Abstract
Gentamicin (GM) is an aminoglycoside antibiotic used to treat bacterial infections. Nephrotoxicity refers to the impairments of the kidneys caused by the use of GM and can result in decreased kidney function and in severe cases, kidney failure. Aronia melanocarpa extract (AME), also known as the black chokeberry, has been used for its protective effects on the kidneys. AME concentration of 3.38 mg/kg (max antioxidant activity in vitro) was used to determine its effectiveness against induced nephropathy during 30 days. GM treatment caused significant hypoalbuminemia and high values of globulins, creatinine, and urea compared to the control group. GM application lead to hemolysis occurrence, echinocytosis, and platelets aggregation. Significantly high values of segmented neutrophils and low values of non-segmented neutrophils were recorded in the blood of rats treated with chokeberry extract (AME). In the pre-treatment (AME + GM), severe hypochromic anemia and a significant improvement in hematological parameters, as well as a reduction of anemia in the post-treatment (GM + AME), were noted. Post-treatment AME also significantly regulates urea and creatinine values. Statistically significantly low hemoglobin values were found in all groups treated with AME. Current study suggests that compounds in the AME have a moderate beneficial effect against renal injury and anti-inflammatory properties that may help protect the kidneys from injury caused by GM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An aminoglycoside antibiotic, gentamicin (GM), is used to treat severe gram-positive and gram-negative bacterial infections. It has been shown that direct tubular necrosis, which is mostly confined in the proximal tubule, is a hallmark of GM-induced nephrotoxicity [1]. Due to its high affinity for renal proximal convoluted tubules, gentamicin is particularly harmful to the kidneys [2]. GM-induced nephropathy is characterized by morphological and functional changes, including increased serum creatinine and proximal tubular edema and tubular necrosis [3]. It has been demonstrated that GM increases the production of reactive oxygen species. In numerous diseased conditions, including several models of renal injury, ROS have been proposed as a causal agent of cell death [4]. Nephrotoxicity brought on by GM involves numerous pathways, including the decrease in renal blood flow, oxidative stress, inflammation, nitric oxide (NO) production, lipid peroxidation, the nuclear factor-B pathway, apoptosis, and the reduction in the effectiveness of kidney antioxidant enzymes like superoxide dismutase (SOD), catalase, glutathione peroxidase, and reduced glutathione (GSH) [5,6,7]. After initiation of aminoglycoside therapy, blood urea nitrogen and serum creatinine often rise 7 to 10 days later. The loss of renal function only happens after the medication is finished in more than half of the instances of nephrotoxicity [8].
To successfully prevent GM nephrotoxicity, substances with anti-inflammatory, anti-apoptotic, or antioxidant action were administered. Numerous plant extracts used in traditional medicine have been found to have immunomodulatory and anti-inflammatory properties, which offers a justification for their therapeutic usage such as black chokeberry Aronia melanocarpa (Michx.) Elliott 1821, a member of the rose family (Rosaceae). This is due to the presence of polyphenolic compounds like anthocyanins (cyanidin 3-O-galactoside, cyanidin 3-O-arabinoside, cyanidin 3-O-xyloside, and cyanidin 3-O-glucoside), flavonoids (quercetin 3-O-vicianoside, quercetin 3-O-robinobioside, and other quercetin glucosides), phenolic acids, and vitamins C and E [9]. Chokeberry fruit extracts’ impact on the leukocyte pattern, phagocytic activity, and cytokine system in Wistar rats with induced immunosuppression was investigated. Results showed that A. melanocarpa extracts (AME) encourage fast immune system recovery in rats, normalize the leukocyte count, and enhance monocyte and neutrophil phagocytic indicators [10]. The antioxidant phenolic compounds found in abundance in chokeberry berries have anti-inflammatory properties, suggesting that they may have therapeutic benefits for metabolic disorders and liver impairments [11]. Studies have shown that consumption of chokeberry juice can increase red blood cell count and hemoglobin levels, as well as improve antioxidant status in rats. Additionally, chokeberry supplementation has been shown to reduce oxidative stress and inflammation in rats, which can contribute to improved hematological parameters [12, 13].
The current research aims to examine the nephroprotective effects of AME pre- and posttreatment at biochemical, hematological, and cell morphology levels during GM-induced nephropathy in Wistar rats.
Material and Methods
Maintenance and Animal Models
A total of 30 Wistar rats aged 3 months (mean weight: 198.40 g) were used in this experiment. In the Laboratory for Biochemistry and Physiology (University of Sarajevo-Faculty of Science), animals were separated based on sex and bred in accordance with guidelines. The rats were given free access to water and were fed Oxbow’s Essentials-Adult food, which is designed to meet the nutritional needs of adult rats (crude protein 15%, crude fat 4%, crude fiber (min) 2%, crude fiber (max) 5%, moisture 10%, calcium (min) 1%, calcium (max) 1.5%, phosphorus 0.8%, vitamin A 8000 IU/kg, and vitamin E 125 IU/kg). They were kept in a standard laboratory environment with 12-h light/dark cycles at a temperature of 24 °C. It was necessary to provide the right care, housing, and handling of the animals during the experimental period. According to moral and legal standards, animal abuse was avoided. The “Declaration on Animal Rights,” “Universal Declaration on Animal Welfare,” and “Law on the Protection and Welfare of Animals” were all followed while caring for animals used in study. The Law on the Protection and Welfare of Animals applies to experiments with animals (Official Gazette of BiH, No. 25/2009 and 9/2018) in Bosnia and Herzegovina.
Experimental Design
Five groups of rats (n = 6) with similar body weights were randomly assigned. Animal models are separated into groups as follows:
-
Control group (Ctr): 1 mL of 0.9% saline solution was administered i.p. for 5 days and water was given ad libitum
-
GM group: gentamicin (GM) 80 mg/kg b.w. was applied i.p. and water was given ad libitum
-
AME group: AME extract in concentration of 3.38 mg/kg was applied via oral gavage for 30 consecutive days
-
GM + AME group: GM (80 mg/kg) was administered i.p. for the first 5 days of experiment in parallel with AME extract for 30 days via oral gavage
-
AME + GM group: Pretreatment with AME extract for 25 days via oral gavage was administered and for the last 5 days of the experiment GM (80 mg/kg) was i.p. applied
Dosage criteria for AME (3.38 mg/kg b.w.) showed maximum antioxidant activity in vitro and were chosen upon previous research done in the Laboratory for Organic Chemistry and Biochemistry, Department of Chemistry, Faculty of Science, University of Sarajevo. GM dosage criteria were chosen upon previous investigations [14, 15]. The timeline of the experiment and outlines are presented in Fig. 1.
Aronia Extract Preparation and Radical Scavenging Activity
Aronia melanocarpa fruit ethanol extracts (AME) were made utilizing ultrasound-assisted extraction (UAE). Aronia fruit was air-dried, crushed, and put into a flask with 70% ethanol. The flask was placed in a sonicator water bath at 20 °C for 45 min with aluminum foil covering it. The raw AME was evaporated using a rotary evaporator. Extracts were collected and stored at 4 °C in glass flasks prior to analysis. The DPPH, ABTS, and DMPD techniques were used to evaluate the antioxidant activity of AME [16, 17].
Blood and Serum Collection
The University of Minnesota’s Research Animal Resources recommended using an intraperitoneal mixture of 60 mg/kg ketamine (Intervet International, Boxmeer, The Netherlands) and 7 mg/kg diazepam (Alkaloid, Skopje, North Macedonia) to provide long-lasting analgesia before cardiac puncture. The cervical dislocation was performed following heart puncture in accordance with AVMA Guidelines. Cardiac puncture was performed to get a total of 3 mL of whole blood without anticoagulants. One milliliter of blood was used for hematological analysis and to make blood smears for differential blood count analysis and 2 mL was centrifuged (3000 rpm, 10 min, 4 °C) to separate the serum (as explained below). Before being used in biochemical testing, serum was gently aspirated and stored at − 24 °C.
Analysis of Biochemical Parameters
The blood serum parameters were examined using a Spectronic® Thermo Scientific GenesysTM 20 spectrophotometer (USA) for a total of 6 parameters. Using the Biuret colorimetric test (Quimica Clinica Aplicada-QCA, Spain), which produces the recognizable purple-colored biuret complex when divalent copper interacts with protein peptide bonds in alkaline solution, the total protein level was determined. The ratio of the color intensity to the protein concentration, which may be assessed photometrically, is one. Using the Rodkey’s colorimetric bromocresol green method (Semikem, Bosnia and Herzegovina), albumin levels in serum were measured. To obtain globulins, total proteins are subtracted from albumins (globulins = total proteins − albumins). Albumin and computed globulin values were used to generate the A/G ratio. Urea concentration in serum was determined by Urea DAM kit (Semikem, Sarajevo, Bosnia and Herzegovina). Creatinine (Semikem, Bosnia and Herzegovina) was determined by Jaffe method where it forms a yellow complex with sodium picrate, and is measured at 520 nm spectrophotometrically.
Hematological Parameters Evaluation and Differential Blood Count Analysis
Hematological parameters that were analyzed were as follows: red blood cell count (RBC), white blood cell count (WBC), hemoglobin concentration (Hb) and hematocrit values (Hct), RBCs indices (MCV, MCH, and MCHC), and differential blood count. A total of 10 μl of blood and 1990 μl of Hayem reagent (Semikem, Sarajevo) was used for RBCs counting in five squares on a Neubauer hemocytometer (Merck, Germany). In a total of 20 µL of blood with 380 µl of Türk’s reagent (Semikem, Sarajevo) and counting in four squares on a Neubauer enhanced hemocytometer, the total number of WBCs was determined. Hemoglobin concentration (Hb) was assessed using Drabkin’s method, which generates cyanmethemoglobin and measures it spectrophotometrically at 546 nm (GenesysTM 20 Spectrophotometer, Mercers Row, UK). For hematocrit analysis (Hct), whole blood was collected into a heparinized microhematocrit capillary tube and centrifuged at 16,000 rpm for 10 min at 20 °C. A drop of blood (0.2 mL) was taken to make blood smears. Giemsa solution (Semikem, Bosnia and Herzegovina), diluted 1:10 in distilled water, was used to stain fresh blood smears for 35 min after being dried, fixed with methanol (p.a. 99.9%, Semikem, Bosnia and Herzegovina) for 5 min (Pappenheim method). From a differential blood count of 100 leukocytes, the following leukocyte cells display the relative quantities of each type of leukocyte. An Olympus BX41 optical microscope with a 100 × objective and an Olympus DP12 camera were used to analyze the slides. Using Olympus software (DP12 Soft DP12-CB Ver.01.01.01.42.® Olympus Corp.), a differential blood count was established.
Results
Improvement Effects of AME in Controlling Biochemical Parameters During Pre- Vs. Post-treatment
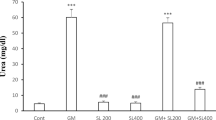
GM treatment leads to significantly lower levels of albumin and A/G ratio, as well as high levels of globulin, urea, and creatinine in comparison to Ctr group (Fig. 2). However, AME treatment showed certain changes in serum parameters and significant differences for urea, creatinine, and A/G ratio compared to Ctr. Comparison of pre- and posttreatment (AME + GM vs. GM + AME) showed significant improvement of biochemical parameters and their regulation towards normal values. In AME + GM group, proteins, globulins, and creatinine level were increased compared to Ctr; albumin and A/G ratio showed downward trend compared to other groups. Values of serum urea are significantly reduced in AME + GM group, and are significantly lower compared to Ctr and GM group.
AME Has Moderate Beneficial Effects on Some Hematological Parameters
Variation in RBCs, Hb and Hct after GM application lead to altered RBCs indices (MCV, MCH and MCHC). The GM + AME group showed a statistically significant decrease (p < 0.00) for RBCs compared to the control (Ctr) group and the other groups. The administration of AME resulted in a statistically significant decrease in Hb values (p < 0.00). MCV values gradually increased in the groups: Ctr > GM > AME > AME + GM > GM + AME. MCHC values decreased in AME-treated rats. Overall, the results indicate that AME had moderate beneficial effects on hematological parameters (Fig. 3).
AME Maintain Normal Level of Eosinophils, Monocytes, and Lymphocytes Despite GM Application
The results of the study indicate that GM treatment leads to a statistically significant increase in WBCs compared to the control group (8.09 × 109/L vs. 6.64 × 109/L). However, the combination of AME and GM showed a decrease in WBCs (3.64 × 109/L) compared to the control group. Additionally, the results indicate that AME treatment leads to an increase in segmented neutrophils and a significant decrease in non-segmented neutrophils. Eosinophils and monocytes were uniformed across all groups, without significant differences. The results also suggest that AME has a significant impact on lymphocytes regulation, which is an indication of a significant inflammatory effect (Fig. 4).
Anisocytosis and Platelet Aggregation Were Reduced During AME Treatment
The main features of GM treatment are changes in the size and morphology of RBCs, as well as the number of platelets. Rare hemolyzed erythrocytes and speculated erythrocytes (echinocytes) are present in the GM group. Also, in addition to the aforementioned morphological alterations, we observed large accumulations—aggregates of platelets (thrombocytosis). The combined effects of the AME extract had a significant role in repairing the GM effects. Large platelet aggregations were not recorded in AME + GM and GM + AME treated rats. Also, hemolyzed RBCs were not detected, and the presence of echinocytes was significantly reduced. Hypochromic erythrocytes were found in both groups (AME + GM and GM + AME) (Fig. 5).
Discussion
A. melanocarpa fruit is valued for its abundance of bioactive substances including polyphenols. They are considered key antioxidants in the diet. Phenolic acids and flavonoids, including anthocyanins, flavanols, flavonols, and proanthocyanidins, are among the most important polyphenols found in chokeberry [18]. In vivo studies in humans or animals have shown that berries’ high antioxidant activity makes them useful in the treatment of chronic diseases linked to oxidative stress, particularly diabetes, cardiovascular conditions, and cancer. They also have a number of other positive effects, including immunomodulatory, antibacterial, and anti-inflammatory properties [19,20,21]. Since GM has significant renal toxicity, its medical use is limited [22]. This aminoglycoside is still the primary treatment option that works against some multi-resistant bacteria despite its nephrotoxic side effects [23]. The exact mechanism of GM nephrotoxicity is still not fully understood. However, both in vitro and in vivo research showed that GM enhanced the formation of reactive oxygen species, which was linked to an increase in lipid peroxidation and a reduction in antioxidant enzyme activity in the kidney [24]. By producing an iron-gentamicin complex, which is a powerful catalyst for radical production, it serves as an iron chelator [25].
GM induced significantly high values of creatinine, urea, and globulin as well as low levels of albumin. Similar biochemical abnormalities in serum are evidence of the kidneys’ severe functional impairment, which was confirmed in earlier studies [15, 26]. GM is nephrotoxic and its effects cause severe injury in the renal tubules. In mice, GM treatment has been associated with apoptosis and tubular epithelial cell necrosis [27, 28]. In rats treated with GM, Dhanarasu et al. [29] discovered kidney damage where GM was administered intraperitoneally for 6 days at a dosage of 100 mg/kg. Blood urea and creatinine metabolic abnormalities may be related to protein metabolic diseases, such as rapid catabolism or urine loss brought on by renal nephrotoxicity. A research of Milutinovic et al. [30] showed significant changes in urea and creatinine levels after 3 and 6 months of diabetes mellitus patients’ treatment with AME. Research showed decrease in the values of parameters related to the initial stage of the renal dysfunction (serum creatinine and urea, urine creatinine and microalbuminuria) after the juice intake and confirmed the protective effects, even though only the decrease in the values of blood creatinine (p < 0.05) was statistically significant. Drinking aronia juice appears to be beneficial for treating urinary tract infections (UTI) that have been treated with antibiotics [31]. Mice treated with AME for 2 weeks improved renal failure to varying degrees (restoring urea and creatinine to reference range values), reduced tubular damage, and suppressed pro-inflammatory cytokines, oxidative stress, lipid peroxidation, and apoptosis [32], while in our case, a better effect was caused by treatment with AME after GM application. Hypoalbuminemia and low A/G ratios may emerge as a result of these metabolic conditions as showed in our study. A/G was statistically lower in AME + GM group which can be caused by an excessive amount of protein that is excreted in the urine. Impairments of renal capillaries that remove waste and excess water from the circulation is typically cause of nephrotic syndrome after GM application. Since serum globulin levels are consistent across groups (except for AME + GM), it is possible that loss or rapid degradation of serum protein fraction is the main factor for that. Overall, post-treatment with AME had beneficial effects on certain biochemical parameters (albumins, globulins, A/G ratio, urea, and creatinine) which is in accordance with previous studies [33, 34].
Current study showed that RBC and Hct values slightly increased in the GM group compared to the control. AME treatment (post treatment) led to a slight decrease in the mentioned parameters. The lowest values of Hb and hematological indices (MCH and MCHC) were observed after pre-treatment with AME, which is a direct consequence of the reduced value of Hb. GM tends to cause an inflammatory condition in the body and leads leukocytosis. In contrast, AME pre-treatment led to a significant decrease in WBC. It indicates to a strong anti-inflammatory effect of AME. Milutinovic et al. [30] conducted a 6-month study on 35 patients with diabetes who consumed aronia-based supplements. The results of hematological parameters showed a significant decrease in WBC (p < 0.05) and lymphocyte count (p < 0.01), while the decrease in other differential cell count was not significant. The significant changes were noticed in the values of Hct, Hb, MCV, MCH, and MCHC, as well as in the RBC count (p < 0.05); Hb levels were increased after the 3-month therapy. Changes in the values of clinically relevant hematological parameters could be the evidence of the chokeberry juice therapy effects. The decrease in the values of WBC and lymphocytes count may indicate a reduction of inflammatory processes that influence the progression of diabetes. GM group had high blood cell counts, reduction in segmented neutrophils, while pretreatment with aronia showed a significant effect on the reduction of lymphocytes, which confirms the theory of anti-inflammatory effects of AME. Additionally, we found that both AME groups had more non-segmented neutrophils than segmented neutrophils. Segmented leukocytes are mature forms, therefore their increase supports the fact that the bone marrow functions normally. Monocytes in the blood were lower in groups treated with AME compared to Ctr and GM groups, but without statistical significance. There is a noticeable lack of studies dealing with the hematological analysis of chokeberry juice or extract in GM-induced kidney injury.
Morphological changes of the erythrocyte membrane are very pronounced, and the presence of echinocytes was recorded. Lipid peroxidation and subsequent alterations in lipid metabolism may be the cause of erythrocyte membrane damage. Erythrocyte corpuscular fragility is a useful parameter for evaluating changes in cell structure and function. Changes in membrane structure and function have been associated with excessive generation of reactive oxygen species. Hemolyzed erythrocytes and echinocytes point to osmotic fragility, which is probably the result of lipid peroxidation. Several studies have shown that GM prevents platelet aggregation [35, 36]. These results are not in accordance with the current study. GM group had visible platelet aggregation and thrombocytosis contrary to the other four groups. In vitro studies have shown the anti-platelet activity of polyphenolic AME fruit extracts [37, 38]. A month of AME supplementation significantly reduced ADP-induced platelet aggregation, according to research conducted by Sikora et al. [39]. Chokeberry’s main chemical constituent, anthocyanins, may chelate iron due to the presence of a hydroxyl group in the C-ring [40]. In current study, hypochromic anemia was observed in rats in AME + GM group. Low values of RBCs may correlate with reduced values of Hb, important for erythrocyte maturation. Hypochromia is caused due to disruption of iron supply or low Hb concentration, which is in correlation with current findings. MCHC is a parameter that indicates the relationship between the RBC size and the concentration of Hb [41]. Our research suggests that the concentration of both Hb and MCHC was reduced in this group.
The results showed moderate protective effects of AME on renal serum markers associated with GM nephropathy. AME appears to contribute to indirect effects against certain hematological disorders, particularly in the development of echinocytosis and platelet aggregation. The inclusion of a larger number of experimental animals and the monitoring of antioxidant parameters are guidelines for further research. However, due to the lack of similar studies, more research in general are needed to fully understand the mechanisms of action and the effects of A. melanocarpa.
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Cuzzocrea, S., Mazzon, E., Dugo, L., Serraino, I., Di Paola, R., Britti, D., De Sarro, A., Pierpaoli, S., Caputi, A., Masini, E., & Salvemini, D. (2002). Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. European Journal of Pharmacology, 450, 67–76. https://doi.org/10.1016/s0014-2999(01)01130-x
Rodrigues, F. A., Prata, M. M., Oliveira, I. C., Alves, N. T., Freitas, R. E., & Monteiro, H. S. (2014). Gingerol fraction from Zingiber officinale protects against gentamicin-induced nephrotoxicity. Antimicrobial Agents and Chemotherapy, 58(4), 1872–1878. https://doi.org/10.1128/AAC.02431-13
Moreira, M. A., Nascimento, M. A., Bozzo, T. A., Cintra, A., da Silva, S. M., & Dalboni, M. A. (2014). Ascorbic acid reduces gentamicin-induced nephrotoxicity in rats through the control of reactive oxygen species. Clinical Nutrition, 33(2), 296–301. https://doi.org/10.1016/j.clnu.2013.05.005
El-Kashef, D. H., El-Kenawi, A. E., Rahim, M. A., Suddek, G. M., & Salem, H. A. (2016). Agmatine improves renal function in gentamicin-induced nephrotoxicity in rats. Canadian Journal of Physiology and Pharmacology, 94(3), 278–286. https://doi.org/10.1139/cjpp-2015-0321
Christo, J. S., Rodrigues, A. M., Mouro, M. G., Cenedeze, M. A., de Jesus Simões, M., Schor, N., et al. (2011). Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide, 24, 77–83. https://doi.org/10.1016/j.niox.2010.12.001
El, M. M., Laurent, G., Mingeot-Leclercq, M. P., et al. (2000). Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicological Sciences, 56, 229–239. https://doi.org/10.1093/toxsci/56.1.229
Dam, V. P., Scott, J. L., & Ross, A. (2012). Inhibition of cystathionine gamma-lyase and the biosynthesis of endogenous hydrogen sulphide ameliorates gentamicin-induced nephrotoxicity. European Journal of Pharmacology, 685, 165–173. https://doi.org/10.1016/j.ejphar.2012.04.030
Sandhu, J. S., Sehgal, A., Gupta, O. A., & Singh, A. (2007). Aminoglycoside nephrotoxicity revisited. Journal Indian Academy of Clinical Medicine, 8(4), 331–333.
Slimestad, R., Torskangerpoll, K., Nateland, H. S., Johannessen, T., & Giske, N. H. (2005). Flavonoids from black chokeberries, Aronia melanocarpa. Journal of Food Composition and Analysis, 18, 61–68. https://doi.org/10.1016/j.jfca.2003.12.003
Bushmeleva, K., Vyshtakalyuk, A., Terenzhev, D., Belov, T., Parfenov, A., Sharonova, N., Nikitin, E., & Zobov, V. (2021). Radical scavenging actions and immunomodulatory activity of Aronia melanocarpa propylene glycol extracts. Plants (Basel), 10(11), 2458. https://doi.org/10.3390/plants10112458
Christiansen, C. B., Mellbye, F. B., Hermansen, K., Jeppesen, P. B., & Gregersen, S. (2022). Effects of Aronia melanocarpa on cardiometabolic diseases: A systematic review of quasi-design studies and randomized controlled trials. The Review of Diabetic Studies, 18, 76–92. https://doi.org/10.1900/RDS.2022.18.76
Seeram, N. P., & Nair, M. G. (2002). Inhibition of lipid peroxidation and structure-activity-related studies of the dietary constituents anthocyanins, anthocyanidins, and catechins. Journal of Agriculture and Food Chemistry, 50(19), 5308–5312. https://doi.org/10.1021/jf025671q
Skarpańska-Stejnborn, A., Basta, P., Sadowska, J., & Pilaczyńska-Szcześniak, L. (2014). Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers. Journal of the International Society of Sports Nutrition, 11(1), 48. https://doi.org/10.1186/s12970-014-0048-5
Silan, C., Uzun, O., Comunoğlu, N. U., Gokçen, S., Bedirhan, S., & Cengiz, M. (2007). Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biological &/and Pharmaceutical Bulletin, 30(1), 79–83. https://doi.org/10.1248/bpb.30.79
Suljević, D., Mitrašinović-Brulić, M., & Fočak, M. (2022). L-cysteine protective effects against platelet disaggregation and echinocyte occurrence in gentamicin-induced kidney injury. Molecular and Cellular Biochemistry, 478, 13–22. https://doi.org/10.1007/s11010-022-04498
Ramić, M., Vidović, S., Zeković, Z., Vladić, J., Cvejin, A., & Pavlić, B. (2015). Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrasonics Sonochemistry, 23, 360–368. https://doi.org/10.1016/j.ultsonch.2014.10.002
Nurcholis, W., Alfadzrin, R., Izzati, N., Arianti, R., Vinnai, B. Á., Sabri, F., Kristóf, E., & Artika, I. M. (2022). Effects of methods and durations of extraction on total flavonoid and phenolic contents and antioxidant activity of java cardamom (Amomum compactum soland ex maton) fruit. Plants, 11, 2221. https://doi.org/10.3390/plants11172221
Jakobek, L., Šeruga, M., Medvidović-Kosanović, M., & Novak, I. (2007). Antioxidant activity and polyphenols of Aronia in comparison to other berry species. Agriculturae Conspectus Scientificus, 72, 301–306.
Jing, P., Bomser, J. A., Schwartz, S. J., He, J., Magnuson, B. A., & Giusti, M. M. (2008). Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. Journal of Agriculture and Food Chemistry, 56, 9391–9398. https://doi.org/10.1021/jf8005917
Ho, G. T., Bräunlich, M., Austarheim, I., Wangensteen, H., Malterud, K. E., Slimestad, R., & Barsett, H. (2014). Immunomodulating activity of Aronia melanocarpa polyphenols. International Journal of Molecular Sciences, 15, 11626–11636. https://doi.org/10.3390/ijms150711626
Xu, J., & Mojsoska, B. (2013). The immunomodulation effect of Aronia extract lacks association with its antioxidant anthocyanins. Journal of Medicinal Food, 16, 334–342. https://doi.org/10.1089/jmf.2012.0151
Ahn, J. M., You, S. J., Lee, Y. M., Oh, S. W., Ahn, S. Y., Kim, S., Chin, H. J., Chae, D. W., & Na, K. Y. (2012). Hypoxia-inducible factor activation protects the kidney from gentamicin-induced acute injury. PLoS ONE, 7, e48952. https://doi.org/10.1371/journal.pone.0048952
Karahan, I., Ateşşahin, A., Yilmaz, S., Çeribaşi, A. O., & Sakin, F. (2005). Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology, 215, 198–204. https://doi.org/10.1016/j.tox.2005.07.007
Banday, A. A., Farooq, N., Priyamvada, S., Yusufi, A., & Khan, F. (2008). Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sciences, 82(9), 450–459. https://doi.org/10.1016/j.lfs.2007.11.014
Khan, S. A., Priyamvada, S., Farooq, N., Khan, S., Khan, M. W., & Yusufi, A. N. K. (2009). Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacological Research, 59, 254–262. https://doi.org/10.1016/j.phrs.2008.12.009
Kalayarasan, S., Prabhu, P. N., Sriram, N., Manikandan, R., Arumugam, M., & Sudhandiran, G. (2009). Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. European Journal of Pharmacology, 606, 162–171. https://doi.org/10.1016/j.ejphar.2008.12.055
Edwards, J. R., Diamantakos, E. A., Peuler, J. D., Lamar, P. C., & Prozialeck, W. C. (2007). A novel method for the evaluation of proximal tubule epithelial cellular necrosis in the intact rat kidney using ethidium homodimer. BMC Physiology, 7, 1. https://doi.org/10.1186/1472-6793-7-1
Li, J., Li, Q. X., Xie, X. F., Ao, Y., Tie, C. R., & Song, R. J. (2009). Differential roles of dihydropyridine calcium antagonist nifedipine, nitrendipine and amlodipine on gentamicin-induced renal tubular toxicity in rats. European Journal of Pharmacology, 620(1–3), 97–104. https://doi.org/10.1016/j.ejphar.2009.08.021
Dhanarasu, S., Selvam, M.S., Alkhalaf, A.A., Aloraifi, A.K.K., Al-Shammari, N.K.A. (2018) Ameliorative and erythrocytes membrane stabilizing effects of Mentha piperita on experimentally induced nephrotoxicity by gentamicin. Egyptian Academic Journal of Biological Sciences 3(10), 23–37. https://doi.org/10.21608/eajbsc.2018.13653.
Milutinovic, M., Velickovic Radovanovic, R., Savikin, K., Radenkovic, S., Arvandi, M., Pesic, M., Kostic, M., Miladinovic, B., Brankovic, S., Kitic, D. (2019) Chokeberry juice supplementation in type 2 diabetic patients - impact on health status. Journal of Applied Biomedicine 17(4), 218–224. https://doi.org/10.32725/jab.2019.020.
Handeland, M., Grude, N., Torp, T., & Slimestad, R. (2014). Black chokeberry juice (Aronia melanocarpa) reduces incidences of urinary tract infection among nursing home residents in the long term-a pilot study. Nutrition Research, 34, 518–525. https://doi.org/10.1016/j.nutres.2014.05.005
Li, L., Li, J., Xu, H., Zhu, F., Li, Z., Lu, H., Zhang, J., Yang, Z., Liu, Y. (2021) The Protective Effect of anthocyanins extracted from Aronia melanocarpa berry in renal ischemia-reperfusion injury in mice. Mediators of Inflammation, e7372893. https://doi.org/10.1155/2021/7372893
Denev, P., Kratchanov, C., Číž, M., Lojek, A., & Kratchanova, M. (2012). Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Comprehensive Reviews in Food Science and Food Safety, 11(5), 471–489.
Kasprzak-Drozd, K., Oniszczuk, T., Soja, J., Gancarz, M., Wojtunik-Kulesza, K., Markut-Miotła, E., & Oniszczuk, A. (2021). The efficacy of black chokeberry fruits against cardiovascular diseases. International Journal of Molecular Sciences, 22(12), 6541. https://doi.org/10.3390/ijms22126541
Sakurai, S., Shiojima, I., Tanigawa, T., & Nakahara, K. (1997). Aminoglycosides prevent and dissociate the aggregation of platelets in patients with EDTA-dependent pseudothrombocytopenia. British Journal of Haematology, 99(4), 817–823. https://doi.org/10.1046/j.1365-2141.1997.4773280.x
Chen, G., Fei, X., & Ling, J. (2012). The effects of aminoglycoside antibiotics on platelet aggregation and blood coagulation. Catheterization and Cardiovascular Interventions, 18(5), 538–541. https://doi.org/10.1177/1076029611430955
Luzak, B., Golanski, J., Rozalski, M., Krajewska, U., Olas, B., & Watala, C. (2010). Extract from Aronia melanocarpa fruits potentiates the inhibition of platelet aggregation in the presence of endothelial cells. Archives of Medical Science, 6, 141–144. https://doi.org/10.5114/aoms.2010.13884
Olas, B., Wachowicz, B., Tomczak, A., Erler, J., Stochmal, A., & Oleszek, W. (2008). Comparative anti-platelet and antioxidant properties of polyphenol-rich extracts from: Berries of Aronia melanocarpa, seeds of grape and bark of Yucca schidigera in vitro. Platelets, 19, 70–77. https://doi.org/10.1080/09537100701708506
Sikora, J., Broncel, M., Markowicz, M., Chałubiński, M., Wojdan, K., & Mikiciuk-Olasik, E. (2012). Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. European Journal of Nutrition, 51(5), 549–556. https://doi.org/10.1007/s00394-011-0238-8
Hider, R., Liu, Z., & Khodr, H. (2001). Metal chelation of polyphenols. Methods in Enzymology, 335, 190–203. https://doi.org/10.1016/s0076-6879(01)35243-6
Alsagaby, S. A. (2022). A comprehensive study on abnormalities associated with red blood cells in Saudi adult patients. International Journal of Health Sciences, 16(1), 30–36.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Muhamed Fočak, Maja Mitrašinović-Brulić, and Damir Suljević. The first draft of the manuscript was written by Muhamed Fočak and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The Law on the Protection and Welfare of Animals applies to experiments with animals (Official Gazette of BiH, No. 25/2009 and 9/2018) in Bosnia and Herzegovina.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fočak, M., Mitrašinović-Brulić, M. & Suljević, D. Aronia melanocarpa (Michx.) Elliott 1821 Extract Has Moderate Ameliorative Influence on Biochemical and Hematological Parameters in Gentamicin-Induced Nephropathy in Wistar Rats. Appl Biochem Biotechnol 196, 896–908 (2024). https://doi.org/10.1007/s12010-023-04573-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04573-z