Abstract
1-Butyl-3-methylimidazolium thiocyanate [BMIM]SCN has been presented on extractive desulfurization of liquid fuel. The FTIR, 1H-NMR, and C-NMR have been discussed for the molecular confirmation of synthesized [BMIM]SCN. Further, thermal, conductivity, moisture content, viscosity, and solubility analyses of [BMIM]SCN were carried out. The effects of time, temperature, sulfur compounds, ultrasonication, and recycling of [BMIM]SCN on removal of dibenzothiophene from liquid fuel were also investigated. In extractive desulfurization, removal of dibenzothiophene in n-dodecane was 86.5 % for mass ratio of 1:1 in 30 min at 30 °C under the mild process conditions. [BMIM]SCN could be reused five times without a significant decrease in activity. Also, in the desulfurization of real fuels, multistage extraction was examined. The data and results provided in the present paper explore the significant insights of imidazolium-based ionic liquids as novel extractant for extractive desulfurization of liquid fuels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, as the environmental regulation on the sulfur content (S-content) in liquid fuels becomes progressively more stringent worldwide. To reduce the S-content from liquid fuels, extractive deep desulfurization (EDS) of gasoline and diesel has fascinated much attention (Nie et al. 2006). S-compounds present in liquid fuels may possibly change into SO X in fuel engine which pollute the air and cause acid rain; the combustion efficiency of fuels may be reduced by S-compounds. Hence, to reduce the S-content into much lower limit (<50 ppm) is substantial task to produce clean sulfur free fuels (Babich and Moulijn 2003).
In industry, traditional hydrodesulfurization (HDS) process is broadly used for EDS of liquid fuels where S-compounds react with H2 and converted into H2S and hydrocarbons (Isao and Choi 2004; Tropsoe and Gate 1997; Ma et al. 1994; Ferrari et al. 2001; Dumeignil et al. 2006; Dharaskar et al. 2013a, b, c, d, a, b, c, d). HDS process is able to remove aliphatic thiols, sulfides, and disulfides effectively, whereas it is not as much competent for some thiophenic S-compounds like dibenzothiophene (DBT) and its derivatives (Pawelec et al. 2001; Dharaskar et al. 2014a, b, c).
In recent years, several technologies such as extractive desulfurization, selective adsorption, catalytic oxidation, and biodesulfurization were proposed. Among these, EDS is a stunning technology, which may be carried out at ambient temperature, pressure, and without H2 as a catalyst. More prominently, a quantity of DBT and BT series S-compounds can be extracted quite efficiently (Nie et al. 2006). A good extractant must have good extractive ability for S-compounds, free of contamination to the fuels, non-toxicity, environmental benignity, and stability for repetitive use (Dharaskar et al. 2013a, b, c, d; Dharaskar 2012). Hence, EDS process may be a complementary technology for the HDS process. Conventional solvents have their own boundaries of environmental issue, reuse capability, etc., which may be overcome by ILs (Jiang et al. 2008; Dharaskar et al. 2013a, b, c, d).
One of the most extensively studied classes of ILs is based upon imidazolium cation. These types of ILs are receiving a great deal of attention and used in many fields including synthesis, catalysis, biochemistry, electrochemistry, energy field’s extraction processes, and industrial applications (Kanai et al. 2009; Jeon et al. 2008; Zhao 2006). They are considered as green solvents with unique physicochemical properties. Some special properties of ILs are high thermal stability, tunable viscosity, low melting point, very high polarity, very large liquids ranging up to 400 °C, non-volatility, favorable solvating properties, non-flammability, high ionic conductivity, and wide electrochemical window. ILs may be immiscible with water and their ionic mobility depends on the structures of cation and anion (Wasewar 2013).
ILs are organic salts with melting points around or below ambient temperature and have been used as “green solvents” in a range of fundamental research and applications owing to their unique and useful properties (Dharaskar et al. 2013a, b, c, d). Many ILs are thermally and chemically stable, and they are in liquid state over a wide range of temperatures. Moreover, a careful selection of their constituent ions can tune the properties of the ionic liquids to a considerable extent, thus allowing the “design” of a specific IL to meet the requirements for a particular target.
The use of ILs for the selective extraction of sulfur compounds from fuels was reported for the first time in 2001 by Wassercheid and co-workers (Esser et al. 2004). Since then, the desulfurization ability of many ionic liquids has been tested. The first studies focused on ILs containing the [PF6]− and [BF4]− anions (Zhang and Zhang 2002; Zhang et al. 2004). However, these ILs are prone to hydrolyze even in the presence of humidity, generating hazardous hydrolysis products (Swatloski et al. 2003; Archer et al. 2005).
Although the chemistry of thiocyanate cations is well recognized, and some imidazolium salts are commercially accessible and amazingly represented in the development and use of ILs. The synthesis and characterization by NMR of 1-butyl-3-methylimidazolium thiocyanate [BMIM]SCN was reported. This imidazolium-based IL has been used in extraction of cobalt from nickel salts, and in liquid membrane (Chen et al. 2012).
In present work, [BMIM]SCN was synthesized and employed as an extractant to explore its extractive efficiency towards fuel containing S-compounds. [BMIM]SCN is insensitive to air/moisture, has high thermal/chemical stability, and its anions are fluorine-free. The effects of time, temperature, sulfur compounds, reusability of [BMIM]SCN, desulfurization of real fuels, and multistage extraction are systematically investigated.
Experimental section
Chemical and materials
IL used in the experiment was synthesized using analytical grade chemicals. The details of source, and grades of the chemicals used are as follows: 1-methylimidazole (CAS 616-47-7, Acros 99 %), 1-chlorobutane (CAS 109-69-3, Acros 99 %), sodium thiocyanate (CAS 540-72-7, Sigma 99 %), ethyl acetate (CAS 20108-L25, SDFCL 99.5 %), n-dodecane (CAS 94094-93-6, Acros 99 %), dibenzothiophene (DBT) (CAS 132-65-0, Acros 98 %), thiophene (TS) (CAS 110-02-1, Sigma-Aldrich 99 %), 3-methylthiophene (3-MT) (CAS 616-44-4, Sigma-Aldrich 98 %), benzothiophene (BT) (CAS 95-15-8, Sigma-Aldrich, 99 %), 4-methyldibenzothiophene (4-MDBT) (CAS 7372-88-5, Sigma-Aldrich, 96 %), 4,6-dimethyldibenzothiophene (4,6-DMDBT) (CAS 1207-12-1, Sigma-Aldrich, 97 %).
All chemicals were used without any further purification. Real fuels were purchased from Local Petroleum Pump House, Nagpur, Maharashtra, India.
Synthesis of IL
Synthesis of [BMIM]Cl
[BMIM]Cl was prepared by the reaction of 1-methylimidazolium (10.497 g), and 1-chlorobutane (17.408 g) are added to a slurry of acetone (30 ml) in a round-bottom flask fitted with a reflux condenser and magnetically stirred under the protection of nitrogen gas at 60 °C for 48 h, and then resulting mixture is allowed to settle for phase splitting. Then, the product was washed thrice with ethyl acetate. The remaining ethyl acetate was removed by heating at 60 °C under vacuum for 2 h. The solution was filtered and the remaining resulting product was obtained [BMIM]Cl (Chen et al. 2012; Mochizuki and Sugawara 2008).
Synthesis of [BMIM]SCN
[BMIM]Cl (7.345 g) and sodium thiocyanate (2.522 g) are place in a round-bottom flask containing acetone (30 ml) to obtain [BMIM]SCN. After reacting at 40 °C for 12 h, a precipitate is formed which is then filtered off, leaving behind a liquid solvent that undergoes rotary vacuum evaporation. The resulting product is washed with dichloromethane, filtered, and again made to undergo rotary vacuum evaporation. [BMIM]SCN is finally obtained after traces of volatile impurities were removed by heating at 60 °C under vacuum for 2 h (Mochizuki and Sugawara 2008). The synthesis route of [BMIM]SCN is shown in Fig. 1.
Preparation of model fuel
A model fuel with 500 ppmw (parts per million by weight) sulfur (DBT as sulfur source) was prepared in n-dodecane. Similarly, the model fuels were prepared by dissolving 4-MDBT, 4,6-DMDBT, BT, TS, and 3-MT individually in n-dodecane, respectively. Actual diesel and gasoline with total S-content of 600 and 90 ppmw respectively were used.
Extractive deep desulfurization
A 100-ml two-necked flask were used for the extractive desulfurization experiments where 10 ml model fuel and describe amount of [BMIM]SCN with mass ratios (model fuel to [BMIM]SCN as 5:1, 3:1, and 1:1) were mixed by vigorous stirring for time range between 5–30 min at 30 °C in a water bath. After completion of the reaction, the upper phase (model fuel) was separated and settling of the reaction mixture. The upper phase was analyzed for S-content. The extraction efficiency is presented in terms of the S-removal based on the initial and final S-content in the fuel.
Instrumentation
A structure of [BMIM]SCN was analyzed by Fourier transform infrared (FTIR) Shimadzu IR-Affinity 1 Spectrometer (Japan), using the method of KBr pellet. [BMIM]SCN was characterized by 1H-NMR, and 13C-NMR using CDCl3 as solvent on a Varian, USA Mercury plus 299.95 MHz for 1H-NMR and 75.43 MHz for 13C-NMR was used for determination of molecular structures and conformations of the compounds. Thermal stability of [BMIM]SCN was determined with SII Co. Exstar TG/DTA (Japan).
Conductivity of [BMIM]SCN was measured by PICO+ (Lab India) pH/conductivity meter. Viscosity of [BMIM]SCN was measured using ARG2 Rheometer (TA Instruments, USA). Solubility of [BMIM]SCN was analyzed by high-performance liquid chromatography (Agilent Technologies, 1200 series equipped with a UV-Vis detector under 237 nm wavelength, column (C-18), mobile phase, methanol/water = 80/20, flow rate, 1.0 ml/min).
S-content in model fuel and real fuels before and after extraction was analyzed by X-ray fluorescence spectrometer, model PW 2404, Phillips (PANalytical, Spectris Technology, Netherlands), Centre of Sophisticated Analytical Instrumental Facility, Indian Institute of Technology, Mumbai, Maharashtra, India. The contact time of 30 min between the model fuel and [BMIM]SCN stage is sufficient to achieve the equilibrium. So the most favorable time required for the desulfurization of model fuel was 30 min. However, [BMIM]SCN was best suitable to remove DBT reflected at 30 °C which was taken as the best possible temperature.
Characterization of [BMIM]SCN
FTIR, 1H-NMR, and 13C-NMR analyses were carried out for the characterization of [BMIM]SCN.
FTIR analysis
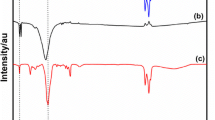
A structure of [BMIM]SCN was analyzed by FTIR Shimadzu IR-Affinity 1 spectrometer (Japan), using the method of KBr pellet. The results are shown in Fig. 2.
The FTIR spectra of the synthesized [BMIM]SCN is shown in Fig. 2. The aliphatic asymmetric and symmetric C–H stretching vibrations were observed at 2960 and 3040 cm−1, respectively, and in-plane bending vibrations at 1390 cm−1 are due to C–H in methyl group. Strong bands at 2084 and 1430 cm−1 is due to C–N and C=N stretching, respectively. Weak band at 815 cm−1 corresponds to C–S vibration stretching. The IR spectra of [BMIM]SCN after extraction of sulfur show similar vibrational bands. From Fig. 2, it was observed that a synthesized IL is [BMIM]SCN.
1H-NMR,and 13C-NMR analyses of [BMIM]SCN
[BMIM]SCN was characterized by 1H-NMR and 13C-NMR using CDCl3 as solvent on a Varian, USA Mercury plus 300 MHz for 1H-NMR and 76 MHz for 13C-NMR spectrometer for the determination of molecular structures and conformations. 1H-NMR data was reported as follows in ppm (δ) from the internal standard (TMS, 0.0 ppm) and chemical shift (multiplicity, integration). The results of 1H-NMR and 13CNMR of [BMIM]SCN as well as the schematic details of NMR analysis which were shown in Scheme 1, are given as follows:
1HNMR (300 MHz, CDCl3): δ (ppm) 9.26 (C2, 1H, t), 7.51 (C4, 1H, t), 7.29 (C5, 1H, t), 4.23 (C6, 2H, t), 2.48 (C7, 2H, m), 1.89 (C8, 2H, m), 1.39 (C9, 2H, m), 0.97 (C10, 3H, s).
13CNMR (76 MHz, CDCl3): δ (ppm) 134.5 (-N=C-S-), 121.3 (C4, d), 126.5 (C5, d), 49.6 (C6, d), 31.8 (C7), 19.2 (C8), 13.3 (C9), 36.0 (C10).
Thermal analyses of [BMIM]SCN
Thermal stability of [BMIM]SCN was determined with thermogravimetric analyzer in order to know their upper temperature limit. The sample (10 mg) was placed in an aluminum pan and heated over a temperature range of 30–500 °C at a heating rate of 20 °C min−1. The total time given to analyze the thermal stability was 23.5 min over temperature range from 30 to 500 °C. The onset of thermal decomposition started at 244.6 °C and decomposition ends around 390.6 °C with weight loss of 38.9 % as shown in Fig. 3. No further decomposition was observed; this shows a high thermal stability of [BMIM]SCN (Dharaskar et al. 2013a, b, c, d).
Physical properties of [BMIM]SCN
The physical properties like moisture content, conductivity, and solubility and viscosity analyses of [BMIM]SCN were studied. The production of pure [BMIM]SCN is very important since impurities have a strong influence on their physical properties and stability.
Water content analysis
Certain amount of water content was found more or less in all heavy oil reservoirs. It is key point whether water has effect on the viscosity reduction of heavy fuel by ILs. However, when the water content in heavy fuel by improving the mass fraction of [BMIM]SCN. Water content of [BMIM]SCN was measured by MA-101-C Karl Fischer Titration System (Spectralab Instruments Pvt. Ltd. India). To upgrade and decrease the viscosity of heavy fuel using [BMIM]SCN, less than 10 % of water content is better (Fan et al. 2009). The water content in [BMIM]SCN was found to be 6.2 %.
Conductivity analysis
[BMIM]SCN conductivity mainly depends on mobility of its cation because the diffusion coefficients of ILs cations are higher than anions. ILs based on imidazolium cations have the highest ionic conductivity. The conductivity and thermal stability are required in a separation or extraction process of imidazolium-based ILs with stable thiocyanate anions (Faridbod et al. 2011). [BMIM]SCN shows conductivity value of 110 (μs cm−1) which is comparatively small. After exchanging of anion, the conductivity of [BMIM]SCN could be increased. Consequently, [BMIM]SCN has great advantages as compared to conventional organic solvents (Liu et al. 2008; Dharaskar et al. 2014a, b, c).
Viscosity analysis
Factors that affect the viscosity of an IL are poorly unwritten, but the chemical structure of the anion is known to have a particularly strong influence. Lowest viscosity of ILs is formed from small anions that have a diffuse negative charge and are unlikely to take part in any hydrogen bonding (Forsyth et al. 2004). The high viscosity of few imidazolium ILs may be decreased by an increase in temperature and/or addition of diluents (Seddon et al. 2000). It has been pointed out that the viscosity of [BMIM]SCN is governed by van der Waals interactions. As a result, both the structure and basicity of anion affect the viscosity. The decrease of size of anion decreases the van der Waals interaction but increases the electrostatic interaction through hydrogen bonding (Hagiwara and Ito 2000).
Viscosity of [BMIM]SCN was measured using ARG2 Rheometer. Viscosity of [BMIM]SCN varies inversely with respect to shear rate, and it is well recognized that larger imidazolium cations make IL more viscous because of the increased intermolecular van der Waals interactions. Figure 4 indicates that as shear rate increases from 0 to 250 (s−1) viscosity of [BMIM]SCN decreases from 0.1878 to 0.1690 (Pa.s), respectively. Also, it was observed that most favorable viscosity found to be at shear rate 250 (s−1). The similar trends were observed in the available literature (Dharaskar et al. 2013a, b, c, d).
Viscosity of [BMIM]SCN decreases with respect to time at constant temperature (298.2 K). From Fig. 5, it was observed that the most favorable viscosity was obtained at 340 s (Dharaskar et al. 2014a, b, c).
Solubility analysis
The extraction of organic product from aqueous media requires ILs with low solubility of water since the higher the water solubility in ILs the lower their extractive potential. This is due to the intrinsic equilibrium competition between water and organic compounds under study for IL, which is related to the IL hydrophobic nature. Hence, the data of mutual solubility’s between water and ILs is of major significance prior to their consideration for extractive applications. [BMIM]SCN comes up as a new option of extremely hydrophobic ILs, especially when large alkyl chain length is present, and thus their use can be advantageous for the extraction (Freire et al. 2008).
Thus, solubility of [BMIM]SCN with six conventional solvents was studied. [BMIM]SCN may be dissolved in some conventional organic solvents such as methanol, acetonitrile, ethanol, acetone, and water, but not all the organic solvents (e.g., [BMIM]SCN not dissolved in ethyl acetate). The [BMIM]SCN solubility might be changed by changing the anions. Novel two-phase system can be created and used for various applications such as synthesis and extraction (Freire et al. 2008). For the application of [BMIM]SCN extractant, the solubility mechanism of IL is needed. [BMIM]SCN solubility in liquid fuel may give rise to extractant loss and liquid fuel contamination. These results suggest that the solubility of [BMIM]SCN in liquid fuel has to be optimized for future applications.
Result and discussion
Effect of time on sulfur removal
The extractions of model fuel (DBT in n-dodecane) with [BMIM]SCN were carried out for 5, 10, 20, 30, and 35 min at 30 °C with mass ratios of 5:1, 3:1, and 1:1 (mass ratio of model liquid fuel to [BMIM]SCN as shown in Fig. 6). The desulfurization process went quite quickly and S-concentration in model fuel decreased with increased in extraction time and reduced from 500 to 195 ppmw (S-removal 61 %), 143.5 ppmw (S-removal 71.3 %), and 104 ppmw (S-removal 79.2 %) with mass ratios of 5:1, 3:1, and 1:1, respectively, in 20 min. However, S-concentration decreased continuously with increased in extraction time and reduced from 500 to 170 ppmw (S-removal 66 %), 104.5 ppmw (S-removal 79.1 %), and 67.5 ppmw (S-removal 86.5 %) with mass ratios of 5:1, 3:1, and 1:1, respectively, in 30 min. No further reduction was observed in the S-removal as equilibrium achieved at 30 min (Dharaskar et al. 2013a, b, c, d).
At the initial stage of the reaction, S-content in the model fuel was very high; hence, the extraction rate becomes high with high S-removal rate. As the reaction proceeds, extraction rate becomes low with S-removal rate no longer noticeably increases. The results, in Fig. 6, show that viscous IL took 30 min contact time to achieve liquid-liquid equilibrium between the model fuel and [BMIM]SCN phase. So the most favorable time required for the desulfurization of model fuel was 30 min.
[BMIM]SCN is more capable of efficiently extracting DBT than other S-containing compounds (Fraser and MacFarlane 2009). This observation was also reported in other ILs that extraction process for [BMIM]SCN was attributed for higher polarizable π-electron density of DBT which tends to insert the molecular structure of ILs (Nie et al. 2006). DBT extraction with [BMIM]SCN is recognized to the π-π interaction between the aromatic ring of imidazolium and thiophenic ring of DBT (MuMurry 1992).
The sulfur partition coefficient (K N ) is used to evaluate the extractive desulfurization performance. K N is based on gravity, which is defined as ratio of S-content in IL to S-content in model fuels. K N measurements were carried out in both model fuel and real fuel (gasoline and diesel). The DBT concentration in model fuel and [BMIM]SCN was directly determined using X-ray fluorescence spectrophotometer. Partition coefficient values of [BMIM]SCN with good DBT removal are shown in Fig. 7.
Effect of temperature on sulfur removal
Temperature plays a vital role in the extractive desulfurization process; Fig. 8 shows the effect of the temperature (20 °C, 25 °C, 30 °C, 35 °C, 40 °C) on the removal of sulfur. As shown in Fig. 8, with the increasing temperature from 20 to 30 °C, the removal efficiency of sulfur increases initially and then decreased. This effect may be attributed that when the temperature was less than 30 °C, as temperature increases, the viscosity of [BMIM]SCN was reduced and then the flexibility of [BMIM]SCN was also improved which may form a viscous flow layer. Thus, DBT removal efficiency in the model fuel by IL increased. When temperature exceeds 30 °C, the flexibility of [BMIM]SCN is not noticeably improved. Moreover, S-removal rate will no longer increase and even to some extent decline (Cun et al. 2011).
Therefore, [BMIM]SCN was best suitable to remove DBT reflected at 30 °C which was taken as the most favorable temperature. The S-content of the model fuel decreased from 500 to 215.5 ppmw (56.9 % S-removal), 150 ppmw (70 % S-removal), and 115 ppmw (77 % S-removal) with mass ratios of 5:1, 3:1, and 1:1, respectively, as shown in Fig. 8. Significant drop in S-removal was observed when temperature reached to 40 °C, and the S-removal was only 30.1, 44, and 48 % with mass ratios of 5:1, 3:1, and 1:1, respectively. Insensitivity to temperature was also observed in other extraction systems such as [BPy]BF4, [(CH2)4SO3HMIM]Tos, [BMIM]BF6, and [BMIM]PF6 (Cun et al. 2011). Subsequently, sulfur extraction may be performed at room temperature, which is encouraging for less energy consumption. K N values of [BMIM]SCN by varying temperatures with good DBT removal are shown in Fig. 9.
Effect of S-compound on sulfur removal
Table 1 represents the molecular structures and properties of S-compounds usually found in real fuels such as diesel and gasoline on extraction with pure hydrocarbons. It might be seen that results for thiols, sulfides, and related compounds are quite low (Liu et al. 2008). However, the results for DBT, 4-MDBT, 4,6-DMDBT, TS, BT, and 3-MT are excellent. The most likely mechanism for the extraction of S-compounds with [BMIM]SCN is the formation of liquid clathrates and π-π interactions between aromatic structures of the extraction target and the imidazolium ring system (Liu et al. 2008).
In real fuels, many nitrogen, oxygen, and aromatic compounds have been existed, which decreased the extraction performance of [BMIM]SCN for S-containing compounds. In real diesel, there were different kinds of alkyl-substituted DBTs present such as 4-MDBT, BT, 4,6-DMDBT, TS, and 3-MT. The removal of DBT reached 79 % in 30 min. However, the removal of 4-MDBT, 4,6-DMDBT, BT, TS, and 3-MT was only 74, 72.9, 71.3, 68, and 64.1 % within 30 min, respectively, as shown in Table 1. Compared with DBT, the electron density on the sulfur atom on 4-MDBT, 4,6-DMDBT, BT, TS, and 3-MT is lower, which leads to the lower reactivity of S-compounds. However, the reactivity of the DBT decreased with increasing methyl substitutes at the derivative substitute positions, and the reactivity sequencing was DBT > 4-MDBT > 4,6-DMDBT > BT > TS > 3-MT (Ge et al. 2011; Lu et al. 2014).
Recycling of spent [BMIM]SCN
In practical processes, considering the high cost of ILs, recycling or regeneration process is needed. The S-extraction performance of spent [BMIM]SCN was investigated and the results are shown in Fig. 10. It is observed that the desulfurization efficiency of [BMIM]SCN was reused up to five cycles. It was seen that the spent [BMIM]SCN was able to remove DBT from liquid fuel, nevertheless, at a lower efficiency of 38.1 % from 66 %, 50.1 % from 79.1 %, and 61.5 % from 86.5 % with mass ratios of model fuel to IL as 5:1, 3:1, and 1:1, respectively, with spent [BMIM]SCN. Reduction of S-removal might recognize DBT which is dissolved in [BMIM]SCN and decreased the extraction performance of [BMIM]SCN. The results indicated that after the [BMIM]SCN was recycled five times, the rate of S-removal decreases slightly (Lu et al. 2014; Liu et al. 2008).
Effect of ultrasonication on sulfur removal using [BMIM]SCN
Ultrasonic bath was used as ultrasonic source under ultrasonication experiments. The ultrasonic microcleaner-103 (OSCAR ultrasonic Pvt. Ltd., Mumbai, India) is a rectangular container (23.5 × 13.3 × 10.2 cm), to which 50 kHz transducers were annealed at the bottom. The bath power rating was 250 W on the scale of 0–100 %. The extraction of DBT is performed by adding the exact amount of [BMIM]SCN solutions in a 50-ml flask. The flask is partially immersed into the ultrasonic bath, which contains 2.5 l water. The water in the ultrasonic bath is circulated and regulated at the desired temperature to maintain the water temperature at a constant value and prevent it from being influenced by the ultrasonic exposure.
After ultrasonication, the model fuel was cooled down to the room temperature. The most favorable ultrasonic time for ultrasonic treatment and the extraction efficiency of S-removal are systematically studied. Table 2 shows that maximum S-removal was 91 % when ultrasonication time was 30 min. Hence, 30 min is the most favorable ultrasonication time required to achieve highest % of S-removal. However, S-removal was 80.1 %, when ultrasonication temperature reached at 30 °C. Hence, it was also noted that 30 °C is the most favorable ultrasonication temperature to achieve highest S-removal (Yang et al. 2011; Ma et al. 2011; Sun et al. 2013).
Desulfurization of real fuels using [BMIM]SCN
Real fuels extraction such as diesel and gasoline is much more difficult due to its typical content of various S-compounds and other impurities. The results of EDS of diesel and gasoline with imidazolium ILs are also promising. [BMIM]SCN displays a high S-removal capability from gasoline and diesel in single-stage extraction in 30 min at 30 °C with mass ratios of 5:1, 3:1, and 1:1 as shown in Table 3. [BMIM]SCN exhibits the best sulfur extraction ability for S-removal in gasoline which was reduced from initial sulfur of 90 ppmw to 39.7 ppmw (55.9 % S-removal), 26.9 ppmw (70.1 % S-removal), and 21.1 ppmw (76.5 % S-removal) with mass ratios of 5:1, 3:1, and 1:1 in a single-stage extraction, respectively. However, in diesel, it was reduced from initial sulfur of 600 to 359.4 ppmw (40.1 % S-removal), 297 ppmw (50.5 % S-removal), and 232.2 ppmw (61.3 % S-removal) with mass ratios of 5:1, 3:1, and 1:1 in a single-stage extraction, respectively. Diesel and gasoline contain more heteronuclear compounds than the model fuel, such as nitrogen and S-containing compounds (alkylthiophene, benzothiophene) which decrease the ability of [BMIM]SCN for S-removal. Because of steric effect of alkyl group in the aromatic rings, methyl-thiophene, methyl-benzothiophene, methyl-dibenzothiophene, etc., S-containing compounds in gasoline and diesel are extracted less than DBT in the model fuel by ILs (Cun et al. 2011; Dharaskar et al. 2014a, b, c).
Multistage extraction using [BMIM]SCN
Although a high S-removal by [BMIM]SCN is obtained as shown in Table 3, the final S-content in fuels cannot meet the definite requirement of low sulfur fuels (e.g., <10–50 ppmw). Consequently, multistage extractions are performed, and the results are shown in Fig. 11. The S-content in gasoline drops significantly from 90 to 16.5 ppmw (81.6 % of S-removal) after 4 cycles, and the S-content in diesel reduced from 600 to 192 ppmw (68 % of S-removal) after 4 cycles with fixed mass ratio of model fuel to IL as 3:1, within 30 min at 30 °C. As a result, multiple extractions are effective for the reduction of S-content of liquid fuel to considerably negligible amount. Similar results were reported in the literature (Cun et al. 2011).
Conclusion
[BMIM]SCN can be used for EDS of liquid fuels, mainly with regard to those S-compounds that are very complicated to eliminate by common HDS process. [BMIM]SCN is the most efficient in the removal of DBT containing liquid fuels and it can reach to 86.5 % for a single-stage extraction at 30 °C in 30 min with mass ratio of 1:1, which is the noteworthy progress of EDS over HDS process. The data and results of the presented work could provide significant insights of imidazolium-based ILs. Thus, the EDS method could be developed into a simple, mild, and environmentally benign method for deep desulfurization.
Abbreviations
- [BMIM]SCN:
-
1-Butyl-3-methylimidazolium thiocyanate
- IL:
-
Ionic liquids
- DBT:
-
Dibenzothiophene
- TS:
-
Thiophene
- BT:
-
Benzothiophene
- 3-MT:
-
3-Methythiophene
- 4-MDBT:
-
4-Methyldibenzothiophene
- 4,6-DMDBT:
-
4 6-Dimethyldibenzothiophene
- EDS:
-
Extractive desulfurization system
- HDS:
-
Hydrodesulphurization system
References
Archer D, Widegren J, Kirklin D, Magee J (2005) Enthalpy of solution of 1-octyl-3-methylimidazolium tetrafluoroborate in water and in aqueous sodium fluoride. J Chem Eng Data 50:1484–1491
Babich I, Moulijn J (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 82:607
Chen X, Liu G, Yuan S, Asumana C, Wang W (2012) Extractive desulfurization of fuel oils with thiazolium based ionic liquids. Sep Sci Technol 47:819–826
Cun Z, Feng W, Xiao-yu P, Xiao-qin L (2011) Study of extraction-oxidation desulfurization of model oil by acidic ionic liquid. J Fuel Chem Technol 39:689–693
Dharaskar S (2012) The green solvents for petroleum and hydrocarbon industries. Res J Chem Sci 2(8):80–85
Dharaskar S, Wasewar K, Varma M, Shende D, Yoo C (2013a) Ionic liquids: the novel solvent for removal of dibenzothiophene from liquid fuel. Procedia Eng 51:314–317
Dharaskar S, Wasewar K, Varma M, Shende D, Yoo C (2013b) Deep removal of sulfur from model liquid fuels using 1-butyl-3-methylimidazolium chloride. Procedia Eng 51:416–422
Dharaskar S, Wasewar K, Varma M, Shende D, Yoo C (2013c) Synthesis, characterization and application of 1-butyl-3 methylimidazolium chloride as green material for extractive desulfurization of liquid fuel. Sci World J 2013:1–9
Dharaskar S, Wasewar K, Varma M, Shende D (2013d) Extractive deep desulfurization of liquid fuels using Lewis based ionic liquids. J Energy 2013:1–4
Dharaskar S, Wasewar K, Varma M, Shende D, Yoo C (2014a) Environmentally benign process for removal of sulfur from liquid fuel using imidazolium based ionic liquids. Res J Chem Environ 18:94–99
Dharaskar S, Wasewar K, Varma M, Shende D, Yoo C (2014b) Extractive desulfurization of liquid fuels by energy efficient green thiazolium based ionic liquids. Ind Eng Chem Res 53(51):19845–19854
Dharaskar S, Wasewar K, Varma M, Shende D, Tadi K, Yoo C (2014c) Synthesis, characterization and application of novel trihexyl tetradecyl phosphonium bis (2,4,4-trimethylpentyl) phosphinate for extractive desulfurization of liquid fuel. Fuel Process Technol 123:1–10
Dumeignil F, Sato K, Imamura M, Matsubayashi N, Payen E, Shimada H (2006) Characterization and hydrodesulfurization activity of CoMo catalysts supported on boron-doped sol-gel alumina. Appl Catal A 315:18–28
Esser J, Wasserscheid P, Jess A (2004) Deep desulfurization of oil refinery streams by extraction with ionic liquids. Green Chem 6:316–322
Fan Z, Wang T, He Y (2009) Upgrading and viscosity reducing of heavy oils by [BMIM][AlCl4] ionic liquid. J Fuel Chem Technol 37(6):690–693
Faridbod F, Ganjali M, Norouzi P, Riahi S, Rashedi H. Application of room temperature ionic liquids in electrochemical sensors and biosensors, ionic liquids: applications and perspectives, Prof. Alexander Kokorin (Ed.), ISBN: 978-953-307-248-7, InTech 2011, China
Ferrari M, Maggi R, Delmon B, Grange P (2001) Influences of the hydrogen sulfide partial pressure and of a nitrogen compound on the hydro-deoxygenation activity of a CoMo/carbon. J Catal 198:47–55
Forsyth S, Prigle J, MacFarlane D (2004) Ionic liquids—an overview. Aust J Chem 57:113–119
Fraser K, MacFarlane D (2009) Phosphonium-based ionic liquids: an overview. Aust J Chem 62:309–321
Freire M, Carvalho P, Gardas R, Luis M, Santos B, Marrucho I, Coutinho J (2008) Solubility of water in tetradecyl trihexyl phosphonium based ionic liquids. J Chem Eng Data 53:2378–2385
Ge J, Zhou Y, Yang Y, Xue M (2011) Catalytic oxidative desulfurization of gasoline using ionic liquid emulsion system. Ind Eng Chem Res 50:13686–13692
Hagiwara R, Ito Y (2000) Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J Fluor Chem 105:221–227
Isao M, Choi K (2004) An overview of hydrodesulfurization and hydrodenitrogenation. J Jpn Pet Inst 47:145–163
Jeon Y, Sung J, Kim D, Seo C, Cheong H, Ouchi Y, Ozawa R, Hamaguchi H (2008) Structural change of 1-butyl-3-methylimidazolium tetrafluoroborate þ water mixtures studied by infrared vibrational spectroscopy. J Phys Chem B 112:923–928
Jiang X, Nie Y, Li X, Wang Z (2008) Imidazolium based alkylphosphate ionic liquids—a potential solvent for extractive desulfurization of fuel. Fuel 87(1):79–84
Kanai K, Nishi T, Iwahashi T, Ouchi Y, Seki K, Harada Y, Shin S (2009) Electronic structures of imidazolium-based ionic liquids. J Electron Spectrosc Relat Phenom 174:110–115
Liu D, Gui J, Song L, Zhang X, Sun Z (2008) Deep desulfurization of diesel fuel by extraction with task specific ionic liquids. Petr Sci Technol 26:973–982
Lu H, Deng C, Ren W, Yang X (2014) Oxidative desulfurization of model diesel using [(C4H9)4N]6Mo7O24 as a catalyst in ionic liquids. Fuel Process Technol 119:87–91
Ma X, Sakanishi K, Mochida I (1994) Hydrodesulfurization reactivities of various sulfur compounds in diesel fuel. Ind Eng Chem Res 33:218–222
Ma C, Liu T, Yang L, Zu Y, Wang S, Zhang R (2011) Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal Chim Acta 689:110–116
Mochizuki Y, Sugawara K (2008) Removal of organic sulfur from hydrocarbon resources using ionic liquids. Energy Fuel 22:3303–3307
MuMurry JJ (1992) Organic chemistry, 3rd edn. Brooks/Cole Publishing Company, Belmont
Nie Y, Li C, Sun A, Meng H, Wang Z (2006) Extractive desulfurization of gasoline using imidazolium based phosphoric ionic liquids. Energy Fuel 20:2083–2087
Pawelec B, Mariscal R, Fierro J, Greenwood A, Vasudevan P (2001) Carbon-supported tungsten and nickel catalysts for hydrodesulfurization and hydrogenation reactions. Appl Catal A 206:295–307
Seddon K, Stark A, Torres M (2000) Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl Chem 72:2275–2285
Sun X, Jin Z, Yang L, Hao J, Zu Y, Wang W, Liu W (2013) Ultrasonic-assisted extraction of procyanidins using ionic liquid solution from Larix gmelinii bark. J Chem 2013:1–9
Swatloski R, Holbrey J, Rogers R (2003) Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem 5:361–363
Tropsoe H, Gate B (1997) Reactivities in deep catalytic hydrodesulfurization: challenges, opportunities, and the importance of 4-methyldibenzothiophene and 4,6-dimethyldibenzothiophene. Polyhedron 16:3213–3217
Wasewar K (2013) Low sulfur liquid fuel by deep desulfurization using ionic liquids. J Future Eng Technol 1:8–14
Yang L, Wang H, Zu Y, Zhao C, Zhang L, Chen X, Zhang Z (2011) Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem Eng J 172:705–712
Zhang S, Zhang Z (2002) Novel properties of ionic liquids in selective sulfur removal from fuels at room temperature. Green Chem 4:376–379
Zhang S, Zhang Q, Zhang Z (2004) Extractive desulfurization and denitrogenation of fuels using ionic liquids. Ind Eng Chem Res 43:614–622
Zhao H (2006) Innovative applications of ionic liquids as “green” engineering liquids. Chem Eng Commun 193:1660–1677
Acknowledgments
The authors gratefully acknowledge the financial support by the Council of Scientific Industrial Research (CSIR), grant number (22(0492)/09/EMR-II), and the Government of India (principal investigator: Dr. Kailas L. Wasewar).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Santiago V. Luis
Highlights
• [BMIM]SCN was synthesized and employed as extractant for S-removal.
• DBT containing model fuel in [BMIM]SCN could reach 86.5 % of S-removal with mass ratio 1:1 at 30 °C in 30 min., which was the remarkable enrichment of EDS process over HDS.
• [BMIM]SCN could be reused without regeneration more than five times with a slight decrease in activity.
• The EDS process could be an option for environmentally benign method for deep desulfurization.
Rights and permissions
About this article
Cite this article
Dharaskar, S.A., Wasewar, K.L., Varma, M.N. et al. Synthesis, characterization, and application of 1-butyl-3-methylimidazolium thiocyanate for extractive desulfurization of liquid fuel. Environ Sci Pollut Res 23, 9284–9294 (2016). https://doi.org/10.1007/s11356-015-4945-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4945-1