Abstract
Increasing human activity continues to threaten peatlands, and as the area of natural mires declines, our obligation is to restore their ecosystem functions. Several restoration strategies have been developed for restoration of extracted peatlands, including “The moss layer transfer method”, which was initiated on the Tässi extracted peatland in central Estonia in May 2012. Three-year study shows that despite the fluctuating water table, rainfall events can compensate for the insufficient moisture for mosses. Total plant cover on the restoration area attained 70 %, of which ~60 % is comprised of target species—Sphagnum mosses. From restoration treatments, spreading of plant fragments had a significant positive effect on the cover of bryophyte and vascular plants. Higher water table combined with higher plant fragments spreading density and stripping of oxidised peat layer affected positively the cover of targeted Sphagnum species. The species composition in the restoration area became similar to that in the donor site in a natural bog. Based on results, it was concluded that the method approved for restoration in North America gives good results also in the restoration of extracted peatland towards re-establishment of bog vegetation under northern European conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The exploitation of mires over several centuries—the combined effect of drainage and usage for agriculture, forestry and peat mining—has led to the destruction or severe degradation of a large portion of peatlands, especially in Europe (Rochefort and Lode 2006). Although recognised only recently (Constanza et al. 1997; Millennium Ecosystem Assessment 2005), the importance of peatlands in water storage and groundwater recharge, climate regulation, carbon accumulation and support of biodiversity places them among the most valuable ecosystems in the world. Peatlands with a peat thickness over 30 cm cover only ~2.8 % of the European Union territory (Joosten 2008) whereas with continuing human activities, the area of natural peatlands remains in decline. Therefore, the restoration of extracted peatlands does have big importance. Bogs with a thick homogeneous Sphagnum peat layer have been exploited for peat mining; therefore. the consensus is that the principal long-term objective for their restoration is the re-establishment of vegetation typical of natural bogs dominated by Sphagnum mosses leading to the restoration of bog functions, including peat accumulation (Money and Wheeler 1999). Restoration attempts of peatlands began in the Netherlands and Germany with the main focus on re-wetting long-abandoned extracted peatlands with oxidised black residual peat (Verhoven 2014). However, re-wetting and inundation of damaged peatlands usually results merely in large lagoons and does not always lead to recolonisation by even aquatic Sphagnum species (Eggelsmann 1988; Joosten 1992), thereby failing to lead to the establishment of peat-forming Sphagnum species (Robroek et al. 2009). Status and restoration of peatlands in northern Europe is summarised by Vasander et al. 2003.
The active restoration method involving the spreading of plant fragments from a donor site was developed in Canada (Quinty and Rochefort 2003). Under favourable conditions, employment of “The moss layer transfer method” has seen encouraging results in the restoration of bog communities over large areas within 10 years (Gonzáles et al. 2013; McCarter and Price 2013). Early monitoring of the restoration has led to techniques with which to predict the resultant restoration and to make adjustments in restoration strategy to achieve the desired results (Poulin et al. 2013; González and Rochefort 2014). Although several methods to restore extracted peatlands have been developed and summarised in handbooks and restoration guides (Wheeler and Shaw 1995; Stoneman and Brooks 1997; Heikkilä et al. 2002; Quinty and Rochefort 2003; Schumann and Joosten 2008; Paal 2011; Pakalne and Strazdina 2013), a gap remains between applied ecological science and practical implementation of peatland restoration (Anderson 2014), e.g., most experimental restorations are limited to small areas, less than 1 ha (Wagner et al. 2008). Local meteorological conditions and characteristics of individual extracted peatlands (residual peat type and depth, hydrology, vegetation type on bordering areas, availability of donor sites) should be considered when selecting restoration direction, target species, donor areas and adjusting methods that have given successful results under different environmental conditions.
Estonia is considered to be one of the most peatland-rich countries in the world where peatlands in various conditions are estimated to cover almost 22 % of the country (Orru 1992). However, a recent inventory has revealed that peatlands in a near-natural state cover only ~5.5 % (Paal and Leibak 2011), and the remainder has been affected by drainage for forestry, agriculture and peat extraction. Historically in Estonia, peat was used mostly as fuel for domestic heating and was excavated manually from trenches on bog margins without drainage, resulting in spontaneous re-vegetation (Triisberg et al. 2011). The situation has been changing drastically since the 1950s with the adoption of milling and vacuum mining techniques that required large areas and lowering of the water table for peat extraction. As a result, there are about 9400 ha of abandoned extracted peatlands in Estonia (Ramst and Orru 2009), which is likely to double in the coming decades as the resources on several ongoing peat extraction sites become depleted (Paal and Leibak 2011). Most extracted peatlands, located largely on public land, were abandoned without restoration during or shortly after the end of the Soviet period at the beginning of the 1990s. Abandoned extracted peatlands have a negative effect on the local environment as well as at a global scale, being a notable source of greenhouse gases (Salm et al. 2009; Maljanen et al. 2010). Drained and extracted peatlands are, after industry, the second largest CO2 emitters in Estonia, exceeding that from traffic several-fold (Ilomets 1996). Although some studies indicate that mining sites can sometimes successfully re-vegetate spontaneously (Prach et al. 2013), owing to their large area, deep drainage, lack of viable propagules and hostile environmental conditions, the spontaneous re-vegetation of milled extracted peatlands is very slow and uneven (Lavoie et al. 2005a; Triisberg et al. 2011; 2013; Konvalinková and Prach 2014). Therefore, there is an urgent need for their managed restoration. So far in Estonia only few extracted peatland areas have been restored for forestry, berry plantation or the energy grass whereas only few small scale experiments are done to restore mire initiation (Vasander et al., 2003; Paal 2011). Now, the European Union has allocated resources for the restoration in Estonia of at least 2000 ha of extracted peatlands during 2014–2020.
Therefore, the purpose of this study was to determine experimentally whether the moss layer transfer method can be successfully applied in restoration of extracted peatlands in Northern European conditions. To our knowledge, no assessment has yet been published of the application of this method in these conditions. The study’s aims were (a) to assess the main factors affecting re-vegetation character and dynamics over the course of restoration, and (b), based on the results, to suggest recommendations for application of the restoration method.
Material and methods
Study site
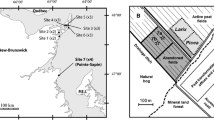
The restoration experiment was undertaken at the eastern edge of the Tässi peat extraction area (264 ha) in central Estonia (58° 32′ 16.97″ N 25° 51′ 43.78″ E). Peat extraction using the milling method was terminated on about a 500 m long and 90 m wide strip between an ongoing extraction area and adjoining forest in the beginning of the 1980s. The residual peat depth is ~2.5 m, of which the uppermost 1 m is Sphagnum bog peat, suitable for restoration of bog communities (Quinty and Rochefort 2003; Triisberg et al. 2014). Some parts of the abandoned peatland were seasonally flooded; during the growing season, the water table was usually 0.5–1 m below the peat surface. Large surface areas were affected by frost heaving. Spontaneous re-vegetation over more than 30 years has been slow and uneven with total plant cover still less than 1 % of the area, most of which consists of single tussocks of Eriophorum vaginatum and some 0.5–1 m tall Pinus sylvestris trees.
Restoration method
Restoration was done on a 60 × 40 m area in late April—early May 2012 following the moss layer transfer method (Quinty and Rochefort 2003) with some adjustments in surface preparation (with or without surface layer stripping) and spreading density of plant fragments (see in more detail below). The topmost about 20-cm thick oxidised peat layer was stripped by bulldozer, and the surface was flattened. The stripped peat was pushed to the sides of the restoration area bordered by drainage ditches and then compressed by tractor to reduce the lateral water outflow from the area. To reveal the likely effect of water-table depth (WTD) on re-vegetation, the surface of one third of the restoration area was levelled about 10 cm lower (hereafter called the wetter sector (HW)) than the rest (drier sector (LW)).
Plant fragments for the restoration were collected from a donor site in Soosaare bog (12 671 ha) about 10 km away. The donor site is located in a bog area bordered by an active peat extraction area being prepared for expansion. Therefore, the cutting of plant fragments did not damage the intact natural bog. Drainage ditches were dug in the donor site 1 year earlier but had so far little effect on WTD and plants. The donor site was a typical open hollow-ridge bog with a few shallow pools and sparse up to 2–3 m tall P. sylvestris trees on its ridges. The ground layer was dominated by Sphagnum mosses: Sphagnum fuscum and S. rubellum on hummocks, S. magellanicum and S. balticum in lawns and S. cuspidatum in hollows (a full list of species is given in Table 1).
To restore extracted peatlands towards bog communities, introduction of hummock-growing Sphagnum species are recommended (Quinty and Rochefort 2003) owing to their greater tolerance to dry conditions and desiccation (Hájek and Beckett 2008). Using trimmers, plant fragments were cut from the topmost about 10 cm of Sphagnum-dominated hummocks containing also some vascular plants (mainly Calluna vulgaris and Oxycoccus palustris). Plant fragments were collected using rakes and transported in plastic bags to the restoration area where they were spread manually over the following few days. On most restoration sites, plant fragments collected from 1 m2 in the donor site were spread over 10 m2 (dense cover, 1:10); fragments on the remaining sites were spread over 15 m2 (sparse cover, 1:15). Prior to spreading, plant fragments were manually disaggregated to cover the restoration area more evenly. In sites with dense and sparse plant fragment-spreading densities, the cover of fragments was estimated at 60 and 40 %, respectively. Plant fragments were spread manually on about 1.5-m wide strips and covered immediately with a fluffy layer of straw mulch for protection from solar radiation and to create better moisture conditions (Quinty and Rochefort 2003). Care was taken to spread straw evenly so that plant fragments remained exposed, thereby receiving adequate light for photosynthesis and growth.
Experiment design
The restoration area contained sites with six treatments (surface preparation, WTD and density of plant fragments): (1) abandoned extracted peatland (Control); (2) site without surface stripping, dense plant fragment spreading (1:10), covered by straw on drier sector of restoration area (Not stripped, LW); (3) site with all restoration steps (stripped surface, dense plant fragment spreading, covered by straw) on wetter sector of restoration area (Restor., HW); and (4) all the same restoration steps as in treatment 3, but on drier sector of restoration area (Restor., LW); (5) site with all restoration steps but sparse plant fragment spreading (1:15) on drier sector of restoration area (Sparse, LW); (6) site with only stripped surface and covered by straw on drier sector of restoration area (No fragments, LW). After the end of restoration measures, the only outflow drainage ditch from the restoration area was blocked with peat. To minimise the effect of trampling, no fieldwork was conducted in 2012, and snow shoes were worn in the sites in following years.
Environmental and vegetation analysis
WTD was measured mostly every few weeks during May–October 2013 (8 times) and in April–October 2014 (10 times) with a tape measure in six perforated plastic tubes placed at ~70 cm depth into the residual peat in the centre of each site with a different treatment. Precipitation data from the nearest (~20 km) meteorological station in Viljandi were provided by the Estonian Meteorological Service. In spring 2013, ten 50 × 50 cm permanent plots were established at random locations in the donor site hummocks as well as in the restoration area on each of the six treatment sites, totalling 70 plots altogether. Vegetation analysis was conducted at the beginning (June) and end (September) of the vegetation seasons in 2013 and 2014. The total plant cover (%) and the covers of vascular plants and of bryophytes were recorded in each plot as well as for every plant species. Nomenclature of vascular plants follows Kukk and Kull (2005) and for bryophytes Hill et al. (2006) and Söderström et al. (2007).
Statistical methods
Altogether, data from 60 permanent plots in the restoration area were subject to statistical analysis. Repeated measures ANOVA, which included all restoration treatments, as well as all four data collecting periods, was used to test the significance of restoration treatments on plant cover. Main effect ANOVA, including only sites with spread plant fragments, was used to elucidate the importance of stripping, plant fragment density and water level depth on the cover of different plant groups. Repeated measures ANOVA including only sites with plant fragments was used to evaluate the effect of time on the cover of plant vegetation and on Sphagnum moss carpet and to quantify differences in covers of vascular plant and bryophyte species. Differences between the four data collecting periods were tested by Tukey HSD test. STATISTICA version 7.1. (StatSoft, Inc 2005) was used to perform all statistical analyses.
For every species community preference was defined according to their occurrence in Estonia, and bog-specific species were selected according to Kask (1982) and Ingerpuu et al. (2014). Jaccard’s similarity indices were calculated between species composition in the donor site and in the six restoration sites at the beginning (spring 2013) and end (autumn 2014) of study. The species distribution of the three communities: donor site, restoration sites at the beginning and at the end of study, were compared by chi-square test. The normality of variables was checked using the Shapiro-Wilk test.
All differences were considered significant at p < 0.05.
Results
Seasonal changes in water table
Water-table depth in the restoration area fluctuated seasonally within 33 cm, being generally higher in early spring after the snowmelt when some areas were flooded for several weeks and lower mostly in summer depending on precipitation (Fig. 1). WTD in the drier sector of the restoration area was on average 7 ± 4 cm deeper than in the wetter sector (mean 30.4 ± 8.9 cm and 23.5 ± 10.1 cm, respectively). This difference was greater (max 12 cm) during wetter periods and less (1–5 cm) during drier periods. WTD in the wetter sector fluctuated between 11 and 41 cm but always remained higher than in the drier sector with WTD fluctuation between 18 and 44 cm. In addition to WTD, moisture conditions for spread plant fragments coincided greatly with the amount of rainfall (Fig. 1) as moisture content of Sphagnum mosses was assessed visually and by touch.
Changes in plant cover during the study period
Vegetation studies on Tässi restored peatland showed that active restoration is crucial for initiating vegetation recovery on extracted peatland. The restoration treatments have a significant effect on plant cover (F(10, 466) = 60.82, p < 0.001). As expected, the spreading of plant fragments had a significant positive effect not only on the cover of bryophytes but also on vascular plants (Fig. 2). The bryophyte cover is significantly greater in sites containing plant fragments, whereas the site without treatment (control site) and sites only stripped and covered by straw (no fragments) underwent no significant change. The vascular plant cover shows the same trend (Fig. 2).
Cover of vascular plants and bryophytes in control site and in sites with different restoration treatments. Significant differences (p < 0.05) among treatments according to Tukey HSD test are indicated by different letters (a, b, c for vascular plants; e, d, f for bryophytes). Vertical bars denote 0.95 confidence intervals
The density of plant fragment spreading had a significant positive effect on the vascular plant cover, whereas the cover of bryophytes was significantly greater in sites with a higher water table (Table 2). Surface stripping did not significantly affect the cover of vascular plants or the total cover of bryophytes. However, stripping had a significant effect on the cover of three main Sphagnum species (F(3; 233) = 15.42, p < 0.001). In addition, the density of plant fragment spreading and the water level affected significantly the cover of these Sphagnum species (F(3233) = 52.88 and F(3233) = 8.60 respectively; p < 0.001). The cover of S. magellanicum was significantly greater on the site with a higher water table (Fig. 3). Two species experienced opposite reactions to the density of plant fragment spreading; the cover of S. rubellum was significantly greater in sites with sparse plant fragment density, whereas the cover of S. fuscum increased with denser fragment cover (Fig. 3).
Cover of three dominant Sphagnum species in restoration sites restored with plant fragments spreading. Significant differences (p < 0.05) among treatments according to Tukey HSD test are indicated by different letters (a, b for S. fuscum; c, d for S. magellanicum and e, f for S. rubellum). Vertical bars denote 0.95 confidence intervals
Time since the start of restoration had also a significant influence on total plant cover (F(3, 156) = 17.2; p < 0.001). The cover of both vascular plants and bryophytes increased significantly during the study period. However, bryophyte cover increased significantly during the first vegetation period of the study; the subsequent increase was non-significant (Fig. 4). Time had a significant positive effect also on the total cover of Sphagnum carpet (F812, 616, 75) = 2.79; p = 0.001). The cover of S. rubellum increased significantly during the study period, while changes in the cover of S. magellanicum and S. fuscum were non-significant (Fig. 5). By autumn 2014, their mean cover in sites with all restoration steps attained 15.3 ± 3.7 %, 14.0 ± 2.6 % and 23.0 ± 7.2 %, respectively.
Total plant cover and cover of bryophytes and vascular plants on restoration sites restored with plants fragment spreading from the spring 2013 until autumn 2014. Different letters (A, B, C) indicate significant differences according to Tukey HSD test (p < 0.05). Vertical bars denote 0.95 confidence intervals
Total cover of Sphagnum mosses and the cover of three Sphagnum species in restoration sites restored with plants fragment spreading from the spring 2013 until autumn 2014. Different letters indicate significant differences according to Tukey HSD test (p < 0.05). Vertical bars denote 0.95 confidence intervals
During the study period, 52 plant species altogether were identified, 49 in the restoration area and 27 in the donor site (Appendix). The number of vascular plant species in the restoration area was similar to that in the donor site; only forb species were fewer than in the donor site. However, many more bryophyte species were identified in both groups, liverworts and mosses, in the restoration area than in the donor site (Table 3). The species composition in all restoration sites became more similar to that in the donor site with time. The vegetation composition in three sites (Restor., HW; Restor., LW and Sparse, LW) can be considered similar to that in the donor site, as their species composition largely coincided (similarity indices attained more than 60 %) by the end of the study period (Fig. 6). Only the similarity between vascular plant species composition in the restoration site without stripping and the donor site decreased. The composition similarity of bryophyte species with the donor site decreased in the restoration site without fragments by the autumn of 2014.
The share of bog-specific plant species in the restored area increased markedly during the study period (Fig. 7). The initial communities in spring 2013 were significantly different from the donor site (χ 2 = 7.03, df = 2, p < 0.05). By the end of the study, two vegetation seasons later (autumn 2014), there were no significant differences between the restoration area and the donor site (χ 2 = 5.07, df = 2, p = 0.079). The share of bog-specific species in the restoration area increased in 2014 mainly due to the disappearance of some species but also to the emergence of some bog-specific species. Altogether, 11 species identified in 2013, of which eight were bryophytes, were not recorded in 2014 (Table 1). Three bog-specific species, Rhynchospora alba from vascular plants and two hepatics, Cephaloziella elachista and Cladopodiella fluitans, became established in 2014.
Discussion
The main goal of restoration is to re-establish functions of disturbed ecosystem, e.g., to convert extracted peatlands into peat-forming communities. Propagule banks are considered crucial for re-establishment of pre-disturbance communities (Caners et al. 2009; Kalamees et al. 2012). Millennia-old peat layers exposed during peat extraction contain no viable propagules (Salonen 1994), and propagules arrived after peat extraction will be removed during stripping of the surface peat layer. Therefore, it is not surprising that spreading of plant fragments had a substantial effect on the re-vegetation of extracted peatland. Results show that plant fragment spreading had a significant effect on bryophyte cover, which regenerated better than vascular plants. It was also found that the spreading density of plant fragments affected the cover differently: the cover of both vascular plants and bryophytes remained slightly lower in sites with sparse spreading density compared to the cover in sites with densely spread fragments. This could be caused by the availability of free substrate and less competition for space in sites with sparse plant fragments, as well as by the size of plant fragment bunches, although this aspect was not addressed in this study. Given identical total cover, smaller bunches have bigger total edge length and, therefore, even when expanding at the same rate, the total effect on the cover increase could be greater for smaller bunches. Nonetheless, plant fragments should not be disaggregated too finely or into individual shoots; otherwise, they risk drying out. We recommend that a sparse (1:15) spreading density of smaller bunches over the proposed 1:10 density for more heterogenic plant fragments (Quinty and Rochefort 2003), especially when collecting fragments with homogeneous composition from bog hummocks only or when donor sites are scarce.
It was found that surface peat stripping had no significant effect on the cover of vascular plants or on the total cover of bryophytes. However, stripping significantly affected the cover of the target species—Sphagnum mosses. This may be due to the specific ecological demands of the collected species. Stripping can help Sphagnum mosses achieve better contact with fresh peat surface with greater moisture through improved capillary rise than loose oxidised surface peat in abandoned extracted peatlands, which are often affected by frost heaving (Groeneveld and Rochefort 2005; Rochefort and Lode 2006). Stripping is not particularly laborious (Quinty and Rochefort 2003), and since it is also required to level surfaces, stripping should be applied for faster recovery of carpets of Sphagnum mosses. The results confirmed earlier findings from a greenhouse experiment (Triisberg et al. 2013) that the germination of propagules and spontaneous re-vegetation of extracted peatlands is affected mainly by moisture conditions. The water-table depth had a significant effect (p = 0.03, Table 2) on the cover of bryophytes, although this effect may be species-specific. Only the cover of S. magellanicum, which grows in natural bogs in lawns and low hummocks, was significantly positively associated with higher water level (Fig. 3), whereas S. fuscum and S. rubellum, which grow in raised bogs on hummocks and are better adapted to lower and fluctuating water table, were not. Results accords well with the simulated results of McCarter and Price (2014a), who showed that while S. fuscum and S. rubellum were able to maintain relatively moist capitula with a water table below 40 cm, i.e., lower than measured in our restoration area for most of the growing periods, the upper parts of S. magellanicum dry up at that level. It was predictable that the cover of vascular plants was not affected significantly by water table because species growing on bog hummocks are naturally better adapted to dryness and can use roots to obtain water from deeper peat layers.
Bryophytes are known to have two growth peaks coinciding with humid seasons (Clymo and Hayward 1982; Gerdol 1996). Moisture conditions for the survival of plant fragments depend not only on WTD, but also on the characteristics of the surface peat layer, which determine capillary water rise, and the amount and distribution of precipitation. The established Sphagnum layer in our restoration area does not yet replace the regulatory mechanisms of acrotelm in natural bogs, and its re-establishment may take longer time for decomposition and compaction of uppermost residual peat layer and plant remnants (McNeil and Waddington 2003; McCarter and Price 2014b). The fluctuating water table in both studied years was likely compensated by rain events in summer and autumn, while favourable moisture conditions for growth of Sphagnum mosses in spring was likely provided mostly by snowmelt. Combined with decreasing temperature and evapotranspiration, relatively light but frequent rain or autumn fog can keep mosses moist and photosynthetically active and prolong the growth of humidity-dependent Sphagnum mosses (Van Gaalen et al. 2007). The growth of Sphagnum mosses in early spring and late autumn is also evident by their increase in cover between September and the following June. Regular rainfall is especially important (Backėus 1988) since Sphagnum mosses require several weeks to recover their photosynthesis after each prolonged drought (McNeil and Waddington 2003). Therefore, even a few droughts can substantially hamper the recovery and expansion of Sphagnum mosses. Also, Chirino et al. (2006) showed that combined humidity and temperature conditions control moss carpet re-establishment, especially during the first years of restoration. The results of the study also show that the combined effect of raised water table, straw mulch and precipitation provide plant fragments in the restoration with favourable moisture conditions during most of the growing season; as estimated visually, plants were dry only during short periods in summer. Since plant fragment spreading in dry years or periods leads to persistently retarded re-establishment of plant cover, it could be recommended to initiate restoration at the end of summer, so the frequent autumn rain and lower temperature reduces the risk of drying out. As shown by Corson and Campbell (2013), the restoration protocol can be adjusted according to local conditions; mulch is not required in regions with lower temperature and higher humidity. It was found that just stripping the surface peat layer and applying straw mulch without spreading plant fragments did not facilitate re-vegetation during the study period. Since the protective effect of straw mulch decreases with each year, it is doubtful that re-vegetation would improve in the longer term. However, the nutrient release from decomposing straw can facilitate the establishment of plants, even Sphagnum species from spores (Sundberg and Rydin 2002), if spores arrive from surrounding mire communities.
It has been shown that the time since the end of peat extraction has a positive effect on spontaneous re-vegetation of extracted peatlands (Triisberg et al. 2013). There has been a remarkable establishment of Sphagnum cover for the third year of restoration compared to the scanty plant cover in abandoned extracted peatland (control site). The total plant cover (70 %) and especially the cover of Sphagnum mosses (60 %) on restored sites is a promising result in this short time also when compared to results from the application of the same method in Canada (Rochefort et al. 2013; Rochefort et al. 2013; González and Rochefort 2014). The cover of bryophytes increased significantly during the second vegetation season after restoration partly due to species not characteristic of bogs. Their number and cover decreased during the third vegetation season while the number of bog-specific species and their cover increased steadily. Similar tendency on restored peatlands is documented also elsewhere (e.g., Aronson and Galatowitsch 2008; Poulin et al. 2013; González and Rochefort 2014). Seedlings undergoing germination are affected by the surrounding vegetation, and some species may act as competitors, whereas others act as nursery plants for the establishment of bog-specific species. Two bryophyte species, Polytrichum strictum and Pohlia nutans, can improve the conditions for Sphagnum growth but upon exceeding a threshold cover may outcompete Sphagnum mosses and lead to the failure of restoration (Groeneveld and Rochefort 2005; Gonzáles et al. 2013; Rochefort et al. 2003). The cover of these species in the Tässi restoration area has remained within a few percentages, and all changes in vegetation point towards a successful start of restoration (e.g., González and Rochefort 2014; Gonzáles et al. 2014). In several plots, the continued increase in total cover was inhibited by an overly thick straw layer with no plants beneath it.
One of the main results from the restoration experiment is that Sphagnum cover increased significantly during the study period; while the cover of S. fuscum and S. magellanicum in restored sites is still less than that in the donor site, the cover of S. rubellum nearly equalled that of the donor site. This species grows in Estonia usually on low hummocks and can tolerate desiccation and short-term flooding (Vellak et al. 2013). Poulin et al. (2013) also have found that S. rubellum can become more abundant on restored sites than in reference ecosystems and increases its cover comparing to that of S. fuscum. The cover of S. fuscum was quite stable on the restoration sites during the first 3 years. The further growth and cover of Sphagnum mosses could be facilitated via creating better microclimate, reducing frost heave, providing support and ladder effect by the vascular plants, especially Ericaceae and E. vaginatum, established on restoration areas (McNeil and Waddington 2003; Pouliot et al., 2011; Laberge et al. 2013). Vascular plants will also initiate the formation of hummocks, thereby further supporting the growth of S. fuscum (Robroek et al. 2009; Hájek and Beckett 2008; Pouliot et al. 2012). A little surprising was the scanty increase in S. magellanicum cover, since this species is among the most widespread Sphagnum mosses with a broad ecological amplitude (Crum 1984). This result could be caused by the difficulties in maintaining capitula wetness at low water table (McCarter and Price 2014a), as evident from the slight cover reduction between autumn 2013 and the following spring (Fig. 5). The decrease may be caused by the different moisture conditions during the time of analysis; large capitula of S. magellanicum saturated with water in autumn can cause greater estimates in cover than dry capitula in spring.
The main aim of spreading plant fragments from a donor site is to accelerate the establishment of Sphagnum carpets. The cover of Sphagnum mosses attained up to 60 %, in the restoration area within 3 years with only S. tenellum unidentified of the six Sphagnum species growing in the donor site. This species grows in Estonian bogs mainly in hollows and wet lawns, but may survive in lower hummocks between dense shoots of other Sphagnum species (Vellak et al. 2013). Therefore, this species could be collected from the donor site as occasional companion shoots. From ten bog-specific vascular plant species, only Drosera anglica and Ledum palustre occurring in the donor site were not identified in the restoration area. D. anglica, which grows in Estonia in natural bogs in hollows and lawns, is more sensitive to the ecological conditions of the surroundings than D. rotundifolia (Hoyo and Tsuyuzaki 2014) and is not yet established on restoration sites because of that. D. rotundifolia grows on hummocks and is therefore also better adapted to changing conditions and temporal dryness on restoration sites. L. palustre could be absent because of its difficulties to regenerate from small fragments (Bret-Harte et al. 2002). E. vaginatum is among the first species to arrive on extracted peatlands. It can act as a nurse plant or at greater cover may inhibit restoration (Tuittila et al. 2000; Campbell et al. 2003; Lavoie et al. 2005b), but since its cover in this study remained just a few percent, a potential negative effect is likely to be minimal.
Conclusions
Although the first edition of “The moss layer transfer method” was published in 1997 and results from its successful application in Canada with further suggestions for restoration have been published in numerous scientific publications, to our knowledge, these here are the first results published of its application in northern Europe. The application of this method in Europe; however, has been suspect owing largely to the differences in climate and characteristics of extracted peatlands, as well as lack of suitable donor sites. The results show that this method gives good results also in the restoration of extracted peatland towards re-establishment of bog vegetation under northern European conditions. Within three years after restoration, total plant cover already exceeds 70 % consisting mostly of targeted Sphagnum mosses. Still, these results are limited to the three first years and, despite several indicators of successful restoration, complete restoration in the coming years depends also on precipitation and raising water table and in time less from restoration treatment. Nonetheless, the results of this study should be encouraging to start restoration of extracted peatlands on larger areas where deemed suitable.
References
Anderson P (2014) Bridging the gap between applied ecological science and practical implementation in peatland restoration. J Appl Ecol 51:1148–1152
Aronson MFJ, Galatowitsch S (2008) Long-term vegetation development of restored prairie pothole wetlands. Wetlands 28(4):883–895
Backėus I (1988) Weather variables as predictors of Sphagnum growth on a bog. Holarctic Ecol 11(2):146–150
Bret-Harte MS, Shaver GR, Chapin FS III (2002) Primary and secondary stem growth in arctic shrubs: implications for community response to environmental change. J Ecol 90:251–267
Campbell DR, Rochefort L, Lavoie C (2003) Determining the immigration potential of plants colonizing disturbed environments: the case of milled peatlands in Quebec. J Appl Ecol 40:78–91
Caners RT, Macdonald SE, Belland RJ (2009) Recolonization potential of bryophyte diaspore banks in harvested boreal mixed-wood forest. Plant Ecol 204:55–68
Chirino C, Campeau S, Rochefort L (2006) Sphagnum establishement on bare peat: the importance of climatic variability and Sphagnum species richness. Appl Veg Sci 9(2):285–294
Clymo RS, Hayward PM (1982) The ecology of Sphagnum. In: Smith AJE (ed) Bryophyte ecology. Chapman and Hall, London, pp 229–289
Constanza R, d’Arge R, de Groots R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Corson A, Campbell D (2013) Testing protocols to restore disturbed Sphagnum—dominated peatlands in the Hudson Bay Lowland. Wetlands 33:291–299
Crum H (1984) North American Flora. Series II, part 11, Sphagnopsida. Sphagnaceae. The New York Botanical Garden, New York
Eggelsmann R (1988) The rewetting of raised bogs. Geowissenschaften 11:317–322
Gerdol R (1996) The seasonal growth pattern of Sphagnum magellanicum Brid. In different microhabitats on a mire in the southerb Alps (Italy). Oecologia 5(1):13–20
Gonzáles E, Rochefort L, Boudreau S, Hugron S, Poulin M (2013) Can indicator species predict restoration outcomes early in the monitoring process? A case study with peatlands. Ecol Indic 32:232–238
Gonzáles E, Rochefort L, Boudreau S, Poulin M (2014) Combining indicator species and key environmental and management factors to predict restoration success of degraded ecosystems. Ecol Indic 46:156–166
González E, Rochefort L (2014) Drivers of success in 53 cutover bogs restored by a moss layer transfer technique. Ecol Eng 68:279–290
Groeneveld EVG, Rochefort L (2005) Polytrichum strictum as a solution to frost heaving in disturbed ecosystems: a case study with milled peatlands. Restor Ecol 13(1):74–82
Hájek T, Beckett RP (2008) Effect of water content components on desiccation and recovery in Sphagnum mosses. Ann Bot-London 101(1):165–173
Heikkilä H, Lindholm T, Jakkola S (2002) Soiden ennallistamisopas. A guide for the restoration of peatland habitats. Metsähallituksen luonnonsuojelulkaisuja 66:1–124
Hill MO, Bell N, Gruggeman-Nannenga MA, Brugues M, Cano MJ, Enroth J, Flatberg KI, Frahm J-P, Gallego MT, Garilleti R, Guerra J, Hedenäs L, Holyoak DT, Hyvönen J, Ignatov M, Lara F, Mazimpaka V, Muňoz J, Söderström L (2006) An annotated checklist of the mosses of Europe and Macaronesia. J Bryol 28:198–267
Hoyo Y, Tsuyuzaki S (2014) Habitat differentiation between Drosera anglica and D. rotundifolia in a post-mined peatland, Northern Japan. Wetlands 34:943–953
Ilomets M (1996) Temporal changes of Estonian peatlands and carbon balance. In: Punning J-M (ed) Estonia in the system of global climate change. Institute of Ecology. Publications 4:65–74
Ingerpuu N, Nurkse K, Vellak K (2014) Bryophytes in Estonian mires. Est J Ecol 63(1):3–14
Joosten JHJ (1992) Bog regeneration in The Netherlands: a review. In: Bragg OM, Hulme PD, Ingram HAP, Robertson RA (eds) Peatland Ecosystems and Man: An Impact Assessment. Department of Biological Sciences, University of Dundee, UK pp 367–373
Joosten H (2008) The IMCG Global Peatland Database. http://www.imcg.net/pages/publications/imcg-materials.php?lang=EN. Assessed 20 February 2015
Kalamees R, Püssa K, Zobel K, Zobel M (2012) Restoration potential of the persistent soil seed bank in successional calcareous (alvar) grasslands in Estonia. Appl Veg Sci 15(2):208–218
Kask M (1982) A list of vascular plants of Estonian peatlands. In: Frey T, Masing V, Roosaluste E (eds) Peatland ecosystems. Academy of Sciences of the Estonian SSR, Tallinn, pp 39–49
Konvalinková P, Prach K (2014) Environmental factors determining spontaneous recovery of industrially mined peat bogs: A multi-site analysis. Ecol Eng 69:38–45
Kukk T, Kull T (eds) (2005) Atlas of the Estonian Flora. Institute of Agricultural and Environmental Sciences of the Estonian University of Life Sciences, Tartu
Laberge V, Rochefort L, Poulin M (2013) Ericaceae stabilize peat and foster Sphagnum majus establishment at pool margins in restored peatlands. Aquat Bot 111:1–8
Lavoie C, Marcoux K, Saint-Louis A, Price JS (2005a) The dynamics of a cotton-grass (Eriophurum vaginatum L.) cover expansion in a vacuum-mined peatland, southern Québec, Canada. Wetlands 25(1):64–75
Lavoie C, Saint-Louis A, Lachance D (2005b) Vegetation dynamics on an abandoned vacuum-mined peatland: five years of monitoring. Wetl Ecol Manag 13(6):621–633
Maljanen M, Sigurdsson BD, Gudmundsson J, Óskarsson H, Huttunen JT, Martikainen PJ (2010) Greenhouse gas balances of managed peatlands in the Nordic countries—present knowledge and gaps. Biogeosciences 7:2711–2738
McCarter CPR, Price JS (2013) The hydrology of the Bois-des-Bel bog peatland restoration: 10-years post-restoration. Ecol Eng 55:73--81
McCarter CPR, Price JS (2014a) Ecohydrology of Sphagnum moss hummocks: mechanisms of capitula water supply and simulated effects of evapotranspiration. Ecohydrology 7(1):33–44
McCarter CPR, Price JS (2014b) The hydrology of the Bois-des-Bel peatland restoration: hydrophysical properties limiting connectivity between regenerated Sphagnum and remnant vacuum harvested peat deposit. Ecohydrology 8(2):173–187
McNeil P, Waddington M (2003) Moisture controls on Sphagnum growth and CO2 exchange on a cutover bog. J Appl Ecol 40:354–367
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: wetlands and water synthesis. World Resources Institute, Washington DC
Money RP, Wheeler BD (1999) Some critical questions concerning the restorability of damaged raised bogs. Appl Veg Sci 2(1):107–116
Orru M (1992) Eesti turbavarud (Estonian peat resources). Eesti Geoloogiakeskus, Tallinn
Paal J (ed) (2011) Jääksood, nende kasutamine ja korrastamine. Keskkonnainvesteeringute Keskus & Eesti Turbaliit, VALI trükikoda, Tartu
Paal J, Leibak E (2011) Estonian mires: inventory of habitats. Publication of the Project “Estonian Mires Inventory completion for maintaining biodiversity”. Regio, Tartu
Pakalne M, Strazdina L (eds) (2013) Raised bog management for the biological diversity conservation in Latvia. University of Latvia, Riga
Poulin M, Andersen R, Rochefort L (2013) A new approach for tracking vegetation change after restoration: a case study with peatlands. Restor Ecol 21(3):363–371
Pouliot R, Rochefort L, Karofeld E, Mercier C (2011) Initiation of Sphagnum hummocks in bogs and the presence of vascular plants: is there a link? Acta Oecol 37(4):346–354
Pouliot R, Rochefort L, Karofeld E (2012) Initiation of microtopography in re-vegetated cutover peatlands: evolution of plant species composition. Appl Veg Sci 15(3):369–382
Prach K, Lencová K, Ŕehounková K, Dvořáková H, Jirová A, Konvalinková P, Mudrák O, Novák J, Trnková R (2013) Spontaneous vegetation succession at different central European mining sites: a comparison across seres. Environ Sci Pollut R 20(11):7680–7685
Quinty F, Rochefort L (2003) Peatland restoration guide. Second edition. Canadian Sphagnum Peat Moss Accociation, New Brunswick Department of Natural Resources and Energy, Québec
Ramst R, Orru M (2009) Eesti mahajäetud turbatootmisalade taastaimestumine. Eesti Põlevloodusvarad ja – jäätmed 1:6–7
Robroek BJM, van Ruijven J, Schouten MGC, Breuwer A, Crushell PH, Berendse F, Limpens J (2009) Sphagnum re-introduction in degraded peatlands: the effects of aggregation, species identity and water table. Basic Appl Ecol 10(8):697–706
Rochefort L, Lode E (2006) Restoration of degraded boreal peatlands. In: Wieder RK, Vitt DH (eds) Boreal peatland ecosystems. Ecological studies 188. Springer, Berlin Heidelberg, pp 381–423
Rochefort L, Nondedeu FI, Boudreau S, Poulin M (2013) Comparing survey methods for monitoring vegetation change through time in a restored peatland. Wetl Ecol Manag 21(1):71–85
Rochefort L, Quinty F, Campeau S, Johnson K, Malterer T (2003) North American approach to the restoration of Sphagnum dominated peatlands. Wetl Ecol Manag 11:3--20
Salm J-O, Kimmel K, Uri V, Mander Ü (2009) Global warming potential of drained and undrained peatlands in Estonia: a synthesis. Wetlands 29(4):1081–1092
Salonen V (1994) Revegetation of harvested peat surfaces in relation to substrate quality. J Veg Sci 5:403–408
Schumann M, Joosten H (2008) Global peatland restoration manual. http://www.imcg.net/media/download_gallery/books/gprm_01.pdf. Assessed 20 February 2015
Söderström L, Urmi E, Váňa J (2007) The distribution of Hepaticae and Anthocerotae in Europe and Macaronesia—update 1–427. Cryptogam Bryol 28(4):299–350
Stoneman R, Brooks S (1997) Conserving bogs. The Management Handbook. The Stationery Office Ltd, Edinburgh
Sundberg S, Rydin H (2002) Habitat requirements for establishment of Sphagnum from spores. J Ecol 90:268–278
Triisberg T, Karofeld E, Paal J (2011) Re-vegetation of block-cut and milled peatlands: an Estonian example. Mires and Peat 8: Article 5, 1–14
Triisberg T, Karofeld E, Paal J (2013) Factors affecting the re-vegetation of abandoned extracted peatlands in Estonia: a synthesis from field and greenhouse studies. Est J Ecol 62(3):192–211
Triisberg T, Karofeld E, Liira J, Orru M, Ramst R, Paal J (2014) Microtopography and properties of residual peat are convenient indicators for restoration planning of abandoned extracted peatlands. Restor Ecol 22(1):31–39
Tuittila ES, Rita H, Vasander H, Laine J (2000) Vegetation patterns around Eriophorum vaginatum L. tussocks in a cut-away peatland in southern Finland. Can J Botany 78:47–58
Van Gaalen KE, Flanagan LB, Peddel DR (2007) Photosynthesis, chlorophyll fluorescence and spectral reflectance in Sphagnum moss at varying water contents. Oecologia 153:19–28
Vasander H, Tuittila E-S, Lode E, Lundin L, Ilomets M, Sallantaus T, Heikkilä R, Pitkänen M-L, Laine J (2003) Status and restoration of peatlands in northern Europe. Wetl Ecol Manag 11(1):51–63
Vellak K, Ingerpuu N, Karofeld E (2013) Eesti turbasamblad. The Sphagnum mosses of Estonia, Tartu Ülikooli Kirjastus
Verhoven JTA (2014) Wetlands in Europe: perspectives for restoration of a lost paradise. Ecol Eng 66:6–9
Wagner KJ, Gallagher SK, Hayes M, Lawrence BA, Zedler JB (2008) Wetland restoration in the new millennium: do research effort match opportunities? Restor Ecol 16(3):367–372
Wheeler BD, Shaw SC (1995) Restoration of damaged peatlands. Wiley, London
Acknowledgments
This study was co-financed by the following research projects: SF0180012s09, SF0180025s12, IUT34-7, IUT34-9 and by the EU Regional Development Fund (Centre of Excellence FIBIR) and Kalloveen BvBa. We thank for H. Oosterkamp, workers from AS Kraver and others for their help in the field and R. Burton for proof reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Karofeld, E., Müür, M. & Vellak, K. Factors affecting re-vegetation dynamics of experimentally restored extracted peatland in Estonia. Environ Sci Pollut Res 23, 13706–13717 (2016). https://doi.org/10.1007/s11356-015-5396-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5396-4