plasma and serum samples were gained.
Analysis of serum biochemical parameters
The measurement of serum total protein, albumin, glucose, cholesterol, triglyceride, blood urea nitrogen (BUN), protein, uric acid, creatinine, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), potassium, magnesium, sodium, and chloride levels/activities were performed using a Konelab60i model auto-analyzer.
A Konelab60i model auto-analyzer was used for the measurement of biochemical parameters in serum, and measurements were performed spectrophotometrically. The test speed of the auto-analyzer used is 600 photometric tests per hour. The reactive compartment possesses a cooling property and maintains the reactive bearing capacity at 45. The sample compartment of device has a segmental structure, and with its six segments, each having a capacity of 14 samples, it enables the loading of 84 samples. The total loading capacity of the tube is 2400. The device enables the use of a low amount reactive and samples and also has separate reactive, mixing probes, automatic predilution and postdilution property, and 11 + 4 interference filters. The calibrator with 20 positions and the control serum compartment with 19 positions have cooling properties and enable the maintenance of calibrator and control serums without any alteration.
Histoptahological examinations
Analysis of tissue (kidney) samples was performed at the Laboratory of the Department of Histology and Embryology in the Medical Faculty of Erciyes University. The specimens were fixed in 10 % neutral buffered formalin and embedded in paraffin. Sections (5 μm thick) were mounted on poly-l-lysine-coated microscope slides and then processed for routine hematoxylin–eosin (H&E) staining. They were placed under the microscope with a digital camera connected to a computer system.
Statistical analysis
The SPSS 11.00 for Windows software package was used for statistical analyses. Data were given in the form of arithmetical mean values and standard deviations. One-way analysis of variance was performed, and variant groups were determined by means of the Duncan test.
Results
Honey samples contained pollens of Ericaceae (Rhododendron spp.), Apiaceae, Asteraceae, Fabaceae, Fagacaeae (Castanea sativa L), and Rosaceae families. Rhododendron pollen frequencies varied from 1.7 to 75.06 % in the samples. Among the samples, honeys containing >45 rhododendron pollen frequency (six monofloral rhododendron honeys) were used for the GTX III analysis. GTX concentrations of honey samples were between 2.114 and 11.371 μg GTX III/g honey sample, and the average value was 6.225 μg GTX III. Therefore, the closest to the average RH1 (5.905 μg GTX III/g honey) was used for biochemical and histopathological evaluations (Table 1).
The change in triglycerides, cholesterol, and total protein levels in the experimental groups were not statistically significant in comparison with the control group. On the other hand, compared to the control group, a significant increase (p < 0.05) in glucose levels were observed in group 2 and group 5. However, glucose levels in group 3 and group 4 were similar to the control group while decreased in comparison with group 2 and group 5 (Table 2).
The change in AST, ALT, and ALP levels in the experimental groups was not statistically significant in comparison with the control group, while LDH levels of the experimental groups were significantly different from the control group (p < 0.05). The lowest LDH levels were achieved in group 3 (Fig. 2). Uric acid levels of all the groups were not significantly different from each other (p > 0.05) (Table 3).
The change in Ca, Na, and Cl levels in the experimental groups was not statistically significant in comparison with the control group, while P levels of the experimental groups were significantly different from the control group (p < 0.05). P levels were decreased in group 3 and group 4 in comparison with the control group, while P levels in group 2 and group 5 were similar to the control group (Table 4). When compared with the control group, no significant change was observed in Mg and K levels in all groups (p > 0.05).
The change in BUN, creatinine, and T-bilirubin levels in the experimental groups was statistically significant in comparison with the control group (p < 0.05). BUN levels were increased in group 2 and group 5 in comparison with the control group, while BUN levels in group 3 and group 4 were similar to the control group. Creatinine levels in group 3, group 4, and group 5 were similar to the control group or lower, as creatinine levels were increased in group 2 in comparison with the control group (p < 0.05). Total bilirubin levels were increased in experimental groups in comparison with the control group, while the difference with the control group was smaller by the dosage increase in rhododendron groups (Table 5).
The change in HDL cholesterol, LDL cholesterol, creatine phosphokinase (CPK), and pseudocholinesterase levels in the experimental groups was not statistically significant in comparison with the control group (p > 0.05). Although in rhododendron groups LDL cholesterol levels were less than that of the control group, an increase was observed dose-dependently. By the dosage increase in rhododendron groups, CPK levels showed an arithmetic decrease while pseudocholinesterase levels showed an arithmetic increase (Table 6).
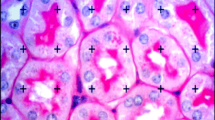
Analysis of kidney tissue samples was performed after the specimens were fixed in neutral buffered formalin and embedded in paraffin. In all groups, the glomeruli in periodic acid–Schiff (PAS) stains had a normal appearance under light microscopy (Fig. 3). In group 3 (RH1), the glomeruli and the proximal tubulus in PAS stains have a normal appearance under light microscopy; however, a minimal loss of cells (arrow) of the distal tubulus is observed. In group 4 (RH2), the glomeruli in PAS stains have a normal appearance under light microscopy; however, a loss of cells (arrow) of the distal tubulus is observed to some extent. In group 5 (RH3), the glomeruli in PAS stains have a normal appearance under light microscopy; however, the proximal tubular lumen dilation is present due to the partial degeneration of cuboidal epithelial cells lining the proximal tubule. An evident loss of cells (arrow) of the distal tubulus is observed. In group 2 (GTX), the glomeruli in PAS stains have a normal appearance under light microscopy; however, a loss of cells (arrow) of both the proximal and more evidently of the distal tubulus is observed. Shedding of desquamated epithelial cells is also present in tubular lumina.
(x40 a4 pas1) a In group 5 (RH3), the glomeruli in PAS stains have a normal appearance under light microscopy; however, the proximal tubular lumen dilation is present due to the partial degeneration of cuboidal epithelial cells lining the proximal tubule. An evident loss of cells (arrow) of the distal tubulus is observed. b In group 4 (RH2), the glomeruli in PAS stains have a normal appearance under light microscopy; however, a loss of cells (arrow) of the distal tubulus is observed to some extent. c In group 3 (RH1), the glomeruli and the proximal tubulus in PAS stains have a normal appearance under light microscopy; however, a minimal loss of cells (arrow) of the distal tubulus is observed. d In group 2 (GTX), the glomeruli in PAS stains have a normal appearance under light microscopy; however, a loss of cells (arrow) of both the proximal and more evidently of the distal tubulus is observed. Shedding of desquamated epithelial cells are also present in tubular lumina
Discussion
Honey samples were collected according to statements of local beekeepers. However, the pollen analysis showed that rhododendron pollen frequencies varied in the samples. Because pollen frequency in honey depends on the floral diversity and density, bees collect rhododendron nectar especially if the other nectar sources around are not enough. Therefore, it is not very common to find mad honey that contain high-frequency rhododendron pollens since the bees prefer to visit the flowering chestnut trees that are common in that season. In fact, nearly half of the samples collected in this study showed multi-floral characteristics. Therefore, this does not concur with some of the previous studies (Cagli et al. 2009) that state that mad honey contains rhododendron pollens. Namely, it is also an important percentage of rhododendron pollen in mad honey.
There have been only a limited number of studies on the chemical properties and biological activities of rhododendron honey. In a study, in order to characterize and authenticate the volatile profile of rhododendron honey, honey samples from the Black Sea Region of Turkey were analyzed and a variety of volatiles, particularly organic acids, phenols, esters, ketones, and alcohols, was detected. A total of 72 volatile compounds were identified, and 1,2 benzenedicarboxylic acid, tributhyl phosphate, stearic acid, propanoic acid, benzene, ethyl phenylacetate, and benzophenone were recognized as specific floral origin markers of rhododendron honey (Silici 2010). In another research, rhododendron honey samples obtained from the Black Sea Region of Turkey were screened for total phenolic content as well as potential antioxidant, antiradical, and antimicrobial activities (Silici et al. 2010). According to Silici et al. (2008), Rhododendron and eight multi-flower honeys obtained from Black Sea Region of Turkey and revealed that rhododendron honeys exhibited higher concentrations of Cu, Co, Cr, Ni, Se, Zn, Ca, and Mg but lower concentrations of Al, Mn, Fe, and K than in the multi-flower honeys. Furthermore, Silici and Karaman (2014) showed that the major amino acids in rhododendron honey are aspartic acid, lysine, and arginine. In addition, Silici et al. (2014a, b) determined the phenolic profile of rhododendron honeys.
Combined biochemical and histopathological analysis is considered for most diagnostic investigations. Many biochemical parameters tend to have specificity for an organ and/or a limited range of pathological processes. Investigative biochemical parameters are designed to provide all the data necessary for a broad investigation of internal disease (Harper 1983).
Blood glucose is an important source of energy for many cells. The maintenance of normal plasma glucose requires delicate balance of glucose availability with glucose utilization. However, glucose is not only an energy source which fuels the energy requirements of the body tissues but also an obligate fuel for the central nervous system. Consequently, maintenance of a normal blood glucose concentration is essential for the survival of brain tissue (Harper 1983). Glucose levels in group 3 and group 4 were similar to that of the control group and decreased in comparison with group 2 and group 5. The increase in the blood glucose levels in group 5 can be explained by the higher carbohydrate content. GTX group had the highest glucose level in experimental groups. Salman and Akbas (2010) reported that grayantoxin may cause hypoglycemia. In addition, Uzun et al. (2013) reported a case of a child with mad honey poisoning whose blood glucose was below the normal level.
LDH is an intracellular enzyme which is widely distributed throughout the body and is found at high levels in tissues that utilize glucose for energy. Elevated LDH activity indicates tissue damage (Harper 1983). An increase in LDH can reflect damage to a number of different tissues (skeletal or cardiac muscle, kidney, liver). In this study, LDH levels increased dose-dependently in the honey groups. The lowest LDH levels were achieved in group 3 (RH2). Also, the level of GTX group was higher than the control group. Similarly, on the mad honey poisoning case reported by Cetin et al. (2009), LDH level (851 U/L) was observed above the normal values.
The majority of the blood urea is synthesized in the liver from ammonia. Once formed, urea diffuses freely throughout all body fluids. The kidney is the most important route of urea excretion, and as a result, urea has long been used as a barometer of renal function. BUN levels were increased in group 2 (GTX) and group 5 (RH3) in comparison with the control group, while BUN levels in group 3 and group 4 were similar to the control group. Eken (2004) reported a case that mad honey showed normal liver and kidney function tests. Ascioglu et al. (2000) reported nephrotoxic effect of acute GTX I administration in rats. However, they stated that low dose level of GTX I (0.25 mg/kg) does not cause a hepatic damage, but exposure to high doses (1 mg/kg) produces hepatotoxicity.
Most creatinine originates from the non-enzymatic conversion of creatine in muscle. Creatinine is freely filtered by the glomerulus, and clearance of creatinine from the plasma to the urine can be used to provide an approximation of the glomerular filtration rate. A small amount of creatinine is secreted by proximal tubules in the kidney, but, in contrast to urea, none is resorbed by the tubules (Harper 1983). Creatinine levels in honey groups were similar to the control group or lower, as creatinine levels were increased in GTX group in comparison with the control group. Ohgaki et al. (1987) suggested that intraperitoneal administration of the GTX III (0.1–0.25 mg/kg bw) resulted in a dose-dependent manner in a significant and reversible muscle relaxation. However, Uzun et al. (2013) reported a case of child with mad honey poisoning whose blood creatinine level (0.9 mg/dL) was normal as well.
Phosphorus is an important ion but is most physiologically active as the phosphate radical. It is used in the structural proteins of the cell wall, bone, and other tissues and in active metabolic enzymes and pathways. Serum concentrations of phosphorus are regulated primarily by the renal tubules responding to parathyroid hormone stimulation. There are various disease processes that alter phosphorus levels and many inter-relationships with other systems. The renal system is closely involved in the control of phosphorus levels, and thus, urea and creatinine are important adjunct determinations (Harper 1983). Any high phosphorus should be correlated with renal evaluations. P levels were decreased in group 3 (RH1) and group 4 (RH2) in comparison with the control group while P levels in GTX group and RH3 group increased for this study. Kim et al. (2010) reported that GTX increases Ca2+ channel influx through voltage-dependent Ca2+ channels secondary to activation of voltage-dependent Na+ channels in inhibitory and excitatory nerve terminals synapsing on VMH neurons, and the increased release of GABA on glutamate from these terminals may be responsible for the autonomic symptoms of GTX intoxication.
Bilirubin and its components may be helpful when evaluating liver function or hemolysis. These tests may be useful in distinguishing prehepatic from hepatic or posthepatic hyperbilirubinemia. Bilirubin is mainly formed from the breakdown of erythrocytes. Total bilirubin level rise may indicate hepatatoxicty. In our study, total bilirubin levels were increased in experimental groups in comparison with the control group. GTX, RH2, and RH3 groups had higher levels of total bilirubin than control and RH1 groups. Likewise, in the case study reported by Cetin et al. (2009), the level of total bilirubin was found as 2.75 mg/dL which is above the normal values. It is therefore necessary to pay attention to the dose of rhododendron honey that is recommended.
Also, histopathological examination of kidney sections from animals revealed normal structures. In all groups, the glomeruli had a normal appearance under light microscopy while some minimal changes in the proximal and the distal tubulus were observed in the experimental groups.
GTX found in rhododendron plants and rhododendron honey or mad honey are lipid-soluble toxins that exert selective effects on voltage-dependent sodium channels by eliminating fast sodium inactivation and causing a hyperpolarizing shift in voltage dependence of channel activation. Mad honey intoxication is commonly seen in the Black Sea Region of Turkey. However, due to increase in popularity of that honey and possibility of its easy and fast transport, it is becoming a worldwide problem.
There have been previous studies on the acute and chronic effects of RH on some vital organs such as the liver, heart, and nervous system (Ascioglu et al. 2000; Onat et al. 1991; Seyama and Narahashi 1981). To the best of our knowledge, this is the first comparable study on the effects of GTX and RH on kidney, and therefore, it can contribute to scientific literature. Additionally, not having toxic effect on biochemical and histological parameters in the determined doses is important in terms of healthy/safe usage of that honey in the traditional medicine. Similarly, Ascioglu et al. (2000) previously reported that there is not an acute hepatotoxic effect of RH on certain doses.
However, studies are available on the effects of GTX on the kidneys and livers of laboratory animals. Nishikawa et al. (1989) gave GTX III intraperitoneally to rats at a dose of 0.8 or 2.8 mg/kg. To study the effects of GTX III on rats, biological tests in serum for functions of liver and kidney and their pathological observation were performed 1 h after the administration. Activities of glutamic-pyruvic transaminase, guanase, and leucine aminopeptidase and concentrations of total protein, albumin, creatinine, uric acid, and K increased significantly. These parameters showed dose–effect relations with GTX III. Although GPT and free fatty acid increased significantly, dose–effect relations were not shown. The activity of choline esterase and the concentrations of bilirubin, urea-N, lipoperoxide, cholesterol, triglycerides, Na, and Cl were not significantly different. Pathological changes were not observed in the liver and kidney of rats.
Ohgaki et al. (1987) searched the effect of systemic administration of GTX III on general behaviors of animals. Intraperitoneal administration of the GTX (0.1–0.25 mg/kg bw) resulted in a dose-dependent manner in a significant and reversible muscle relaxation and a profound and long-lasting depression of locomotor activity. They suggested that GTX III might elicit a central depressant action in mice through a molecular mechanism other than activation of the voltage-sensitive sodium channels in the brain. Our study differs from previous studies by being a comparable study on the effects of GTX and RH, considering possible dosages of GTX in RH.
General histo-analysis of kidney tissue samples was performed, and in addition to the glomeruli, epithelial cells, distal tubulus, and proximal tubulus were all investigated as well. In all groups, the glomeruli had a normal appearance under light microscopy.
Due to the lack of detailed literature review on the subject, the results of the research could be compared only with case reports. However, it should be noted that these reports usually offer the result of tests carried out on one or just a few people. Therefore, this study may be useful for researches to be conducted thereafter. Research on the medicinal use of the rhododendron honey, while still in its early stages, has revealed initial results confirming folk belief to some extent. Yet, medicinal use of grayanotoxin is not well understood, and care should be taken when consuming mad honey.
References
Aliyev F, Turkoglu C, Celiker C (2009) Nodal rhythm and ventricular parasystole: an unusual electro cardiographic presentation of mad honey poisoning. Clin Cardiol 32:e52–e54
Ascioglu C, Ozesmi C, Dogan P et al (2000) Effects of acute grayantoxin-I administration on hepatic and renal functions in rats. Turk J Med Sci 30:23–27
Cagli KM, Tufekcioglu O, Sen N et al (2009) Atroventricular block induced by mad-honey intoxication: confirmation of diagnosis by pollen analysis. Tex Heart Inst J 36(4):342–344
Cetin NB, Marcıl E, Kıldıran M et al (2009) Hepatotoxicity with mad honey. Turk J Emerg Med 9(2):84–86
Durmus I, Turedi S, Gunduz A et al (2007) Mad honey poisoning related asystole. Emerg Med 24(8):592–593
Eken C (2004) Grayanotoxin poisoning. Turk J Emerg Med 4(2):76–77
Erdemoglu N, Akkol EK, Yesilada E et al (2008) Bioassay-guided isolation of anti-inflammatory and antinociceptive principles from a folk remedy, Rhododendron ponticum L. leaves. J Ethnopharmacol 119(1,2):172–8
Gunduz A, Turedi S, Uzun H et al (2006) Mad honey poisoning. Am J Emerg Med 24:595–598
Gunduz A, Tatlı O, Turedi S (2007) Mad honey poisoning from the past to the present. Turk J Emerg Med 8(1):46–49
Harper AH (1983) Review of Bio Chemistry. 20 th ed. Lange Medical Publications Co, pp.1012
Jansen SA, Kleerekooper I, Hofman ZLM et al (2012) Grayanotoxin poisoning: “mad honey disease” and beyond. Cardiovasc Toxicol 12(3):208–215
Jing X, Zhonghua Z, Jianshe L et al (2009) The effect of root of rhododendron on the activation of NF-κ B in a chronic glomerulonephritis rat model. J Nanjing Med Uni 23:73
Kim SE, Shin MC, Akaike N et al (2010) Presynaptic effects of grayanotoxin III on excitatory and inhibitory nerve terminals in rat ventromedial hypothalamic neurons. NeuroToxicology 31(2):230–238
Louveaux J, Maurizio A, Vorwohl G (1978) Methods of melissopalynology. Bee World 59:139–157
Nishikawa Y, Fukumoto K, Tetsumi T et al (1989) Effects of grayanotoxin III on liver function and renal function in rats. Yakugaku Zasshi 109(5):340–3
Ohgaki T, Meguri H, Ogita K et al (1987) Tetradoxin- sensitive central depression by grayanotoxin III in mice. Brain Res 425(2):364–368
Okuyan E, Uslu A, Ozan Levent M (2010) Cardiac effects of “mad honey”: a case series. Clin Toxicol (Phila) 48(6):528–532
Onat F, Yeğen BC, Lawrence R et al (1991) Mad honey poisoning in man and rat. Rev Environ Health 9:3–9
Ozhan HO, Akdemir R, Yazıcı H et al (2004) Cardic emergency caused by honey ingestion: a single centre experience. Emerg Med J 21:742–744
Oztasan N, Altinkaynak K, Akcay F et al (2005) Effect of mad honey on blood glucose and lipid levels in rats with streptozocin-induced diabetes. Turk J Vet Anim Sci 29:1093–1096
Qiang Y, Zhou B, Gao K (2011) Chemical constituents of plants from the genus Rhododendron. Chem Biodivers 8:792–814
Salman C, Akbas M (2010) Bradycardia, hypotension and hypoglycemia caused by grayanotoxin (andromedotoxin) intoxication: case report. Turk Klin J Anest Reanim 8(1):64–8
Scott PM, Coldwell BB, Wilberg GS (1971) Grayanotoxins. Occurrence and analysis in honey and a comparison of toxicities in mice. Food Cosm Toxicol 9:179–184
Seyama I, Narahashi T (1981) Modulation of sodium channels of squid nerve membranes by grayanotoxin I. J Pharmacol Exp Ther 219:614–24
Silici S (2010) Characterization of volatile compunds of Rhododendron honey. Mellifera 10–19:17–23
Silici S, Karaman K (2014) Chemometric approaches for the characterization of Turkish Rhododednron and honeydew honeys depending on amino acid composition. J Liq Chromatogr Relat Technol 37(16):864–877
Silici S, Uluözlü ÖD, Tüzen M et al (2008) Assessment of trace element levels in Rhododendron honeys of Black Sea Region, Turkey. J Hazard Mater 156:612–618
Silici S, Sagdic O, Ekici L (2010) Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem 121(1):238–243
Silici S, Sarıoglu K, Karaman K (2014a) Determination of polyphenols of some Turkish honeydew and nectar honeys using HPLC-DAD. J Liq Chromatogr Relat Technol 36(6):2330–2341
Silici S, Sarioglu K, Dogan M, Karaman K (2014b) HPLC-DAD analysis to identify the phenolic profile of rhododendron honeys collected from different regions in Turkey. Int J Food Proper 17(5):1126–1135
Sogut O, Sayhan MB, Mordeniz C et al (2009) Mad honey poisoning: a case report and review of the literature. Anatol J Clin Investig 3(1):100–102
Uzun H, Sari I, Gunes C, Kocabay K et al (2013) A child with bradycardia and hypotension related to mad honey intoxication. Turk Arch Ped 48:53–54
Von der Ohe W, Oddo IP, Piana ML et al (2004) Harmonized methods of melissopalynology. Apidologie 35:18–25
Yarlioglues M, Akpek M, Ardic I et al (2011) Mad-honey sexual activity and acute interior myocardial infarctions in a married couple. Tex Heart Inst J 38:577–580
Yilmaz O, Eser M, Sahiner A et al (2006) Hypotension, bradycardia and syncope caused by honey poisoning. Resuscitation 68:405–408
Acknowledgments
This research was supported by Erciyes University Research Fund through Project No. FBY-11-3774. Huseyin SAHIN was supported by TUBITAK BIDEB for his Ph.D. study. We also thank R.T.E University for helping us with the LC-MS/MS device.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
Low dose (0.1 g/kg bw) of rhododendron honey produces no adverse effect on biochemical parameters in rats.
Rights and permissions
About this article
Cite this article
Silici, S., Doğan, Z., Sahin, H. et al. Acute effects of grayanotoxin in rhododendron honey on kidney functions in rats. Environ Sci Pollut Res 23, 3300–3309 (2016). https://doi.org/10.1007/s11356-015-5534-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5534-z