Abstract
The cytotoxicity and genotoxicity of abamectin, chlorfenapyr, and imidacloprid have been evaluated on the Chinese hamster ovary (CHOK1) cells. Neutral red incorporation (NRI), total cellular protein content (TCP), and methyl tetrazolium (MTT) assays were followed to estimate the mid-point cytotoxicity values, NRI50, TCP50, and MTT50, respectively. The effects of the sublethal concentration (NRI25) on glutathione S-transferase (GST), glutathione reductase (GRD), glutathione peroxidase (GPX), and total glutathione content have been evaluated in the presence and absence of reduced glutathione (GSH), vitamin C, and vitamin E. The genotoxicity was evaluated using chromosomal aberrations (CA), micronucleus (MN) formation, and DNA fragmentation techniques in the presence and absence of the metabolic activation system, S9 mix. Abamectin was the most cytotoxic pesticide followed by chlorfenapyr, while imidacloprid was the least cytotoxic one. The glutathione redox cycle components were altered by the tested pesticides in the absence and presence of the tested antioxidants. The results of genotoxicity indicate that abamectin, chlorfenapyr, and imidacloprid have potential genotoxic effects on CHOK1 cells under the experimental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive and/or improper uses of pesticides cause pollution of water, air, soil, and food. The environmental pollution with pesticides results in detrimental effects on non-target organisms (Gilden et al. 2010). Pesticide production workers, applicators, and farmers are the main risk groups of high-dose exposure; however, the common population is exposed to lower doses via the contaminated food and water or during the household use. It was documented that the exposure to low doses of pesticides produces various biochemical alterations leading to adverse health effects in the exposed organisms (Banerjee et al. 1999; Al-Sarar et al. 2009, 2014).
The pesticides tested in the present study are systemic and belong to three new classes with different modes of action. Abamectin, a macrocyclic lactone derived from the soil microorganism Streptomyces avermitilis, is a mixture of B1a and B1b avermectins (Meister 1992). In agriculture, abamectin is used as a systemic acaricide and insecticide worldwide; it is also used as a parasiticide for the lung worm and nasal bots and against gastrointestinal nematodes in cattle and sheep (Elbetieha and Da’as 2003; Novelli et al. 2012). Its mode of action is associated with its effect on the ɣ-aminobutyric acid (GABA) receptors and glutamate-gated chloride channels increasing the permeability of chloride ions, hyperpolarizing the nerve and muscle cells, and disturbing the neuromuscular transmission leading to death (Cully et al. 1994).
Chlorfenapyr, a novel N-substituted halogenated pyrrole, is a wide-spectrum insecticide with an acaricidal activity (Hunt and Treacy 1998). Chlorfenapyr is a proinsecticide activated by the in vivo oxidative elimination of its N-ethoxymethyl group by mixed function oxidases (Black et al. 1994). Chlorfenapyr inhibits the production of mitochondrial ATP via uncoupling the mitochondrial oxidative phosphorylations, interrupting the conversion of ADP to ATP, which might generate reactive oxygen radicals. Although chlorfenapyr is classified as slightly hazardous insecticide as per WHO (Tomlin 2000), it was banned in Europe because of its high persistence in the environment (van Leeuwen et al. 2004).
Imidacloprid is a neonicotinoid insecticide. In 2010, the world production of imidacloprid was estimated to be ca. 20,000 t; it was the world’s largest selling insecticide in 120 countries with registered uses for more than 140 crops (Pollack 2011; Jeschke et al. 2011; Simon-Delso et al. 2015). Imidacloprid acts as a selective agonist of the nicotinic acetylcholine receptor subtypes in insects; it is classified as a toxicity class II agent (EPA 1994; WHO 2002) with low mammalian toxicity (Tomizawa and Casida 2003). So, it is widely used in animal health and crop protection with soil, seed, and foliar applications (Tomizawa and Casida 2005; Costa et al. 2009). However, imidacloprid was found to induce DNA damage in a dose-related manner in earthworms as well as to increase the frequency of adducts in calf thymus DNA, indicating agent-induced genotoxicity (Shah et al. 1997; Zang et al. 2000).
In vitro toxicity tests, using animal cell cultures, are useful tools in the screening of environmental contaminants, risk assessment, and safety evaluation in a rapid and cost-effective way (Spielmann and Goldberg 1999; Bols et al. 2005). Many cellular endpoints have been utilized as biomarkers such as cellular lysosomes via the neutral red incorporation (NRI) assay, changes in total cellular protein (TCP) content, and mitochondrial function through methyl tetrazolium (MTT) assay (Saito et al. 1991; Wataha et al. 1991; Bertheussen et al. 1997).
The glutathione redox cycle components, glutathione S-transferase (GST), glutathione reductase (GRD), glutathione peroxidase (GPX), and glutathione, are involved in the cellular detoxification of xenobiotics; the oxidative stress of pesticides has been reported (Bayoumi et al. 2000, 2001; Garcia-Fernandez et al. 2002), and the role of GSH, vitamin C, and vitamin E in the protection of cells and tissues against the oxidative stress of xenobiotics was documented (Aly et al. 2010; Elsharkawy et al. 2013).
The mutagenic and genotoxic behaviors of several pesticides have been studied both in vivo and in vitro using cytogenetic endpoints such as chromosomal aberrations (CA), cytokinesis-block micronuclei (CBMN), sister chromatid exchange (SCE), and DNA fragmentation assays (Soloneski et al. 2008; Soloneski and Larramendy 2010; Calderón-Segura et al. 2012; Pandey and Guo 2014).
The increasing use of abamectin, chlorfenapyr, and imidacloprid and the presence of their residues in fruits, vegetables, and milk (Dakova 2005; Arias et al. 2014) demand detailed studies to evaluate their potential toxic risks to non-target organisms, particularly mammals. The present study was conducted to evaluate the in vitro cytotoxicity and genotoxicity of abamectin, chlorfenapyr, and imidacloprid using the Chinese hamster ovary (CHOK1) cells. We employed three cytotoxicity assays: neutral red incorporation (NRI), total cellular protein (TCP) content, and methyl tetrazolium (MTT) assays. Furthermore, the effects of the sublethal cytotoxicity value (NRI25) on GST, GRD, GPX, and total cellular glutathione content were evaluated in the presence and absence of GSH, vitamin C, and vitamin E. The genotoxicity assays CA, CBMN, and DNA fragmentation were employed as different cytogenetic endpoints to evaluate the genotoxic effects exerted by the tested pesticides.
Materials and methods
Chemicals
All reagent-grade chemicals and cell culture components were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal calf serum (FCS) was obtained from Boehringer Ingelheim GmbH (Germany). The pesticides abamectin, chlorfenapyr, and imidacloprid were purchased with purity of 98 % from Dr. Ehrenstorfer-Schafers (Augsburg, Germany).
Cell culture
CHOK1 cells were purchased from Cell Line Service Company (Eppelheim, Germany) and cultured in humidified atmosphere of 5 % CO2 at 37 °C. Culture medium (Ham F-12) was supplemented with HEPES buffer (25 mM, pH 7.4), 10 % heat-inactivated FCS, 100 μg/ml streptomycin, and 100 U/ml penicillin. The cells were counted by an improved Neubauer hemocytometer, and cell viability was checked by trypan blue dye exclusion (Freshney 1987).
Pesticide treatments
The cytotoxicity of the tested compounds was determined after 24- and 48-h exposure times in a serum-free medium or in a medium supplemented with 10 % FCS. Stock solutions of pesticides (10 mM) were prepared in sterilized dimethyl sulfoxide (DMSO), and a series of ten concentrations (0.97–500 μM) were freshly prepared. Cells were allowed to reach 65 % confluence before being pulsed with indicated concentrations.
Cytotoxicity assays
Neutral red incorporation assay
According to Borenfreund and Puerner (1985), 200-μl medium containing 65 % confluence of CHOK1 cells was added to 96-well tissue culture plates and incubated for 24 h. The medium was replaced with pesticide containing medium, serum-free, or supplemented with 10 % FCS. After 24 and 48 h, the medium was substituted with a medium containing 40 μg/ml NR dye, which had been prepared and preincubated overnight at 37 °C. Three hours after incubation, the cells were washed twice with 0.5 % formaldehyde in 1 % CaCl2. To extract the dye from the cells, 200 μl acetic acid (1 %) in 50 % ethanol was added. After 20 min of agitation, the absorbance was measured at 570 nm using microplate reader (Dialab model ELx 800, Wiener Neudorf, Austria).
Total cellular protein content assay
TCP content was measured according to the method of Pelletier et al. (1988). After 24- and 48-h exposure times, the wells were washed with 200 μl of phosphate-buffered saline (PBS) and fixed with 200 μl of 10 % v/v formaldehyde in PBS. After 10 min, the cells were washed with 200 μl of borate buffer (0.01 M, pH 8.4) and stained with 100 μl of methylene blue (1 % w/v in borate buffer). After 10 min, the dye was removed and the cells were washed five times with borate buffer. The stain was solubilized with 200 μl of 0.1 N HCl, and plates were shaken for 15 min. The optical density was measured at 660 nm.
Methyl tetrazolium assay
This assay was performed according to the method of Plumb et al. (1989) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye. After 24- and 48-h exposure times, the medium was removed and 50 μl of 5 mg/ml MTT dye in PBS was added. Plates were incubated at 37 °C for 4 h; the dye solution was removed, and 200 μl of DMSO was added followed by 25 μl Sorensen’s glycine buffer (0.1 M glycine and 0.1 M NaCl, pH 10.5). After 15 min of agitation, the optical density was measured at 570 nm.
Determination of glutathione redox cycle parameters
Treatment and preparation of cellular extracts
Cells were plated (104 cells/cm2) in tissue culture Petri dishes. On the second day, the medium was replaced with serum-free medium containing the antioxidants, GSH (1 mM), vitamin C (70 μM), and vitamin E (30 μM). After 24 h, an aliquot of serum-free medium containing the tested concentration (NRI25) was added. After 24 h, the cells were trypsinized using 500 μl of a 0.25 % trypsin/EDTA solution in sterilized PBS. Cells were washed twice via centrifugation at 990 g for 5 min at 4 °C using sterilized PBS (Bayoumi 1998). To determine the enzymatic activities, the cells were resuspended in 500 μl of a sucrose solution (0.25 M) containing 1 mM EDTA. Finally, the cell suspension was sonicated for three intervals of 10 s separated by 10 s in the ice bath. The suspension was recentrifuged for 15 min at 13,680 g, and the supernatant was used as the enzyme extract. For determination of the total glutathione content, the same procedure was followed after suspending the cells in 500 μl perchloric acid (1 M) containing EDTA (2 mM). The cell suspension was sonicated and recentrifuged as mentioned above; the supernatant (100 μl) was used in the assay. Before determining the glutathione levels, all samples were neutralized to pH 7 using KOH (2 M) and MOPS buffer (0.3 M), according to Bayoumi et al. (2001).
Assessment of the enzymatic activity and glutathione content
Glutathione S-transferases
This assay was performed according to Habig et al. (1974). The reaction mixture consisted of 800 μl phosphate buffer (0.2 M, pH 6.5), 50 μl of GSH solution (20 mM), 50 μl of 1-chloro-2,4-dinitrobenzene solution (20 mM), and 100 μl of the enzyme extract. Absorbance was recorded for 3 min at 340 nm.
Glutathione reductase
The activity of GRD was determined according Carlberg and Mannervik (1985). The reaction mixture contained 500 μl phosphate buffer (0.2 M, pH 7.0), 50 μl β-NADPH, (2 mM) in Tris-HCl (10 mM, pH 7.0), 50 μl oxidized glutathione (20 mM), 300 μl redistilled water, and 100 μl of the enzyme extract. The reaction mixture was incubated for 3 min at 30 °C; the absorbance was measured for 3 min at 340 nm.
Glutathione peroxidase
GPX activity was determined according to Wendel (1981). The reaction mixture composed of 500 μl phosphate buffer, (0.25 M, pH 7.0), 100 μl GRD, 100 μl GSH (10 mM), 100 μl β-NADPH (2.5 mM in 0.1 % NaHCO3), and 100 μl of the enzyme extract. After incubation for 10 min at 37 °C, 100 μl of tert-butyl hydroperoxide (12 mM) was added. The variation in the absorbance was recorded at 366 nm for 3 min.
Total glutathione content
The method of Akerboom and Sies (1981) was followed. The following reagents were added to a quartz cuvette: 900 μl phosphate buffer (0.1 M) with EDTA (1 mM), 200 μl of the cellular extract, 50 μl NADPH (4 mg/ml in 0.5 % NaHCO3), 20 μl of DTNB (1.5 mg/ml in 0.5 % NaHCO3), and 20 μl of GRD. The variation of absorbance was measured at 412 nm after 3 min.
Total protein determination
The microassay procedure was performed according to the method described by Bradford (1976) using bovine serum albumin as standard.
Chromosome aberration assay
CHOK1 cells were seeded in 60-mm plates at 4 × 104 cells/dish. The cultures were treated at the NRI25 values for 24 h in the absence and presence of the S9 fraction. Methyl methanesulfonate (MMS, 40 μg/ml) and benzo[a]pyrene (BP, 2 μg/ml) were the positive controls in the absence and presence of S9 fraction, respectively. The chromosomes were prepared according to the conventional method (IAE 1986). Three hours before harvesting the culture, cells were treated with colchicine (0.2 μg/ml). The cells were trypsinized and collected by centrifugation; then, the hypotonic solution (0.075 M KCl at 37 °C for 17 min) was added, followed by fixation in methanol/acetic acid (3:1). The suspension of cells was dropped onto cold glass microscope slides and air-dried. The slides were stained with 5 % Giemsa in distilled water for 10 min and scored for structural chromosome aberrations. One hundred metaphases were examined for each treatment.
Cytokinesis-block micronucleus assay
The CBMN test (Fenech 1993) was used to detect the genotoxic effects of abamectin, chlorfenapyr, and imidacloprid. CHOK1 cells were seeded in cell culture dishes (60 mm) at 4 × 104 cells/dish and allowed 24 h to establish normal growth. The cells were treated for 24 h with the NRI25 concentrations and were cultured with cytochalasin-B (4.5 μg/ml) for 24 h before trypsinization, centrifugation, and resuspension in KCl (0.075 M) at 37 °C for 5 min. The cells were then fixed three times in methanol/glacial acetic acid (3:1). Subsequently, the cell solution was dropped onto precleaned slides; after air-drying, the slides were stained by 5 % Giemsa for 10 min. The slides were analyzed under light microscope at × 600 magnification. The number of binucleated cells containing one, two, or more micronucleus (MN) was scored in cytokinesis-blocked cells based on the observation of 500 cells per slide.
Evaluation of DNA fragmentation
The cell cultures were exposed to the NRI25 values for 24 h, followed by trypsinization and centrifugation at 1200 × g for 10 min to harvest the cells. The DNA was extracted and purified using DNA extraction kit (Wizard® Genomic DNA Purification Kit, Promega Corporation, Madison, USA). The quality and quantity of DNA were assessed by Nanodrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The DNA samples were analyzed on 1.1 % agarose gels containing ethidium bromide. The gels were visualized with a long wave transilluminator and photographed.
Statistical analysis
For estimation of cytotoxicity values, NRI25, NRI50, TCP50, and MTT50, the absorbance values were correlated to the percentages of cell mortality by plotting the toxicity regression lines in the form of a log/probit relationship according to the method of Bayoumi (1998) using Sigma Plot® Version 2.0 software (Systat Software Inc., CA, USA). The statistical analysis of cytotoxicity, chromosome aberrations and MN results was performed by the Student’s t test. For effects of treatments on glutathione redox cycle components, analysis of variance (ANOVA) and the Newman-Keuls test were used to determine significant differences between the treatment groups using the statistical program (CoStat 2®, CoHort Software, Monterey, USA).
Results
Cytotoxicity
The median lethal cytotoxicity values, NRI50, TCP50, and MTT50, for abamectin, chlorfenapyr, and imidacloprid are shown in Table 1. The NRI assay showed that abamectin after 24 and 48 h was the most cytotoxic pesticide followed by chlorfenapyr and imidacloprid; the cytotoxicity was time-dependent. The FCS caused a significant increase in the NRI50 values of abamectin and chlorfenapyr calculated by 5.2- and 2.4-fold after 24 h and 5.5- and 1.9-fold after 48 h, respectively. The TCP50 values showed that abamectin was the most cytotoxic pesticide followed by chlorfenapyr and imidacloprid. The presence of FCS reduced the cytotoxicity of abamectin by 12.2- and 14-fold after 24 and 48 h, respectively; the TCP50 values of chlorfenapyr increased by 2.9- and 2.1-fold after 24 and 48 h, respectively. The MTT50 values showed that abamectin was significantly the most cytotoxic pesticide followed by chlorfenapyr and imidacloprid. Abamectin was more toxic than chlorfenapyr by 35.8- and 661-fold and more toxic than imidacloprid by 297.1- and 5513-fold after 24 and 48 h, respectively.
Effects on the glutathione redox cycle components
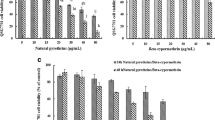
The effects of the sublethal concentration (NRI25) of abamectin, chlorfenapyr, and imidacloprid on the glutathione redox cycle components, after 24 h, in the absence and presence of GSH, vitamin C, and vitamin E are illustrated in Table 2 and Fig. 1. For the effect of antioxidants on the untreated cells, the extracellular GSH caused significant elevations in the activities of GST (26.8 %), GRD (93.4 %), and intracellular total glutathione content (34.7 %) compared with the antioxidant-free control. In addition, vitamins C and E caused significant inductions by 175.4 and 94.96 % in GRD activity and 26.1 and 29.2 % in GPX activity, respectively, compared with the antioxidant-free control.
Effect of imidacloprid (A), chlorfenapyr (B), and abamectin (C) on the glutathione redox cycle components, GST, GRD, GPX, and GSH content, in CHOK1 cells in absence and presence of the tested antioxidants, GSH, vitamin C, and vitamin E. *Significantly different relative to controls at the 0.01 level. GST glutathione S-transferase, GRD glutathione reductase, GPX glutathione peroxidase, GSH reduced glutathione, Vit. C vitamin C, Vit. E vitamin E
The three pesticides caused a significant inhibition in the activity of GST in the antioxidant-free and GSH-treated cells compared with the antioxidant-free or GSH-treated control cells. Abamectin and imidacloprid significantly inhibited the activity of GST in vitamin C-treated cells compared with the corresponding control. In addition, the specific activity of GST significantly increased in the presence of vitamin E in the treatment of abamectin compared with antioxidant-free/abamectin-treated cells.
Abamectin and imidacloprid significantly inhibited the activity of GRD in the antioxidant-free and antioxidant-treated cells compared with their corresponding controls; chlorfenapyr significantly inhibited the GRD activity in the presence of vitamins C and E compared with their corresponding controls. A significant induction in the GRD activity was observed in abamectin-treated cells in the presence of GSH (95.9 %), vitamin C (72.4 %), and vitamin E (51.4 %) compared with the antioxidant-free/abamectin-treated cells. GSH and vitamin E significantly increased the activity of GRD in the chlorfenapyr-treated cells by 87.1 and 33.3 %, respectively, compared with the antioxidant-free/chlorfenapyr-treated cells. In the presence of vitamin E, imidacloprid induced the GRD activity by 118.6 % compared with the antioxidant-free/imidacloprid-treated cells.
All tested pesticides caused significant decreases in the activity of GPX in the absence and presence of all tested antioxidants compared with their parallel controls. In the imidacloprid treatments, presence of GSH, vitamin C, and vitamin E caused inductions in the GPX activity by 131.1, 130.3, and 168.7 % compared with the antioxidant-free treatment, respectively. In addition, presence of vitamin E in the abamectin treatment increased the GPX activity by 48.3 % over the antioxidant-free/abamectin-treated cells.
Abamectin and chlorfenapyr significantly increased the glutathione content by 22.2 and 34.7 %, respectively, compared with the antioxidant-free control. Moreover, abamectin and chlorfenapyr caused a significant elevation in the glutathione content in the presence of vitamin E compared with the corresponding control. The presence of vitamin C in abamectin and chlorfenapyr treatments significantly reduced the glutathione content compared with antioxidant-free/abamectin- and chlorfenapyr-treated cells, respectively, reducing its level to that of the control. No significant differences were observed between all imidacloprid treatments and their controls.
Chromosome aberrations
The effects of abamectin, chlorfenapyr, and imidacloprid at the sublethal concentrations (NRI25) on the chromosome aberrations were evaluated in the CHOK1 cells in the presence and absence of S9 mix (Table 3). Abamectin and chlorfenapyr significantly (p < 0.05) induced the total CA compared with the control in the absence and presence of S9 mix. Chromosome and chromatid gaps were the most obvious aberrations in all affected cells. Imidacloprid did not exert any significant (p > 0.05) CA in the CHOK1 cells in the absence and presence of S9 mix.
Induction of micronucleus
The results of pesticide-induced micronuclei in binucleated cytokinesis-blocked CHOK1 cells are shown in Table 4. There were significant increases (p < 0.01) in the frequencies of micronuclei in the positive controls, MMC-, or BP-treated cells, compared with the control cells. Abamectin, chlorfenapyr, and imidacloprid significantly induced the frequencies of micronuclei in the absence of S9 mix (p < 0.05). In the presence of S9 mix, a highly significant induction (p < 0.01) in the micronuclei frequencies was observed after exposure to abamectin and chlorfenapyr, while imidacloprid treatment resulted in a significant induction (p < 0.05). Cells with one MN were predominant in the binucleated cells. No cells with more than two micronuclei were observed in all treatments.
DNA strand breakage
Using the DNA laddering technique, a single band was obtained in the treated CHOK1 cells for the three pesticides (Fig. 2). This single band is a characteristic of intact genomic DNA. So, no apparent DNA breakage was observed in abamectin, chlorfenapyr, and imidacloprid-treated CHOK1 cells using this technique.
Discussion
The present study was planned to assess the cytotoxic and genotoxic effects of three systemic insecticides, abamectin, chlorfenapyr, and imidacloprid, using in vitro-cultured CHOK1 cells via ten different endpoints. In addition, the ameliorative effect of the antioxidants, GSH and vitamins C and E, on the glutathione redox cycle components was evaluated. The results indicate that abamectin was the most cytotoxic pesticide in all cytotoxicity assays, followed by chlorfenapyr and imidacloprid. Maioli et al. (2013) reported a cytotoxic effect of abamectin on isolated rat hepatocytes. Abamectin is a mixture of B1a and B1b avermectins (Meister 1992). Avermectin exerted in vivo and in vitro cytotoxic actions in hepatocytes and brain neurons of King pigeon (Chen et al. 2013; Zhu et al. 2013). Ivermectin, a member of the avermectin family, and its commercial formulation Ivomec® originated cytotoxic effects in CHOK1 cells (Molinari et al. 2009). The present results showed that the MTT was the most sensitive tested bioassay for assessing the cytotoxicity of abamectin. The MTT assay is an indicator for the mitochondrial function. In accordance with our results, Maioli et al. (2013) reported that abamectin inhibited the mitochondrial activity in the rat hepatocytes, decreasing ATP synthesis, leading to the cell death. Little metabolism of avermectin has been observed in animals, and up to 80–98 % of the parent compound was found in the feces (Sun et al. 2005). Maioli et al. (2013) proved that abamectin, not its metabolites, is responsible for the cytotoxic effect on isolated hepatocytes. The ultrastructural examination showed swollen mitochondria and unclear structure of the inner membranes in abamectin-treated cells from Spodoptera frugiperda (Sf9) (Huang et al. 2011). The authors supposed that mitochondria may be involved in the apoptosis initiation effect of abamectin. Our results indicate that abamectin cytotoxicity was time-dependent, particularly in the MTT assay (p < 0.001). In agreement with our findings, Huang et al. (2011) reported that abamectin decreased the cell viability in a time-dependent manner in Sf9 cell line. Chlorfenapyr exhibited cytotoxic effects in the three assays. Chlorfenapyr is a pyrrole proinsecticide; it is activated by oxidative elimination of N-ethoxymethyl group, which inhibits mitochondrial ATP production through uncoupling of the mitochondrial oxidative phosphorylation that might produce reactive oxygen radicals (Black et al. 1994). Studies on the cytotoxicity of chlorfenapyr are very limited. Chlorfenapyr caused a significant inhibition in the cell growth and a marked decrease in the ATP concentration in Sf9 cells (Saito 2005; Saito et al. 2005). Aljabr et al. (2014) reported a cytotoxic action of chlorfenapyr against the midgut cell culture (RPW-1) from the red palm weevil Rhynchophorus ferrugineus. Our results showed that imidacloprid is less cytotoxic to the CHOK1 cells compared with abamectin and chlorfenapyr, but it still displays a cytotoxic action to some extent, especially with the MTT assay. Su et al. (2007) showed that imidacloprid is able to inhibit the growth of flounder gill (FG) cell culture causing severe injury to the mitochondria. They suggested that the mitochondria are probably the primary target of imidacloprid.
The presence of FCS in the culture medium significantly reduced the cytotoxicity of abamectin and chlorfenapyr in the NRI and TCP assays, indicating the important effect of the protein binding on the availability of these compounds to reach their targets. On contrary, FCS increased the cytotoxicity of imidacloprid, indicating the toxifying interaction between FCS and imidacloprid. Freshney (1987) showed that FCS is a complex mixture containing different protein molecules, which may bind with the tested substance and reduce its bioavailability and penetration to the target cells; however, both reduction and enhancement of pesticide cytotoxicity by binding proteins were reported (Sogorb et al. 2002; Gülden et al. 2003).
The glutathione redox cycle is playing a vital role in protecting cells from oxidative injury (Meister 1983). Alteration in the activity of glutathione redox cycle components is affected by the chemical structure and concentrations of pesticides that cells are exposed to (EL-Shenawy 2010). The present results indicate that abamectin and imidacloprid significantly reduced the GRD activity in the presence and absence of antioxidants compared with the control. However, all tested antioxidants in abamectin treatment and vitamin E in imidacloprid treatment ameliorated the GRD activity to levels over that of the antioxidant-free control. Abdollahi et al. (2004) observed a reduction of GRD activity in pesticide workers and explained this reduction by the implication of this enzyme in regeneration of GSH. GRD is an important enzyme that reduces GSSG to its sulfhydryl form, GSH, which is crucial for cell proliferation, viability, and protection from oxidative damage (Tandoğan and Ulusu 2006). Inhibition of GRD disturbs cellular prooxidant/antioxidant steadiness and may contribute to the genesis of several diseases (Tandoğan and Ulusu 2006). Significant inhibition of GPX activity was observed with all tested pesticides in the absence or presence of tested antioxidants compared with their corresponding controls. However, the tested antioxidants significantly increased the activity of GPX in imidacloprid-treated cells compared with the antioxidant-free/imidacloprid-treated cells. Also, vitamin E ameliorated the abamectin-reducing effect on GPX activity. GPX is one of the most important enzymes of the cell antioxidant defense system; it catalyzes the GSH-dependent reduction of H2O2 and other peroxides (Pigeolet et al. 1990; Lei 2002). Reduced levels of GPX activity following pesticide exposure have been reported in the neuronal (SH-SY5Y) cells (Jia and Misra 2007). All the tested pesticides caused a significant inhibition of GST activity in the presence and absence of GSH compared with their corresponding controls. Abamectin and imidacloprid significantly inhibited the GST activity in the presence of vitamin C compared with the parallel controls. With all tested pesticides, the ameliorative effect of vitamin E was clear; it increased the GST activity to levels close to that of antioxidant-free control. GST plays a key role in the cellular detoxification of endogenous and xenobiotic chemicals (Chronopouloua et al. 2012). Inhibition and induction of human and animal GST by pesticides have been reported (Ezemonye and Tongo 2010; Chronopouloua et al. 2012); the differential alteration in the GST activity is pesticide- and tissue-specific (Ezemonye and Tongo 2010). Abamectin and chlorfenapyr significantly increased the glutathione content; the presence of vitamin C ameliorated this effect. The induction of the glutathione content was also observed by Barlow et al. (2005) in the rat PC12 cells after exposure to the fungicide, maneb; the authors attributed this effect to the increase of ɣ-glutamyl cysteine synthetase, the enzyme involved in the production of GSH precursor. Glutathione is a critical constituent of the cellular antioxidant defense system, and its reduced form is the predominant reducing agent in animal cells (Sebastià et al. 2003; Wu et al. 2004). Alterations of the glutathione status by toxic agents cause oxidative stress leading to mammalian cytotoxicity (Vettori et al. 2005). Therefore, the in vitro studies with mammalian cells often depend on the cellular glutathione status as an early biomarker of cytotoxicity (Schoonen et al. 2005). Antioxidants differ in their binding site inside the organism as well as in their affinity to the free radicals; for example, GSH and vitamin C are powerful scavengers of reactive oxygen species (ROS), particularly in aqueous medium. In contrast, vitamin E functions principally in the membrane lipid bilayers (Inoue 2001). Therefore, no common effective antioxidant is found as a universal treatment (Matkovics 2001)
The present results revealed a significant induction of CA and MN frequencies in CHOK1 cells exposed 24 h to abamectin. On the other hand, no DNA damage was observed for the same treatment. Abamectin induced single-strand DNA breaks in the rat hepatocytes (EPA 1989); there are rare studies on the genotoxicity of abamectin. Molinari et al. (2009, 2013) found that ivermectin did not induce SCE in the CHOK1 cells; however, it induced single-DNA-strand breaks. Ivermectin showed genotoxic effects on the somatic cells of the mother Wister rats and higher MN frequencies in the red blood cells from the embryos’ umbilical cord compared with the control (El-Ashmawy et al. 2011). Chlorfenapyr caused significant inductions of CA and MN in the presence and absence of S9 mix. Studies on the genotoxicity of CPF are very limited. The induction of stress protein genes in cultured cells of cabbage armyworm Mamestra brassicae treated with chlorfenapyr was concentration- and time-dependent (Sonoda and Tsumuki 2007); the authors attributed this induction to defend the cells from the reactive oxygen radicals. The present results showed that imidacloprid did not induce CA or DNA damage; however, a significant induction of MN was detected in the absence and presence of S9 mix. On contrary, Stivaktakis et al. (2010) reported no significant changes in the MN frequencies in the imidacloprid-treated blood cells. Four neonicotinoid pesticides, including imidacloprid, significantly induced DNA damage when measured by the comet frequency and the tail length (Calderón-Segura et al. 2012). Admire, the commercial product of imidacloprid, produced calf thymus DNA adducts upon activation by S9 mix (Shah et al. 1997). Exposure of the human peripheral blood lymphocytes to imidacloprid (0.05 to 0.5 mg/l) significantly increased the levels of SCE and MN and enhanced DNA strand breaks (Feng et al. 2005); however, imidacloprid (0.1 to 100 μg/ml) did not affect the frequency of SCE and MN formation (Demsia et al. 2007). In the presence and absence of S9 mix, imidacloprid (20 μM) significantly increased the frequency of MN in the peripheral blood lymphocytes and DNA strand breaks in the leukocytes (Costa et al. 2009). Recently, De Arcaute et al. (2014) demonstrated that imidacloprid can be considered a harmful agent with genotoxic effects at both DNA and chromosomal levels. The molecular mechanisms motivating the genotoxicity of the neonicotinoid insecticides are generally unknown. In vitro studies of Yao et al. (2006) have indicated that acetamiprid may induce ROS generation in bacteria. However, the incubation of imidacloprid with Yurkat cells and lymphocytes did not increase the production of ROS (Costa et al. 2009). Although these results are inconsistent, Valko et al. (2006) suggested that the neonicotinoid insecticides are direct genotoxic compounds that could act as a source of ROS or free radicals in the treated human cells.
Generally, the induction of genotoxicity may lead to further problems of mutagenic and carcinogenic activities (Valko et al. 2006). Previous epidemiological studies demonstrated a relationship between pesticide exposure and the occurrence of cancer (Dich et al. 1997). Therefore, the current awareness of the real or potential hazards of pesticides considering their cytotoxic/genotoxic actions cannot be neglected. Thus, further investigations are needed to gain a comprehensive and complete knowledge of the possible mechanisms through which abamectin, chlorfenapyr, and imidacloprid exert their cytotoxic/genotoxic effects.
References
Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaiee A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 10:RA14–RA147
Akerboom TPM, Sies H (1981) Assay of glutathione, glutathione disulfide and glutathione mixed disulfides in biological samples. Method Enzymol 77:373–382
Aljabr AM, Rizwan-ul-Haq M, Hussain A, Al-Mubarak AI, AL-Ayied HY (2014) Establishing midgut cell culture from Rhynchophorus ferrugineus (Olivier) and toxicity assessment against ten different insecticides. In Vitro Cell Dev Biol Anim 50(4):296–303
Al-Sarar AS, Abobakr Y, Al-Erimah GS, Hussein HI, Bayoumi AE (2009) Hematological and biochemical alterations in occupationally pesticides-exposed workers of Riyadh municipality, Kingdom of Saudi Arabia. Res J Environ Toxicol 3(4):179–185
Al-Sarar AS, Abobakr Y, Bayoumi AE, Hussein HI, Al-Ghothemi M (2014) Reproductive toxicity and histopathological changes induced by lambda-cyhalothrin in male mice. Environ Toxicol 29(7):750–762
Aly N, EL-Gendy K, Mahmoud F, El-Sebae A (2010) Protective effect of vitamin C against chlorpyrifos oxidative stress in male mice. Pestic Biochem Physiol 97(1):7–12
Arias LA, Bojacá CR, Ahumada DA, Schrevens E (2014) Monitoring of pesticide residues in tomato marketed in Bogota, Colombia. Food Control 35:213–217
Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK (1999) Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett 107(1-3):33–47
Barlow BK, Lee DW, Cory-Slechta DA, Opanashuk LA (2005) Modulation of antioxidant defense systemsby the environmental pesticide maneb in dopaminergic cells. Neurotoxicology 26:63–75
Bayoumi AE (1998) Utilization of the alternative methods in the toxicological evaluation of environmental contaminants. Dissertation, University of Leon, Spain
Bayoumi AE, Perez-Pertejo Y, Ordóñez C, Reguera RM, Balaña-Fouce R, Ordóñez D (2000) Changes in the glutathione-redox balance induced by the pesticides heptachlor, chlordane and toxaphene in CHO-K1 cells. Bull Environ Contam Toxicol 65:748–755
Bayoumi AE, Garcia-Fernandez AJ, Ordonez C, Perez-Pertejo Y, Cubria JC, Reguera RM, Balana-Fouce R, Ordonez D (2001) Cyclodiene organochlorine insecticide-induced alterations in the sulfur-redox cycle in CHO-K1 cells. Comp Biochem Physiol C Toxicol Pharmacol 130:315–323
Bertheussen K, Yousef MI, Figenschau Y (1997) A new sensitive cell culture test for the assessment of pesticide toxicity. J Environ Sci Health 32:195–211
Black BC, Hollingworth RM, Ahammadsahib KI, Kubkel CB, Donovan S (1994) Insecticidal action and mitochondrial uncoupling activity of AC-303, 603 and related halogenated pyrroles. Pestic Biochem Physiol 50:115–128
Bols N, Dayeh V, Lee L, Schirmer K (2005) Use of fish cell lines in the toxicology. In: Moon TW, Mommsen TP (eds) Biochemistry and molecular biology of fishes. Elsevier Science, Amsterdam, pp 43–84
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alteration and neutral red absorption. Toxicol Lett 24:119–124
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calderón-Segura ME, Gómez-Arroyo S, Villalobos-Pietrini R, Martínez-Valenzuela C, Carbajal-López Y, Calderón-Ezquerro MD, Cortés-Eslava J, García-Martínez R, Florñes-Ramírez D, Rodríguez-Romero MI, Méndez-Pérez P, Bañuelos-Ruíz E (2012) Evaluation of genotoxic and cytotoxic effects in human peripheral blood lymphocytes exposed in vitro to neonicotinoid insecticides news. J Toxicol. doi:10.1155/2012/612647
Carlberg Y, Mannervik B (1985) Glutathione reductase. Method Enzymol 113:484–490
Chen LJ, Sun BH, Qu JP, Xu S, Li S (2013) Avermectin induced inflammation damage in king pigeon brain. Chemosphere 93(10):2528–34
Chronopouloua EG, Papageorgiou AC, Markoglou A, Labrou NE (2012) Inhibition of human glutathione transferases by pesticides: development of a simple analytical assay for the quantification of pesticides in water. J Mol Catal B Enzym 81:43–51
Costa C, Silvari V, Melchini A, Catania S, Heffron JJ, Trovato A, De Pasquale R (2009) Genotoxicity of imidacloprid in relation to metabolic activation and composition of the commercial product. Mutat Res 672:40–44
Cully DF, Vassilatis DK, Liu KK, Paress PS, van der Ploeg LHT, Schaeffer JM, Arena JP (1994) Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371(6499):707–11
Dakova T (2005) Abamectin and closantel residues in milk from sheep treated with abantel-b. Trakia J Sci 3(5):17–19
De Arcaute CR, Pérez-Iglesiasa JM, Nikoloff N, Natale GS, Soloneski S, Larramendy ML (2014) Genotoxicity evaluation of the insecticide imidacloprid on circulating blood cells of Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae) by comet and micronucleus bioassays. Ecol Indic 45:632–639
Demsia G, Vlastos D, Goumenou M, Matthopoulos DP (2007) Assessment of the genotoxicity of imidacloprid and metalaxyl in cultured human lymphocytes and rat bone marrow. Mutat Res Genet Toxicol Environ Mutagen 634(1-2):32–39
Dich J, Zahm SH, Hanberg A, Adami HO (1997) Pesticides and cancer. Cancer Causes Control 8(3):420–443
El-Ashmawy IM, El-Nahas AF, Bayad AE (2011) Teratogenic and cytogenetic effects of ivermectin and its interaction with P-glycoprotein inhibitor. Res Vet Sci 90:116–123
Elbetieha A, Da’as SI (2003) Assessment of antifertility activities of abamectin pesticide in male rats. Ecotoxicol Environ Saf 55(3):307–313
Elsharkawy EE, Yahia D, El-Nisr NA (2013) Sub-chronic exposure to chlorpyrifos induces hematological, metabolic disorders and oxidative stress in rat: attenuation by glutathione. Environ Toxicol Pharmacol 35(2):218–227
El-Shenawy NS (2010) Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicol In Vitro 24(4):1148–57
EPA (1989) Avermectin (Agri-Mek, Affirm) pesticide fact sheet 9/89. Cornell University, Ithaca
EPA (1994) Office of Pesticide Programs, pesticide fact sheet: imidacloprid, Washington, DC
Ezemonye L, Tongo I (2010) Sublethal effects of endosulfan and diazinon pesticides on glutathione-S-transferase (GST) in various tissues of adult amphibians (Bufo regularis). Chemosphere 8:214–217
Fenech M (1993) The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect 101(3):101–107
Feng S, Kong Z, Wang X, Peng P, Zeng EY (2005) Assessing the genotoxicity of imidacloprid and RH-5849 in human peripheral blood lymphocytes in vitro with comet assay and cytogenetic tests. Ecotoxicol Environ Saf 61(2):239–246
Freshney RI (1987) Culture of animal cells. A manual of basic technique. Wielly Liss press, New York, pp 72–81
Garcia-Fernandez AJ, Bayoumi AE, Perez-Pertejo Y, Romeros D, Ordóñez C, Reguera RM, Balaña-Fouce R, Ordóñez D (2002) Changes in glutathione-redox balance induced by hexachlorocyclohexane and lindane in CHO-K1 cells. Xenobiotica 32(11):1007–1016
Gilden RC, Huffling K, Sattler B (2010) Pesticides and health risks. J Obstet Gynecol Neonatal Nurs 39:103–110
Gülden M, Mörchel S, Seibert H (2003) Serum albumin binding at cytotoxic concentrations of chemicals as determined with a cell proliferation assay. Toxicol Lett 137:159–168
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Huang JF, Tian M, Lv CJ, Li HY, Muhammad R, Zhong GH (2011) Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic Biochem Physiol 100(3):256–263
Hunt DA, Treacy MF (1998) Pyrrole insecticides: a new class of agriculturally important insecticides functioning as uncouplers of oxidative phosphorylation. In: Ishaaya I, Degheele D (eds) Insecticides with novel modes of action, mechanism and application. Springer Verlag, Heidelberg
IAE (1986) Biological dosimetry: chromosome aberration analysis for dose assessment, In: Technical reports series no. 260, International Atomic Energy Agency, Vienna, pp 59–63
Inoue M (2001) Protective mechanisms against reactive oxygen species. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Schachter D, Shafritz DA (eds) The liver: biology and pathobiology, 4th edn. Lippincott Williams & Wilkins, Philadelphia
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908
Jia ZH, Misra P (2007) Reactive oxygen species in in vitro pesticide-induced neuronal cell (SH-SY5Y) cytotoxicity: role of NFκB and caspase-3. Free Radic Biol Med 42:288–298
Lei XG (2002) In vivo antioxidant role of glutathione peroxidase: evidence from knockout mice. Methods Enzymol 347:213–225
Maioli MA, de Medeiros HCD, Guelfi M, Trinca V, Pereira FTV, Mingatto FE (2013) The role of mitochondria and biotransformation in abamectin-induced cytotoxicity in isolated rat hepatocytes. Toxicol in Vitro 27:570–579
Matkovics A (2001) Antioxidánsok 2000- elmélet és gyakorlat. Magy Belorv Arch (54):35–40
Meister A (1983) Selective modification of glutathione metabolism. Science 220:472–477
Meister RT (1992) Farm chemicals handbook 92. Meister Publishing Company, Willoughby
Molinari G, Soloneski S, Reigosa MA, Larramendy ML (2009) In vitro genotoxic and cytotoxic effects of ivermectin and its formulation ivome on Chinese hamster Ovary (CHO-K1) cells. J Hazard Mater 165:1074–1082
Molinari G, Kujawski M, Scuto A, Soloneski S, Larramendy ML (2013) DNA damage kinetics and apoptosis in ivermectin-treated Chinese hamster ovary cells. J Appl Toxicol 33:1260–1267
Novelli A, Vieira BH, Cordeiro D, Cappelini LTD, Vieira EM, Espíndola ELG (2012) Lethal effects of abamectin on the aquatic organisms Daphnia similis, Chironomus xanthus and Danio rerio. Chemosphere 86(1):36–40
Pandey MR, Guo H (2014) Evaluation of cytotoxicity, genotoxicity and embryotoxicity of insecticide propoxur using flounder gill (FG) cells and zebrafish embryos. Toxicol In Vitro 28:340–353
Pelletier B, Dhainaut F, Pauly A, Zahnd JP (1988) Evaluation of growth rate in adhering cell cultures using a simple colorimetric method. Biochem Biophys Methods 16:63–73
Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, Raes M, Zachary M, Remacle J (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51:283–297
Plumb JA, Milroy R, Kaye SB (1989) Effects of pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res 49:4435–4440
Pollack P (2011) Fine chemicals: the industry and the business, 2nd edn. Wiley, New Jersey
Saito S (2005) Effects of pyridalyl on ATP concentrations in cultured Sf9 cells. J Pestic Sci 30(4):403–405
Saito H, Iwami S, Shigeoka T (1991) In vitro cytotoxicity of 45 pesticides to goldfish GF-scale (GFS) cells. Chemosphere 23:525–537
Saito S, Sakamoto N, Umeda K (2005) Effect of pyridalyl, a novel insecticide agent on cultured Sf9 cells. J Pestic Sci 30(1):17–21
Schoonen WGEJ, Westerink WMA, de Roos JADM, Debiton E (2005) Cytotoxic effects of 100 reference compounds on Hep G2 and HeLa cells and of 60 compounds on ECC-1 and CHO cells. I. Mechanistic assays on ROS, glutathione depletion and calcein uptake. Toxicol In Vitro 19:505–516
Sebastià J, Cristòfol R, Martín M, Rodríguez-Farré E, Sanfeliu C (2003) Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry A 51:16–25
Shah RG, Lagueux J, Kapur S, Levallois P, Ayotte P, Tremblay M, Zee J, Poirier GG (1997) Determination of genotoxicity of the metabolites of the pesticides guthion, sencor, lorox, reglone, daconil and admire by 32P-post labeling. Mol Cell Biochem 169:177–184
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L et al (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res 22:5–34
Sogorb MA, Carrera V, Benabent M, Vilanova E (2002) Rabbit serum albumin hydrolyzes the carbamate carbaryl. Chem Res Toxicol 15:520–526
Soloneski S, Larramendy ML (2010) Sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary (CHO-K1) cells treated with the insecticide pirimicarb. J Hazard Mater 174(1-3):410–415
Soloneski S, Reigosa MA, Molinari G, González NV, Larramendy ML (2008) Genotoxic and cytotoxic effects of carbofuran and furadan® on Chinese hámster ovary (CHO-K1) cells. Mutat Res 656:68–73
Sonoda S, Tsumuki H (2007) Induction of heat shock protein genes by chlorfenapyr in cultured cells of the cabbage armyworm, Mamestra brassicae. Pestic Biochem Physiol 89:185–189
Spielmann H, Goldberg AM (1999) In vitro methods. In: Marquardt H, Schafer SG, McClellan R, Welsch F (eds) Toxicology. Academic press, USA, pp 113–138
Stivaktakis P, Vlastos D, Giannakopoulos E, Matthopoulos DP (2010) Differential micronuclei induction in human lymphocyte cultures by imidacloprid in the presence of potassium nitrate. Sci World J 10:80–89
Su F, Zhang S, Li H, Guo H (2007) In vitro acute cytotoxicity of neonicotinoid insecticide imidacloprid to gill cell line of flounder Paralichthy olivaceus. Chin J Oceanol Limnol 25(2):209–214
Sun Y, Diao X, Zhang Q, Shen J (2005) Bioaccumulation and elimination of avermectin B1a in the earthworms (Eisenia foetida). Chemosphere 60:699–704
Tandoğan B, Ulusu NN (2006) Kinetic mechanisms and molecular properties of glutathione reductase. FABAD J Pharm Sci 31:230–237
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48:339–364
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45:247–268
Tomlin CDS (2000) The pesticide manual, a world compendium, 12th edn. British Crop Protection Council, London
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress induced cancer. Chem Biol Interact 160(1):1–40
van Leeuwen T, Stillatus V, Tirry L (2004) Genetic analysis and cross-resistance spectrum of a laboratory-selected chlorfenapyr resistant strain of two-spotted spider mite (Acari: Tetranychidae). Exp Appl Acarol 32:249–261
Vettori MV, Caglieri A, Goldoni M, Castoldi AF, Dare E, Alinovi R, Ceccatelli S, Mutti A (2005) Analysis of oxidative stress in SK-N-MC neurons exposed to styrene 7,8-oxide. Toxicol In Vitro 19:11–20
Wataha JC, Hanks CT, Craig RG (1991) The in vitro effects metal cations on eukaryotic cell metabolism. J Biomed Mater Res 25:1133–1149
Wendel A (1981) Glutathione peroxidase. Method Enzymol 77:325–333
WHO (2002) The WHO recommended classification of pesticides by hazard and guidelines to the classification 2000–2002. World Health Organization, Geneva, pp 1–58
Wu G, Fang Y, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Yao XH, Min H, Lv ZM (2006) Response of superoxide dismutase, catalase, and ATPase activity in bacteria exposed to acetamiprid. Biomed Environ Sci 19(4):309–314
Zang Y, Zhong Y, Luo Y, Kong ZM (2000) Genotoxicity of two novel pesticides for the earthworm, Eisenia fetida. Environ Pollut 108:271–278
Zhu WJ, Li M, Liu C, Qu JP, Min YH, Xu SW, Li S (2013) Avermectin induced liver injury in pigeon: mechanisms of apoptosis and oxidative stress. Ecotoxicol Environ Saf 98:74–81
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (09-ENV837-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Markus Hecker
Rights and permissions
About this article
Cite this article
Al-Sarar, A.S., Abobakr, Y., Bayoumi, A.E. et al. Cytotoxic and genotoxic effects of abamectin, chlorfenapyr, and imidacloprid on CHOK1 cells. Environ Sci Pollut Res 22, 17041–17052 (2015). https://doi.org/10.1007/s11356-015-4927-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4927-3