Abstract
The present study examined the effects of gibberellin semi-sensitive reduced height (Rht) alleles on wheat grain yield and quality under high temperature and drought stress during booting and anthesis stages. Near-isogenic lines (NILs) of winter wheat (Rht (tall), Rht-B1b, Rht-D1b, Rht-B1c, Rht-8c, Rht-D1c, Rht-12) having background of Mercia and Maris Widgeon cultivars were compared under variable temperatures (day/night: 20/12, 27/19, 30/22, 33/25, 36/28, and 39/31 °C) and irrigation regimes. Pots were transferred to controlled thermal conditions (Saxcil growth chamber) during booting and anthesis stages and were maintained at field capacity (FC) or had water withheld. High temperature (>30 °C) and drought stress for seven consecutive days during booting and anthesis stages reduced the grain yield, while increased nitrogen (N) and sulphur (S) concentrations. A 50 % reduction in grain yield was fitted to have occurred at 37.4 °C for well-watered plants and at 31.4 °C for drought-stressed plants. The N and S concentrations were higher for severe dwarfs, whereas no significant differences were observed between tall and semi-dwarfs in Mercia. In the taller background (Maris Widgeon), N and S concentrations were significantly higher compared with that in Mercia. In Mercia, the severe dwarf Rht-D1c had higher Hagberg falling number (HFN) and sodium dodecyl sulphate (SDS) sedimentation volume. In both backgrounds, semi-dwarfs and severe dwarfs had higher HFN. Moreover, the SDS sedimentation volumes in Maris Widgeon were also higher than that in Mercia. Greater adaptability and improved grain quality traits suggested that severe dwarf Rht alleles are better able to enhance tolerance to high temperature and drought stress in wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop production and food security are under severe threat owing to futuristic escalations in frequency of extreme climatic events such as heat waves and drought stress (Semenov et al. 2014; Hussain et al. 2015). Wheat (Triticum aestivum L.) is the most prominent cereal food stuff and staple food for more than one third of the world population. A notable susceptibility in wheat to thermal and drought stress at booting and anthesis stages had been observed previously, which are coincident with meiosis (Barnabás et al. 2008). Due to partial susceptibility to higher (>30 °C) temperature at booting or anthesis stages, frequent decline in grain yield and quality has been reported in wheat. Extreme temperature leads to sharp reduction in grain set that were associated with impaired grain viability and functionality of conceptive tissues, resulting in lower production and yield (Houshmand et al. 2014).
Since Green Revolution, wheat breeders are developing dwarf and semi-dwarf wheat genotypes containing Rht genes in order to produce dwarf stature varieties which enhance yield and grain quality and also protect plants against heat, drought, and salt stresses, making them less responsive to plant growth hormone like gibberellin (GA) (Semenov et al. 2014). Flintham et al. (1997) revealed that GA-insensitive alleles derived from Norin 10 such as Rht-B1b (Rht1) and Rht-D1b (Rht2) reduced plant height by 15 % along with 24 % improvement in grain yield. Plant height of wheat can be reduced up to 30 % by introducing Rht-B1c (Rht3) and Rht-D1c (Rht10) sharing the same loci as Rht-B1b and Rht-D1b (Addisu et al. 2010). Plant architecture has been modified by the semi-dwarfing alleles Rht-B1b and Rht-D1b by minimizing the sensitivity to GA. When introduced to tall background, these alleles have reduced plant height and improved nutrient response through decreased lodging and increased grain yield, quality, and harvest index (Gooding et al. 2012; Uppal 2012). This increment has been associated with enhanced photo-assimilate partitioning and translocation to ears and florets of developing spikes of these alleles (Gooding 2009; Addisu et al. 2010). Presence of a number of GA-insensitive alleles at Rht-B1 and Rht-D1 loci has been recognized, and most of them have been applied in agriculture (Tan et al. 2013). These researches have illustrated that the influence of these alleles depends on the varietal background and growing environment. The Rht alleles improved bread-making quality through reducing GA sensitivity and alpha-amylase activity and increasing Hagberg falling number (HFN) (Uppal 2012). Moreover, significant yield improvements with dwarf alleles can be associated with decreased protein concentration of grain, specific weight, and 1000-grain weight (Triboi et al. 2006). In UK, the adoption of varieties carrying Rht-D1b in commercial production between 1974 and 1993 has reduced the concentration of crude protein (Gooding et al. 1999) because of the genetic selection that was mostly for a higher harvest index (Gooding and Davies 1997). The GA-sensitive allele Rht12 (reduced height) was associated with a decreased HFN (Gooding et al. 2012), but the grain nitrogen and sulphur ratio was not affected by Rht alleles (Addisu et al. 2009).
Wheat grain quality represents the suitability of the grain for end users and by product manufacturers such as milling, baking and dough rheology, nutritional value, and storage capacity (Porter and Semenov 2005; Tajnšek et al. 2010). Thus, end-use quality together with grain yield is of significant importance in defining the value of the world’s wheat market. Grain quality relies upon several biochemical processes in crops which have been dictated by the interaction of a large number of genes and the environment during crop growth. Wheat dough and dough-based multiple characteristics are influenced by irreplaceable rheological properties of grain that have strongly been influenced by the quantity and type of storage proteins in the grain endosperm (Shewry and Halford 2002). High-quality wheat for bread-making has a high protein concentration with other features such as high specific weight, protein quality (conferring strong doughs) (Oury and Godin 2007), HFN (indicating lower alpha-amylase activity) (Smith and Gooding 1996), high sodium dodecyl sulphate (SDS) sedimentation volume (indicating gluten strength) (Oelofse et al. 2012), and free of black points and fungal contamination (Gooding and Davies 1997).
Despite the fact that proteins are the most important component representing wheat grain quality, further improvement in wheat yield is commonly brought at the cost of protein quality damage (Oury and Godin 2007; Gooding et al. 2007). Genetic, climatic, and agronomic factors are the major players which control this inverse relationship. A grain quality estimate may be checked through SDS sedimentation volume test which derives the amount of gluten and protein quantity as well as quality (Oelofse et al. 2012). Contrary to SDS sedimentation volume test, grain sulphur (S) concentration assesses the baking quality of cereals more accurately than crude protein concentration. Grains with S concentration lower than 0.12 % are considered to have low SDS sedimentation (McGrath et al. 1993). Any genetic or breeding improvement in grain nitrogen (N) rate is mostly greater than S, therefore resulting in greater N/S ratio (Kettlewell et al. 1998). In wheat, higher N/S ratio is related to poor protein quality for baking. HFN represents the degree of starch breakdown into glucose and maltose by enzyme activity through viscosity changes measurement of heated suspension of flour in water (Hagberg 1960). The higher alpha-amylase activity is undesirable for baking as it causes starch hydrolysis during processing, which results in poor-quality end product (Gupta et al. 2010; Hadnađev et al. 2011).
Wheat grain quality and composition are significantly affected by environmental changes (Panozzo and Eagles 2000). Reduced specific weight at the grain filling stage is one of the examples of quality disturbance under extreme drought and heat variations (Saint Pierre et al. 2008). These stresses not only decrease grain yield but can cause severe quality damage (Zhao et al. 2007). The high temperature at grain filling stage may increase the protein content of the grain, but in contrast, it could decrease protein functionality (Corbellini et al. 1997). Quantity of heat shock protein production, construction of complex protein aggregates, and gliding to gluten ratio have a variable response to temperature streams, and generally higher temperatures have negatively influenced these properties (Daniel and Triboi 2000; Skylas et al. 2002). Consequently, it reduces the dough strength and decreases the time for flour mixing, which lead to reduced tolerance (Wardlaw 2002). Drought stress applied at anthesis stage significantly decreased dough resistance, loaf score, and loaf volume (Randall and Moss 1990). The wheat quality is strongly controlled by genetic factors, but during grain filling period, the climatic conditions can have considerable additional impacts (Gooding 2010). Anthesis is the most sensitive stage for heat or drought stress, which decreased the final yield quantitatively and qualitatively (Zhang et al. 2013). There are a limited number of published reports on the effect of high heat and drought stress applied separately or collectively on wheat grain quality at booting and anthesis. Moreover, little work has been done on the effects of GA-sensitive and GA-insensitive dwarfing alleles on susceptibility to heat and drought stress at sensitive growth stages. Therefore, the present study was undertaken to investigate the responses of 11 near-isogenic elite lines (NILs) with contrasting Rht alleles to high heat and drought stress at booting and anthesis stages. Crop performance under heat and drought stress was investigated in terms of grain yield and quality.
Materials and methods
Site description and crop establishment
Eleven NILs of winter wheat were compared under temperature and drought stress for grain yield and quality at the Plant and Environment Laboratory, University of Reading, UK (51°27′ N latitude, 00°56′ W longitude) during 2010–2011. Plastic pots having 180 mm diameter and 41 m3 volume were used. All of the pots were filled with vermiculite/sand/gravel/compost (2:1:2:0.5) mixed with Osmocote slow-release granules (2 kg m-3) containing N/P2O5/K2O/MgO in a ratio of 15:11:13:2, respectively. During booting and anthesis stage, pots were maintained at 100 % field capacity (FC) or had water withheld (drought stress). Weight of growing media per pot averaged 2.60 and 3.05 kg at 0 and 100 % FC, respectively. Ten seeds were sown in each pot, which were thinned to four plants at the two-leaf stage. The pots were kept in an open environment under a net. A fungicide (Prothioconazole + bixafen) was applied twice to control powdery mildew. Pots were irrigated daily by an automatic drip irrigation system to maintain FC. In order to apply heat stress, the pots were transferred to Saxcil growth chamber cabinets (1.37 × 1.47 m) at booting and anthesis stage. Alternate day and night temperature cycles were repeated for a weak having 16-h day (700 mM photon m−2 s−1, 70 ± 2 % relative humidity, and 350–360 μmol CO2 M air) and 8-h night cycle (the same conditions as for the day, but the temperature was 8 °C below the day time) (Saini and Aspinall 1982).
Plant material
Seeds of 11 NILs varying for dwarf alleles were obtained from multiplication plots of Crop Research Unit, Sonning, University of Reading, UK. Initially, these seeds were provided by the John Inns Centre, Norwich from two different backgrounds (i.e., Mercia and Maris Widgeon). These lines are comprised of parent lines from different sources such as Norin 10, Tom Thumb, Ai-Bian, Akakomugi, Mara, and Karcagi 522. These lines are Rht tall, semi-dwarf, and severe dwarf with GA-sensitive and GA-insensitive backgrounds (Tables 1 and 2; Foulkes et al. 2004).
Observations
Ears were tagged at booting stage and harvested at maturity from each pot separately. Ears and stems were collected and weighted separately. Spikes were manually threshed and cleaned by hand, and grains were weighted for yield determination. Moisture content of 2-g sample from each pot was determined after drying at 80 °C for 48 h. Fresh flour was used for grain quality analysis such as HFN and SDS volume determination. Dried grain samples were used for N and S concentration analysis. Grain N was determined from 0.20-g dried flour sample by oxidative combustion using LECO FP-528, while grain S concentration was measured by using LECO SC 144DR (LECO Instruments UK). The N (%) and S (%) presented the grain N and S concentration per pot. N/S ratio was also computed from these values. The NG (N per grain) and SG (S per grain) were computed as the ratio of mean grain weight and percentage of respective element (N% or S%) per pot and were presented as mg. However, the NY (N in yield) and SY (S in yield) were presented in g and depicted the ratio of grain yield weight per pot (g) to the percentage of respective element per pot.
The SDS volume tests were performed as a rapid method to assess gluten strength (Axford et al. 1979). Whole-meal flour was suspended in a solution of SDS containing lactic acid and was allowed to settle (British Standards Institution 1982). Whole-meal flour equivalent to 6 g (at 15 % moisture content) from each sample was suspended in 50 ml of distilled water in a 100-ml cylinder before the addition of 50 ml SDS reagent (20 g SDS + 20 ml diluted lactic acid in a liter of distilled water) and further suspension. Sediment volume was recorded after 20 minutes of settling. Alpha-amylase activity was estimated by the HFN test (Perten Instruments Falling Number 1500; ISO 3093:1982). Whole-meal flour (7 g) was suspended in 25 ml distilled water by shaking in a Viscometer tube. The Viscometer tube was placed in boiling water, and after 5 s, plungean agitation was automatically run for 55 s. After 60 s, the plunger was released and the time taken from the start of agitation to fall (to a predetermined height) was recorded in seconds as the falling number.
Experimental design and statistical analysis
The NILs with different backgrounds and allelic combinations were compared under different temperature and irrigation regimes in a completely randomized design with factorial arrangement replicated four times. All of the data collected were subjected to Fisher’s analysis of variance using SAS 8.1 (SAS Institute Inc. Campus Drive, Cary, NC, USA). Treatment means were compared using SED. Correlation between different attributes was also estimated by SAS. Pictorial representations of the data were drawn by using Origin 9.5.
Results and discussion
Grain yield
Effects of temperature, irrigation, and temperature × irrigation were highly significant (P < 0.001) for wheat grain yield. High temperature was detrimental for wheat grain yield, and significant reductions in grain yield were observed when temperature at booting or anthesis stage was increased above 30 °C (Figs. 1 and 2). At anthesis stage, 50 % reduction in grain yield was fitted to have occurred at 37.6 °C for well-watered plants and at 32.2 °C for drought-stressed plants. Nevertheless, at booting stages, 50 % yield reduction occurred at 37.2 and 30.6 °C for well-watered and drought stressed plants, respectively. Significant variations were observed between both backgrounds under the influence of temperature × irrigation. The Mercia recorded higher grain yields per pot compared with Maris Widgeon. The negative effects of high temperature and drought stress were more in Maris Widgeon particularly at booting stress. Semi-dwarfing alleles in Mercia (Rht-B1b, Rht-D1b) had grain yields but similar to the tall (rht) and Rht8c lines. Grain yield was significantly reduced by severe dwarfing alleles (Rht-B1c in both backgrounds, Rht-D1c and Rht12 in Mercia). Negative effect of high temperature particularly under water-limited conditions on wheat productivity is well evident. Barnabás et al. (2008) proposed that heat and drought severely reduced photosynthesis and led to subsequent dilution of sucrose in the ear, which were associated with floret abortion and less grain production. Temperature above 30 °C during meiosis can interfere with division and leads to abnormal pollen development (Saini and Aspinall 1982). Recently, Stratonovitch and Semenov (2015) have concluded that drought stress was detrimental to grain set and yield formation in wheat, particularly when combined with heat stress. Previously, Alghabari et al. (2014) stated that higher sensitivity to heat and drought at booting stage was mainly due to grains per spikelet, while at anthesis stage, mean grain weight was more prone to heat and drought.
The effects of Rht alleles (varying for GA sensitivity) on grain yield (g/pot) of wheat NILs from Maris Widgeon background under drought and high temperature stress at booting and anthesis stage. Fitted curves are logistic; error bars are 2 S.E.D; open symbols drought stress, solid symbols well-watered
N and S concentrations
The effects of temperature, irrigation, and temperature × irrigation were significant (P < 0.001) for N (%), S (%), NY, NG, SY, and SG (Table 3; Fig. 3a–f), except the effect of temperature on NG and irrigation on S%. Increase in temperature significantly increased the N (%) as well as S (%), while it decreased the NY and SY. Drought-stressed plants recorded higher N% and S% compared with well-watered plants when temperature was between 22 and 36 %. The NY and SY were higher for well-watered plants at all temperatures (Fig. 3). The N/S ratio of wheat NILs varied significantly (P < 0.001) by the main effects of temperature and irrigation. However, interaction between these two factors was non-significant (P = 0.193). Increase in temperature as well as drought stress significantly increased the N/S ratio (Fig. 3). Inclusion of severe dwarfs in both backgrounds (Rht-D1c and Rht12 in Mercia; Rht-B1c in Maris Widgeon) enhanced the N% and S%. Nevertheless, both of these attributes had a negative relationship with grain yield. Severe dwarfs in both backgrounds recorded lower grain yields but higher N and S concentrations. In Mercia background, the highest N% (2.54), S% (0.156), and N/S ratio (16.2) were observed for Rht12 that was statistically similar with Rht-D1c. In Maris Widgeon background, Rht (tall) had the highest N% (2.60) and S% (0.174 %), while Rht-B1b recorded the highest N/S ratio (16.5). In Mercia, severe dwarfs (Rht-D1c and Rht12) had higher N/S ratio than tall and semi-dwarfs. However, tall (Rht), semi-dwarfs, and severe dwarfs were similar with each other for N/S ratio, N%, and S% in Maris Widgeon.
The effects of irrigation (open symbols drought stress, solid symbols well-watered) and temperature on N % (N concentration per pot), S % (S concentration per pot), NG (N per grain), NY (N in yield), SG (S per grain), SY (S in yield), N/S ratio, HFN (Hagberg falling number), and sodium dodecyl sulphate (SDS) sedimentation volume of winter wheat. Error bars are 2 S.E.D. Points are means of 11 NILs varying for Rht alleles. Fitted lines are linear and/or polynomial. N nitrogen, S sulphur
The response of the studied NILs with reduced height alleles to heat and drought stress was dependent on their level of sensitivity. Genotypes which were insensitive to GA (severe dwarf) resulted in improved grain quality characteristics. The present study demonstrated that the grain N concentration was associated with an increase in temperature, which is consistent with the some recent studies (Haberle et al. 2008; Farooq et al. 2011; Asseng et al. 2014; Tian et al. 2014). Hasanuzzaman et al. (2013) also found that heat stress during grain filling increased both N and S concentrations. The present study focused on the effect of high temperature and drought stress at booting and anthesis stages in relation to reduction in grain quality rather than the stress events during grain filling. These results along with previous studies have demonstrated that the amount of NG was more stable than the mean grain weight when plants were exposed to stress (Gooding et al. 2003; Ihsan et al. 2014). The negative inverse relationship between grain N concentration and grain yield was found in this experiment. For example, Rht (tall) in Mercia, with the lowest N%, had the highest grain yields, while severe dwarfs (Rht-B1c in both backgrounds, Rht-D1c and Rht12 just in Mercia) had the highest grain N concentration and were associated with lower grain yield (Figs. 1, 2, and 3; Table 4). Severe dwarfing alleles had higher N concentration due to reduced dry matter production, leading to the concentration of accumulated N. S has a significant impact on bread-making quality of flour due to the essential role of disulphide bonds in maintaining gluten functionality (Gooding 2010). The range of S concentrations in this study was 0.13–0.17 %, which was well above the critical value (<0.12 %) for S deficiency in wheat crop (Zhao et al. 1999). The severe dwarfs in Mercia (Rht-B1c, Rht-D1c, and Rht12) have increased grain S concentration. The effects of semi-dwarfing alleles on grain S were similar to N concentration; therefore, the N/S ratio did not change (Uppal 2012).
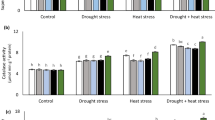
HFN and SDS sedimentation volume
There were no significant effects of temperature (P = 0.725), irrigation (P = 0.121), or their interaction (P = 0.855) on HFN (Fig. 3h). However, variations in HFN were observed due to GA sensitivity level. In both backgrounds, GA-insensitive semi-dwarfs (Rht-B1b and Rht-D1b) and severe dwarfs (Rht-B1c and Rht-D1c only in Mercia) had higher HFN compared with GA-sensitive alleles (tall (rht) in both backgrounds; Rht8c and Rht12 just in Mercia). SDS sedimentation volume was significantly affected by temperature (P = 0.017), but there was no significant effect of either irrigation (P = 0.869) or irrigation × temperature (P = 0.527) (Table 3; Fig. 3). The SDS sedimentation volumes were higher in Maris Widgeon than that in Mercia. In Mercia, tall (Rht), Rht-D1b, Rht8c, and Rht-D1c had similar SDS sedimentation volumes which were higher than Rht-B1b. Among the severe dwarfs, Rht12 had lower SDS volumes.
Correlation matrix estimated a strong positive correlation of N% to S% and N/S ratio (Table 4). This relationship was stronger for Mercia and medium to weak for Maris Widgeon. The N/S ratio was positively correlated to N% and S% for Mercia background, but negatively associated with S% for Maris Widgeon. HFN and SDS were strongly correlated for Maris widgeon (−0.972), but weakly linked for Mercia background (0.124). A weak negative correlation was studied for HFN and SDS to N%, S%, and N/S ratio for Mercia background. The NILs from Maris Widgeon background revealed weak correlations to other studied traits except NY and SY that were negatively associated to HFN and positively associated to SDS sedimentation volume (Table 4).
This study demonstrated that HFN can be affected by Rht allele, and these effects are dependent on the genetic background and mechanism of dwarfing alleles (Gooding et al. 2012). Our results confirmed that HFN for GA-sensitive alleles (Rht8c and Rht12) did not exceed the highest standard (>250 s) required for bread-making quality in the UK (Table 3; Fig. 3h). The effects of Rht-B1b and Rht-D1b on HFN might be higher in the taller backgrounds (Corbellini et al. 1997). In the present study, the semi-dwarfing alleles increased HFN in Mercia background. Gooding et al. (1999) explained that the new varieties (e.g., Mercia) tend to have higher HFN than their predecessors (e.g., Maris Widgeon). In this study, Rht-B1c increased HFN in both backgrounds (Addisu et al. 2009; Uppal 2012). Increase in HFN in Rht-B1c can be related to increased grain dormancy in certain environments (Gooding et al. 2012).
Genotype and environmental and agronomic factors are known to affect SDS sedimentation volume in wheat (Triboi et al. 2000). The present study found that semi-dwarfing alleles in Maris Widgeon scored SDS volumes more than 65 ml [the value marked as A (<65 ml) for bread-making quality], which is consistent with the findings of Corbellini et al. (1997). There was no reduction in SDS sedimentation volume by semi-dwarfing alleles in Maris Widgeon (Nazco et al. 2014). Consistent with earlier studies, SDS volume was not affected by severe dwarfing alleles Rht-B1c and Rht-D1c (Uppal 2012) (Table 3). The SDS sedimentation volume was affected by the presence of the severe dwarf Rht12 possibly because of the shriveled grains, resulting in a high proportion of bran in the whole meal flour (Addisu et al. 2009). The NIL Rht8c did not affect SDS sedimentation volume (Table 3), which is in accordance with Uppal (2012).
Conclusions
High temperature (>30 °C) and drought stress for seven consecutive days during booting and anthesis stages reduced the grain yield, while it increased N and S concentrations in wheat grain. Our results suggested that the variations among genotypes regarding their response to high temperature under field studies are more likely to be due to differences in water availability, root architecture, and growth stages. A 50 % reduction in grain yield was noted to have occurred at 37.4 °C under well-watered conditions and at 31.4 °C under drought stress. Severe dwarfism was associated with lower grain yield and higher N% and S%. Variations were apparent between GA-insensitive and GA-sensitive dwarfing alleles for HFN, and GA-insensitive dwarfing alleles conferred an advantage for HFN. The SDS was not affected by the presence of semi-dwarf Rht8c, but severe dwarf Rht12 increased the SDS sedimentation volume.
References
Addisu M, Snape J, Simmonds J, Gooding M (2010) Effects of reduced height (Rht) and photoperiod insensitivity (Ppd) alleles on yield of wheat in contrasting production systems. Euphytica 172:169–181
Addisu M, Snape J, Simmonds J, Gooding M (2009) Reduced height (Rht) and photoperiod insensitivity (Ppd) allele associations with establishment and early growth of wheat in contrasting production systems. Euphytica 166:249–267
Alghabari F, Lukac M, Jones H, Gooding M (2014) Effect of Rht alleles on the tolerance of wheat grain set to high temperature and drought stress during booting and anthesis. J Agron Crop Sci 200:36–45
Asseng S, Ewert F, Martre P, Rötter R, Lobell D, Cammarano D, Kimball B, Ottman M, Wall G, White J (2014) Rising temperatures reduce global wheat production. Nat Clim Change
Axford D, McDermott E, Redman D (1979) Note on the sodium dodecyl sulfate test of breadmaking quality: comparison with Pelshenke and Zeleny tests. Cereal Chem (USA) 56:582–584
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Corbellini M, Canevar M, Mazza L, Ciaffi M, Lafiandra D, Borghi B (1997) Effect of the duration and intensity of heat shock during grain filling on dry matter and protein accumulation, technological quality and protein composition in bread and durum wheat. Funct Plant Biol 24:245–260
Daniel C, Triboi E (2000) Effects of temperature and nitrogen nutrition on the grain composition of winter wheat: effects on gliadin content and composition. J Cereal Sci 32:45–56
Farooq M, Bramley H, Palta JA, Siddique KH (2011) Heat stress in wheat during reproductive and grain-filling phases. Crit Rev Plant Sci 30:491–507
Flintham J, Börner A, Worland A, Gale M (1997) Optimizing wheat grain yield: effects of Rht (gibberellin-insensitive) dwarfing genes. J Agric Sci 128:11–25
Foulkes M, Sylvester-Bradley R, Worland A, Snape J (2004) Effects of a photoperiod-response gene Ppd-D1 on yield potential and drought resistance in UK winter wheat. Euphytica 135:63–73
Gooding M, Cannon N, Thompson A, Davies W (1999) Quality and value of organic grain from contrasting breadmaking wheat varieties and near isogenic lines differing in dwarfing genes. Biol Agric Hortic 16:335–350
Gooding M, Ellis R, Shewry P, Schofield J (2003) Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J Cereal Sci 37:295–309
Gooding M, Kasyanova E, Ruske R, Hauggaard-Nielsen H, Jensen ES, Dahlmann C, Von Fragstein P, Dibet A, Corre-Hellou G, Crozat Y (2007) Intercropping with pulses to concentrate nitrogen and sulphur in wheat. J Agric Sci 145:469–479
Gooding M, Uppal R, Addisu M, Harris K, Uauy C, Simmonds J, Murdoch A (2012) Reduced height alleles (Rht) and Hagberg falling number of wheat. J Cereal Sci 55:305–311
Gooding MJ (2009) The wheat crop. In: Khan K, Shewry PR (eds) Wheat: chemistry and technology, 4th edn. AACC International; American Association of Cereal Chemists, Inc. (AACC), Minnesota
Gooding MJ (2010) The effects of growth environment and agronomy on grain quality. Cereal grains: assessing and managing quality. Woodhead, Cambridge, pp 393–412
Gooding MJ, Davies WP (1997) Wheat production and utilization: systems, quality and the environment. CAB International
Gupta M, Abu-Ghannam N, Gallaghar E (2010) Barley for brewing: characteristic changes during malting, brewing and applications of its by products. Compr Rev Food Sci Food Saf 9:318–328
Haberle J, Svoboda P, Raimanova I (2008) The effect of post-anthesis water supply on grain nitrogen concentration and grain nitrogen yield of winter wheat. Plant Soil Environ 54:304–312
Hadnađev TD, Pojić M, Hadnađev M, Torbica A (2011) The role of empirical rheology in flour quality control. Wide spectra of quality control. InTech, Rijeka, pp 335–360
Hagberg S (1960) A rapid method for determining alpha-amylase activity. Cereal Chem 37:218–222
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Houshmand S, Arzani A, Mirmohammadi-Maibody S (2014) Effects of salinity and drought stress on grain quality of durum wheat. Commun Soil Sci Plant Anal 45:297–308
Hussain S, Peng S, Fahad S, Khaliq A, Huang J, Cui K, Nie L (2015) Rice management interventions to mitigate greenhouse gas emissions: a review. Environ Sci Pollut Res 22:3342–3360
Ihsan MZ, Khaliq A, Matloob A, El-Nakhlawy FS, Abohassan RA, Daur I, Aslam Z (2014) Influence of herbicides applied alone or supplemented with manual weeding on weed growth, rice yield and grain quality in direct-seeded rice (Oryza sativa L.). Philip Agric Sci 97:377–384
Institution BS (1982) Cereals—determination of falling number BS 4317: part 9. BSI, Hemel Hempstead. 5
Kettlewell P, Griffiths M, Hocking T, Wallington D (1998) Dependence of wheat dough extensibility on flour sulphur and nitrogen concentrations and the influence of foliar-applied sulphur and nitrogen fertilisers. J Cereal Sci 28:15–23
McGrath J, Jancso M, Pichersky E (1993) Duplicate sequences with a similarity to expressed genes in the genome of Arabidopsis thaliana. Theor Appl Genet 86:880–888
Nazco R, Peña R, Ammar K, Villegas D, Crossa J, Moragues M, Royo C (2014) Variability in glutenin subunit composition of Mediterranean durum wheat germplasm and its relationship with gluten strength. J Agric Sci 152:379–393
Oelofse RM, Labuschagne MT, Van-Deventer CS (2012) Influencing factors of sodium dodecyl sulfate sedimentation in bread wheat. J Cereal Sci 52:96–99
Oury FX, Godin C (2007) Yield and grain protein concentration in bread wheat: how to use the negative relationship between the two characters to identify favourable genotypes? Euphytica 157:45–57
Panozzo J, Eagles H (2000) Cultivar and environmental effects on quality characters in wheat. II. Protein. Crop Pasture Sci 51:629–636
Porter JR, Semenov MA (2005) Crop responses to climatic variation. Philos Trans R Soc B: Biol Sci 360:2021–2035
Randall P, Moss H (1990) Some effects of temperature regime during grain filling on wheat quality. Crop Pasture Sci 41:603–617
Saini H, Aspinall D (1982) Sterility in wheat (Triticum aestivum L.) induced by water deficit or high temperature: possible mediation by abscisic acid. Funct Plant Biol 9:529–537
Saint Pierre C, Peterson C, Ross A, Ohm J, Verhoeven M, Larson M, Hoefer B (2008) Winter wheat genotypes under different levels of nitrogen and water stress: changes in grain protein composition. J Cereal Sci 47:407–416
Semenov M, Stratonovitch P, Alghabari F, Gooding M (2014) Adapting wheat in Europe for climate change. J Cereal Sci 59:245–256
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958
Skylas D, Cordwell S, Hains P, Larsen M, Basseal D, Walsh B, Blumenthal C, Rathmell W, Copeland L, Wrigley C (2002) Heat shock of wheat during grain filling: proteins associated with heat-tolerance. J Cereal Sci 35:175–188
Smith GP, Gooding MJ (1996) Relationships of wheat quality with climate and nitrogen application in regions of England (1974–1993). Ann Appl Biol 129:97–108
Stratonovitch P, Semenov MA (2015) Heat tolerance around flowering in wheat identified as a key trait for increased yield potential in Europe under climate change. J Exp Bot. doi:10.1093/jxb/erv070
Tajnšek A, Nikolic EP, Ceh B, Tajnšek L (2010) Baking quality of wheat grain with regard to agrotechnical arrangements and location. Arch Agron Soil Sci 56:589–603
Tan MK, Koval J, Ghalayini A (2013) Novel genetic variants of GA-Insensitive Rht-1 genes in hexaploid wheat and their potential agronomic value. PLoS One 8:e69690
Tian Y, Zheng C, Chen J, Chen C, Deng A, Song Z, Zhang B, Zhang W (2014) Climatic warming increases winter wheat yield but reduces grain nitrogen concentration in East China. PLoS One 9:e95108
Triboi E, Abad A, Michelena A, Lloveras J, Ollier J, Daniel C (2000) Environmental effects on the quality of two wheat genotypes: 1. Quantitative and qualitative variation of storage proteins. Eur J Agron 13:47–64
Triboi E, Martre P, Girousse C, Ravel C, Triboi-Blondel AM (2006) Unravelling environmental and genetic relationships between grain yield and nitrogen concentration for wheat. Eur J Agron 25:108–118
Uppal R (2012) Effect of wheat dwarfing alleles on physiology of yield and nitrogen use efficiency in contrasting tillage systems. Ph.D. thesis, University of Reading
Wardlaw IF (2002) Interaction between drought and chronic high temperature during kernel filling in wheat in a controlled environment. Ann Bot 90:469–476
Zhang B, Liu Y, Xu D, Zhao N, Lei B, Rosa RD, Paredes P, Paço TA, Pereira LS (2013) The dual crop coefficient approach to estimate and partitioning evapotranspiration of the winter wheat–summer maize crop sequence in North China Plain. Irrig Sci 31:1303–1316
Zhao F, Hawkesford M, McGrath S (1999) Sulphur assimilation and effects on yield and quality of wheat. J Cereal Sci 30:1–17
Zhao H, Dai T, Jing Q, Jiang D, Cao W (2007) Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul 51:149–158
Compliance with ethical standards
The manuscript has been prepared in compliance with the ethical standards of the COPE.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Alghabari, F., Ihsan, M.Z., Hussain, S. et al. Effect of Rht alleles on wheat grain yield and quality under high temperature and drought stress during booting and anthesis. Environ Sci Pollut Res 22, 15506–15515 (2015). https://doi.org/10.1007/s11356-015-4724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4724-z