Abstract
The present paper provides an overview of mercury studies performed in the Mediterranean Sea region in the framework of several research projects funded by the European Commission and on-going national programmes carried out during the last 15 years. These studies investigated the temporal and spatial distribution of mercury species in air, in the water column and sediments, and the transport mechanisms connecting them. It was found that atmospheric concentrations of Hg compounds, particularly oxidised Hg species observed at five coastal sites in the Mediterranean Sea Basin, are significantly higher compared with those recorded at five coastal sites distributed across N Europe, most probably due to natural emissions. Hg levels in water are comparable to other oceans. Anthropogenic and natural point sources show locally limited enrichments, while natural diffusive sources influence Hg speciation over larger areas. Results and statistic comparison of mercury species concentrations within Mediterranean compartments will be presented and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
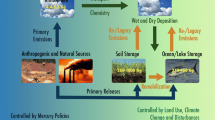
Elemental mercury (Hg0) has a ground-level background concentration which is almost constant over hemispheric scales, although this may be due to rapid cycling at interfaces rather than uninterrupted transport from source to receptor (Hedgecock and Pirrone 2004). Mercury cycling between different environmental compartments indeed depends on the rate of different chemical and physical mechanisms (e.g. dry deposition and wet scavenging) and meteorological conditions, as well as on anthropogenic and natural variables and forcing which affect its fate in the global environment (Pirrone et al. 1996; Pirrone and Mason 2009; Pirrone et al. 2010; Mason and Sheu 2002). Among anthropogenic sources, the most important are fossil fuel combustion, smelting, cement production and waste incineration, whereas the oceans are the largest natural source of Hg to the atmosphere, even though volcanism also makes an important contribution (Pirrone et al. 1996; Pirrone and Mason 2009; Pirrone et al. 2010; Ferrara et al. 2000; Mason 2009; Pacyna et al. 2010). The current contribution of oceanic Hg emissions to the atmosphere is roughly the same as that from anthropogenic sources (Ferrara et al. 2000; Pacyna et al. 2006; Pirrone et al. 2010), and possibly nearly 90 % of oceanic emissions are re-emissions (Strode et al. 2007). While emissions of Hg are in the form of elemental Hg (Hg0), and gaseous and particulate ionic Hg (HgII), most of the Hg in the atmosphere is Hg0 (typically >95 % of the total), and most of the “natural” sources of Hg to the atmosphere are Hg0, although there is a small signal because of inputs of particulate-associated Hg (HgP; i.e. volcanoes and dust). The continued higher atmospheric concentration of Hg in the northern hemisphere is indicative of both the greater industrial activity in the northern hemisphere and also the atmospheric lifetime of mercury, which while it is long enough for Hg to be evenly distributed hemispherically, it is not long enough to survive the longer times required for transport across the “chemical equator”, which does not precisely coincide with the geographical equator. This suggests that Hg deposited to the oceans does not remain there long enough for the global ocean circulation to redistribute it evenly across the globe but continual replenishment of Hg0 (g) must take place, suggesting a “multi-hop” mechanism for the distribution of Hg, rather than solely aeolian transport with little or no chemical transformation between source and receptor (Hedgecock and Pirrone 2004). This transportation mechanism for Hg, where deposition and re-emission occur on a reasonably short-time scale of hours, weeks or months later (Hedgecock and Pirrone 2004), tallies with the estimate of Strode et al. (2007) for the 90 % of Hg deposited to the oceans which is re-emitted to the atmosphere. Re-emissions of mercury from the sea surface have been observed during the last 15 years (Wängberg et al. 2001; Sprovieri et al. 2003, 2010a, b; Gardfeldt et al. 2003; Andersson et al. 2007; Horvat et al. 2003) in the Mediterranean Basin which is a unique sea system with a distinct combination of climatological, meteorological, geographical and geological characteristics which play a role on mercury cycle processes in the Mediterranean boundary layer. It is well known, in fact, that the evasion of elemental mercury from surface waters is primarily driven by (1) the mercury concentration gradient between the top-water microlayer and air above the surface water, (2) solar irradiation which is responsible for the photo-reduction of oxidised mercury in the top-water microlayer and (3) the temperature gradient between the top-water microlayer and air above the surface water (air–water interface) (Pirrone et al. 2003; Sprovieri et al. 2003, 2010a, b). Therefore, sunlight conditions along with the warm and dry climate in the Mediterranean Basin during several months of the year as well as the lower amount of precipitations compared with other areas, such as Northern Europe, would thus enhance the fluxes from the water to the atmosphere (Wängberg et al. 2001; Sprovieri et al. 2003; Gardfeldt et al. 2003; Andersson et al. 2007).
In seawater, mercury behaves as a very reactive element. It can be present in different forms, either dissolved or bound to particulate matter. The main dissolved Hg species in the aquatic environment are elemental Hg (Hg0 (g)), complexes of HgII with various organic and inorganic ligands, and organic Hg forms, mainly the monomethyl mercury compounds (MMHg) and dimethyl mercury (DMHg) (Horvat et al. 2003). The chemical form of Hg in aquatic systems strongly depends on environmental conditions (chemical and physical), and the concentration of inorganic and organic complexing agents. The cycling of Hg in coastal marine systems is comparable to that in the open oceans, although levels of Hg species are enhanced (Fitzgerald et al. 2007). Most mercury enters marine waters by wet or dry deposition or by river discharges, with a significant fraction in oxidised form (Mason et al. 1994; Fitzgerald et al. 2007).
Elemental Hg (Hg0 (g)) is a species that interacts between water and atmosphere and thus it is of crucial importance to understand its cycling between different environments. It is very soluble in natural waters and is usually supersaturated (Schroeder and Munthe 1998), especially in surface waters, where its evasion represents an important source to the global atmosphere. It is measured as dissolved gaseous Hg (DGM) that consists mainly of Hg0 and a very small portion (<5 %) of DMHg. Hg0 is present at all depths in oceanic environments. It has been found that Hg0 in marine waters originates from several biotic and abiotic transformations of labile oxidised HgII (Mason et al. 1995a, b; Costa and Liss 1999, 2000; Amyot et al. 1997) and decomposition of organo-mercury compounds (Mason and Fitzgerald 1993; Mason and Sullivan 1999). HgII can also be reduced photochemicaly. This process is mediated by dissolved organic matter (Fitzgerald et al. 2007). The balance between reduction and oxidation reactions results in diurnal variations of Hg0 in sunlit waters when sea-water exchange remains relatively constant (Fitzgerald et al. 2007). Intensive tectonic activity may also be an important source of Hg0 (Ferrara et al. 2003; Horvat et al. 2003; Kotnik et al. 2007), especially in the Mediterranean which is tectonically very active. Between 10 and 30 % of total Hg can be present in marine waters as Hg0 (Kim and Fitzgerald 1988; Mason and Fitzgerald 1993). Horvat et al. (2003) suggested that Hg0 production is an important mechanism for reducing the availability of HgII as a substrate for microbially mediated methylation.
MMHg is present in open oceanic waters at very low concentrations (Cossa et al. 1997; Mason et al. 1998; Horvat et al. 1999; Cossa and Coquery 2005; Fitzgerald et al. 2007), even lower than the detection limit of 0.05 pM in the North Atlantic (Mason and Sullivan 1999) and North Pacific Oceans (Mason and Gill 2005). In the Mediterranean Sea, MMHg is distributed throughout the water column in significant fractions (Horvat et al. 2003; Cossa and Coquery 2005; Kotnik et al. 2007; Heimbürger et al. 2010). Methylated Hg species are primarily formed in deeper ocean waters, but the process is not restricted to low oxic zones, suggesting that there are additional mechanisms for methylation/demethylation processes (Horvat et al. 2003). Reducing conditions and high salinity promote demethylation of MMHg (Hines et al. 2000; 2006), while it can also be efficiently decomposed by photochemical reactions and microbial activity (Horvat et al. 2003). Benoit et al. (2003) suggests that most MMHg in marine fish has a near-shore sedimentary origin, where it is apparent that biological methylation is more important than abiotic mechanisms. In addition, an abiotic origin of MMHg is also likely as significant levels of MMHg have been observed in hydrothermal vent fluids (Lamborg et al. 2006). However, Fitzgerald et al. (2007) noted that that neither freshwater inputs (riverine or wet atmospheric deposition) nor hydrothermal vent fluids are important sources, but diffusion from deep sediments and advection or “bioadvection” of MMHg from near-shore regions may be an important source. Mason and Fitzgerald (1990) observed a methylated Hg maximum in the low oxygen zone and connected it to in situ microbial methylation of inorganic HgII. Mason and Fitzgerald (1993) also suggested that dimethyl Hg might be a source of MMHg in the water column.
DMHg was reported to be the dominant organic form of Hg in deeper ocean waters (Mason and Fitzgerald 1990; Cossa et al. 1994; Mason et al. 1995a; Horvat et al. 2003; Kotnik et al. 2007). The major source of DMHg at depth remains unknown. It was suggested that some heterotrophically driven production of DMHg might be occurring at depth (Mason et al. 1998). It is readily lost from aquatic environments by evaporation and is rapidly degraded by photochemical mechanisms (Horvat et al. 2003). Evidence indicates that DMHg formation likely occurs in oxygenated environments (Mason et al. 1995a; Cossa et al. 1994). There is little evidence for DMHg in surface waters, but it was found in the central and deepest part of the Adriatic Sea in significant portions. Mason et al. (1998a, b) suggested that DMHg could be brought to the surface, and subsequently emitted to the atmosphere via deep water upwelling or deep thermocline mixing. Additionally, they suggested that DMHg could be formed in situ, but be degraded rapidly as a consequence of its low stability in the presence of light and higher temperatures in surface waters (Fitzgerald and Mason 1997).
The Mediterranean Sea Basin characteristics
The Mediterranean Sea is an enclosed basin connected to the Atlantic Ocean by the narrow Strait of Gibraltar and connected to the Black Sea by the Dardanelles/Marmara Sea/Bosporus system. It is made up of two sub-basins, the Western and the Eastern Mediterranean, connected by the Strait of Sicily. With the exception of the Gulf of Lions, the continental shelves of the Western Mediterranean are narrow. The Tyrrhenian Sea, between the Italian peninsula and its islands of Sardinia and Corsica, is the easternmost and deepest part of the Western Basin, about 3,500 m, and joins the rest of the Western Mediterranean to the south in a wide opening between Sardinia and Sicily. The Eastern Mediterranean is more complicated than the Western Basin and consists of four sub-basins: the Adriatic, Ionian, Aegean and Levantine Seas. The Ionian Sea lies between Italy and Greece to the north and Libya and Tunisia in the south and has depths of about 3,500–4,000 m, with a maximum of 5,000 m south of Greece. The Levantine Sea has a depth of about 2,500–3,000 m in the centre of the basin and a maximum depth of about 4,500 m in a depression south-east of the of the island of Rhodes.
Given the weak cloud coverage, high temperature and warmer climate that characterise the Mediterranean Basin during several months of the year, it is also subject to stronger solar radiation in comparison with oceanic areas of similar latitude. This causes stronger photochemical reactions thus enhancing the Hg fluxes from the water to the atmosphere, either directly, or by interaction of solar radiation with marine biota, both as abiotic and biotic processes that have been suggested to be pathways for the reduction of oxidised HgII to Hg0 (Mason 2009; Andersson et al. 2007; Fantozzi et al. 2007; 2013). The chemical and physical processes characterising the mercury cycle in the Mediterranean are strongly influenced by the particular environmental conditions occurring within this semi-closed sea, which possesses a low capacity interchange with other surrounding seas, such as the Black Sea and the Atlantic Ocean, which latter represents the main external water source through the Strait of Gibraltar influencing water circulation in the basin and some of its sub-basins.
The circulation and hydrography of Mediterranean Sea waters are driven by the net fresh water loss and heat loss to the atmosphere, and the exchange of salinity and heat through the Strait of Gibraltar. Freshwater in the Mediterranean Sea also inputs from the rivers which represent an additional contribution to the water balance of the basin. However, considering that the estimated freshwater budget yields a net negative value ranging between 0.5 and 1 m year−1(Ribera d’Alcalá et al. 2003) and that in the Mediterranean evaporation exceeds precipitation, especially during summer when evaporation has its maximum, Mediterranean water become more saline than the other European seas. During summer, the surface waters are characterised by a thermocline whose depth, temperature gradient, and duration increase slowly from the Western to the Eastern Basin. Deep Mediterranean waters are homogeneous with a temperature of about 12.8 °C and salinity about 38.2. During the last 40 years, an increase of 0.12 °C and 0.05 salinity was measured. The residence time for Mediterranean waters is about 15 years for the Western Basin and 50 years for the Eastern Basin.
The Mediterranean Basin shows in addition a range of geographical complexities and anthropogenic pressures because of the highly populated and industrialised areas of the coastal zone, with large differences in terms of development, environmental status, and management of chemical pollution that has led to concerns that pollutants and contaminants could build up within the Basin and affect biosphere. The high Hg concentrations, above EU and WHO recommendations, observed in muscle tissue of tuna, some rays and swordfish, as well as in other fish during past studies in the Mediterranean Sea caused great concern, particularly because the measured Hg was almost all in the form of methylmercury (MMHg), which is a potent neurotoxin (Storelli et al. 2005, 2002). Its presence in predatory fish is one of the main reasons for Hg being added to the environmental political agenda in recent years.
The Mediterranean is also a rather special sea from the point of view of natural sources of mercury, being characterised by intensive tectonic activity because of the subduction of the African plate under the Eurasian plate, resulting in rigorous volcanic and seismic activity. Emissions from volcanoes, fumaroles and solfataras (e.g. Etna, Volcano, Stromboli, etc.) could represent an important source of mercury released to the atmosphere and marine water in the Mediterranean Basin (Ferrara et al. 2000; Horvat et al. 2003; Kotnik et al. 2007), as well as the contributions from widespread geological anomalies represented by large cinnabar deposits that run from southern Spain (Almaden) to Tuscany in Italy (Mt. Amiata) and into Slovenia (Idrija), forming the “Mediterranean mercury belt”.
The spatial and temporal distribution of different Hg species in the Mediterranean Sea is also affected by the behaviour of different water masses. The hydrology of the Mediterranean is defined by four main water masses: Modified Atlantic Water (MAW), Levantine Intermediate Water (LIW), West Mediterranean Deep Water (WMDW) and East Mediterranean Deep Water (EMDW). The surface waters (MAW) flowing into the Mediterranean through the Strait of Gibraltar are subject to evaporation and mixing with the underlying waters, causing an increase of salinity towards the east from 36.3 in the Strait of Gibraltar to 37.3 in the Strait of Sicily and to values higher than 38.5 in the Levantine Sea (Zavatarelli and Mellor 1995). The origin of LIW is the result of winter convection processes in the Eastern Mediterranean. Its depth range is 300 to 700 m in the E Mediterranean and 200 to 400 m in W Mediterranean. The deep water formation areas of the W and E Mediterranean are geographically separated at the Strait of Sicily, preventing exchange between these deep water masses. The source of the EMDW is the Adriatic Sea, where evaporation and winter cooling processes cause it to sink along the continental slope over the Strait of Otranto and to spread to the whole E Mediterranean Basin. The source of W Mediterranean deep water is located in the Gulf of Lions, where in winter intensive convective movements occur under the influence of cold and dry winds, causing the sinking and mixing of cold and salty surface waters to a depth of about 1,200 to 1,500 m (Zavatarelli and Mellor 1995). This water mass can enter the Mediterranean outflow at Gibraltar without mixing with the LIW (Kinder and Parrilla 1987).
All of these characteristics make the Mediterranean a complex region where the mechanisms and processes characterising the Hg cycle are still unclear and of crucial importance for the marine ecosystem of the Basin. International and national organisations and programmes have been involved for decades in assessing the current status of environmental contamination by mercury in the Mediterranean and in developing strategies and policies to reduce anthropogenic emissions of this pollutant. There have also been research programmes carried out at European and national level that have investigated different aspects of mercury cycling in Mediterranean ecosystems (e.g. the EU-MAMCS/MOE project, EU-MERCYMS project, EU-GMOS project and MedOceanor on-going measurement programme) focused, among other topics, on the identification and possible quantification of the magnitude of major chemical and physical processes involved in Hg transfer between environmental compartments (Pirrone et al. 2003; Pirrone et al. 2005; Sprovieri and Pirrone 2008, 2010a). The present paper aims to provide an overview of mercury studies performed in the Mediterranean Sea region in the framework of several research projects funded by the European Commission and on-going national programmes during the last 15 years to investigating the temporal and spatial distribution of mercury concentrations in atmosphere, the water column and sediments of the Mediterranean Sea from the available Hg datasets related to a number of Mediterranean coastal sites, as well as seasonal oceanographic campaigns performed across the Basin.
Mercury in Mediterranean compartments
Air
Most of the Hg in the atmosphere is Hg0 (typically >95 % of the total), and in addition to Hg0, two other atmospheric Hg fractions have been operationally defined based on physicochemical properties and current methods of measurement—the gaseous ionic HgII fraction, termed reactive gaseous Hg (RGM or GOM), and HgP (or PBM) (Landis et al. 2002; Lindberg et al. 2007; Mason and Sheu 2002). The speciation of GOM is not known in detail, but based on laboratory studies and the methods used for its collection, it is assumed to consist of gaseous neutral HgII complexes, such as HgCl2, HgBr2 and HgOBr (Ariya et al. 2002). The two forms have very different physical properties and this ultimately decides their fate; Hg0 is less reactive, more volatile and most importantly less soluble than GOM. Conversions between different Hg forms provide the basis for understanding the distribution patterns from a local to a global scale. The most important aspect in determining the fate and transport of Hg in the atmosphere is therefore its redox chemistry. The atmospheric oxidants generally considered to be the most important in terms of Hg oxidation are O3, OH and Br. The one reaction which is unanimously accepted is the reaction of Hg with Br (Sprovieri et al. 2010b). This reaction is responsible for the Hg depletion events observed around polar dawn at both Arctic and Antarctic sites (Sprovieri et al. 2002, 2005a, b; Dommergue et al. 2010) which occur at the same time as O3 is destroyed, thereby eliminating both O3 and OH as potential candidates for the observed decrease in Hg0 concentration, thus leaving Br as the only known candidate. Oxidation and reduction processes are extremely important in the overall Hg cycle (evasion–oxidation–deposition–reduction), as the reduction of HgII deposited on sea surfaces permits the evasion of Hg0, ensuring that it is transported (to long haul or multihop mechanism) on the global scale (Mason and Sheu 2002; Hedgecock and Pirrone 2004).
Spatial and temporal trends in the atmosphere
Long-term Hg monitoring is important to provide datasets which can give new insights and information about regional trends of atmospheric Hg. Recognising that Hg measurements are spatially heterogeneous, several studies were designed to set up monitoring networks to compare trends between ground-based monitoring sites in the same region and between regions, as well as to understand the processes that contribute to Hg variability on a diurnal, weekly, seasonal, and annual basis. As part of two European Commission funded projects (MAMCS and MOE), a first attempt was made in 1998 to establish European-wide measurement network. One of the major outcomes of these EU projects was that for the first time, synchronised measurements of mercury species were performed at ten coastal measurement sites, five sites located across Europe (Neuglobsow and Zingst, Germany; Rörvik and Aspvreten, Sweden; and Mace Head, Ireland) and five sites across Mediterranean countries (Mallorca, Spain; Calabria and Sicily, Italy; Antalya, Turkey; and Haifa, Israel) (Fig. 1) during seasonal 2-week intensive campaigns from 1998 to 1999 (Pirrone et al. 2001, 2003; Munthe et al. 2001; Wängberg et al. 2001, 2008; Hedgecock et al. 2006) to identify sources, levels and Hg species behaviour (Pirrone et al. 2001).
Table 1 shows the average total gaseous mercury (TGM)/GEM, TPM and RGM (or GOM) concentrations from the MOE and MAMCS sampling campaigns, performed during the four season between 1998 and 1999. Total gaseous mercury (TGM) concentrations in the Mediterranean area varied between 1.3 and 2.4 ng m−3 and are comparable to the global background concentration of airborne Hg in the Northern Hemisphere (Lindberg et al. 2007; Slemr et al. 2011) with no significant seasonal variations, showing only occasionally higher values because of the influence of major sources. One of the major findings was that more than TGM, oxidised Hg species concentrations were significantly higher at Mediterranean sites compared with those in northern Europe even though the density of industrial and urban centres is higher in northern compared with southern Europe (Pirrone et al. 2001; 2003; Sprovieri et al. 2003; Wängberg et al. 2001; Munthe et al. 2001). These findings are probably a combination of the contribution of natural emissions both from diffuse sources (Hg-enriched minerals, emissions through larger faults, etc.) and volcanism with accompanying phenomena (Ferrara et al. 2000; Pirrone et al. 2001). More active atmospheric transformation processes occurring in the marine boundary layer (MBL) because of the specific meteorological and climatic conditions in the Mediterranean Basin (i.e. warmer climate, high temperature and strong solar radiation) are also strong contributing factors. These findings have been confirmed by the results obtained during oceanographic campaigns carried out along a cruise-path of 6,000 km from summer 2000 to 2007 (Sprovieri et al. 2003). Table 2 shows main statistical parameters for atmospheric mercury species concentrations observed over the Mediterranean Sea Basin during the MedOceanor campaigns. These studies provided the first ever simultaneous measurements of speciated Hg performed over a large area of the Eastern and Western Mediterranean Basins. The meanly higher Hg0 concentrations seen in different cruise campaigns, prevalently those carried out during summer seasons when compared with those observed at the MOE coastal sites during 1998 to 1999 (see Tables 1 and 2) were probably related to Hg0 evasion from seawater because of chemical reduction of oxidised mercury in the water column, suggesting that surface seawater is an important source of Hg release to the global atmosphere. In addition, GOM concentrations observed during the cruises over marine clean air followed a diurnal cycle, increasing after sunrise and decreasing towards evening, with the highest concentrations occurring around midday, clearly indicating the omnipresence of oxidised Hg compounds in the MBL over the Mediterranean Sea (Sprovieri et al. 2003; Sprovieri et al. 2010a, b). As in the Mediterranean, high GOM concentrations following a diurnal cycle have also been observed in clean marine waters of the North Atlantic (Bermuda) (Mason et al. 2001a, b) and the Pacific (Laurier et al. 2003) oceans, indicating the possibility of a minor contribution of anthropogenic influences on a constant “in situ” production of oxidised Hg compounds. It is clear that GOM measurements will always contain a contribution from both (a) “in situ” production and (b) atmospheric transport from emission sources on the mainland. It is not possible to distinguish between them; therefore it is not possible to draw conclusions regarding, for instance, the influence of atmospheric transport on deposition at a given site. However, considering the short lifetime of the GOM in the MBL, primarily driven by the combination of high humidity and high Henry’s Law constant, the relative contribution of atmospheric transport should not be significant; conversely, the clear diurnal fluctuation of RGM concentration with the minimum during the night, could exclude the possibility that the high RGM values is prevalently related to the contribution due to transport from anthropogenic sources. However, in a semi-closed sea such as the Mediterranean it is possible that they do have some influence (Sprovieri et al. 2010a, b).
One feature which all the MBL measurements of GOM have in common is a diurnal variation with a maximum HgII (g) concentration which coincides with the solar irradiation maximum, indicating photolytically produced oxidants are responsible for the gas phase oxidation of Hg0 (g) to HgII (g). This suggests that catalytic destruction involving the BrO(g) radical or another Br-containing radical is responsible for the sharp increase in the oxidation rate of Hg0 (g) (Hedgecock et al. 2003, 2006). Inclusion of reactions between halogen molecules and atoms withHg0 (g) (Ariya et al. 2002) in a modelling study (Hedgecock and Pirrone 2004; Pirrone et al. 2008) suggested a significant temporal reduction of Hg0 (g) lifetime in MBL, implying enhanced deposition rate events and an enhanced emission rate of volatile Hg species from the Mediterranean Sea surface. The enhanced re-emission fluxes of Hg from the sea surface are driven primarily by the higher solar radiation, humidity and temperature in the Mediterranean Basin compared with more northern seas.
To assess the relationship between the atmospheric input of mercury to the Mediterranean region and the formation/production of the most toxic forms of Hg in the marine system, four intensive sampling campaigns at five locations (Cabo de Creus, Spain; Thau Lagoon, France; Piran, Slovenia; Calabria, Italy; and Haifa, Israel) (see Fig. 1) across the Mediterranean Basin were performed from 2003 to 2004 within the EU funded MERCYMS project (Pirrone et al. 2005; Wängberg et al. 2008; Sprovieri et al. 2010a) which also included Mediterranean Sea cruises where mercury in air, water and sediments were measured. Average TGM/Hg0 concentrations from MERCYMS coastal background sites were in the range of 1.6 and 2.0 ng m−3. In addition, three ship measurements performed in summer 2003, spring 2004 and autumn 2004 within the MedOceanor programme yielded similar values. In particular, the average Hg0 concentration obtained from a three week cruise on the Mediterranean Sea during August 2003 was 1.96 ng m−3 (see Table 2) (Sprovieri and Pirrone 2008, 2010a) and an average value of 1.75 ng m−3 was obtained during a two-week cruise in March–April 2004 (Sprovieri and Pirrone 2008, 2010a). These values are close to those observed during the MedOcenaor cruise campaign “summer 2000” highlighting that the mean Hg0 concentration is higher than that recently estimated for the northern hemispheric background, by Slemr et al., (2011). These authors highlight that the worldwide atmospheric mercury concentration have decreased by about 20 to 38 % since 1996 as indicated by continuous measurements performed at a majority of sites in the Northern Hemisphere with records long enough to establish significant trends combined with integrated measurements over the Atlantic Ocean. Therefore, previous estimates on the atmospheric Hg pool in the Northern Hemisphere by Lindberg et al. (2007) should be updated considering other mechanisms and process which can affect the atmospheric mercury cycle as highlighted by Slemr et al. (2011) (i.e. re-emissions) and other reservoirs (i.e. soils and oceans mercury pools and their dynamics) to be able to make projections of future trends. Table 2 shows in addition that average Hg0 values recorded during summer were higher than those observed in winter season, highlighting that the seasonal variation of Hg0 concentrations in the Mediterranean Basin is the reverse of that in Northern Europe (at Mace Head) (Kock et al. 2005). The reasons for this difference are most likely due to the prevailing meteorological conditions during the summer, when a stable high pressure system persists over the Basin causing a low MBL (around 400 m) and a southerly movement of air from northern and central Europe into the region. This southward flow of air is responsible for the high O3 concentrations observed across the Mediterranean during summer, and also carries anthropogenic Hg into the MBL (Sprovieri et al. 2010a, b). The stability of the summer anticyclone may also result in marine emissions of Hg0 remaining trapped in the MBL and contributing to the higher observed Hg0 concentrations as has been suggested by Wängberg et al. (2008).
GOM and PBM concentrations observed at coastal background sites (i.e. Spain and Italy) during the MERCYMS studies were lower than those recorded during the previous MAMCS-MOE sampling campaigns (Wängberg et al. 2001, 2008). These findings could be due to the different measurement methods in the earlier work, which may give different results (Landis et al. 2002; Sheu et al. 2001). In view of recent findings and developments regarding oxidised Hg species measurements, it would be reasonable to believe that the GOM values obtained during previous investigations were overestimated, as suggested by Wängberg et al. (2008). The highest coastal MERCYMS background values were comparable with those obtained from ship board measurements during the summer campaigns. Average GOM of 8.2 and 5.8 pg m−3 were obtained during the summer cruise in 2003 and the spring cruise in 2004, respectively. In addition, GOM behaviour at some coastal sites (i.e. southern Sweden and the Slovenian coastal site) (Wängberg et al. 2008; Sprovieri et al. 2010a) sometimes showed a diurnal variation as has been often observed in the Mediterranean MBL (particularly in clear weather), peaking around midday or soon after.
The cruise campaigns in the Adriatic demonstrated the complexity of Hg cycling in the MBL in general, and the importance of anthropogenic influences, particularly in areas not far from the coast. The Adriatic is more susceptible to these influences than many other parts of the Mediterranean or oceanic environments because it is extremely narrow and in many ways a microcosm of the Mediterranean Sea, with a wide range of anthropogenic pressures. For example, to the north of the Adriatic, the Soča/Isonzo River runs from the severely polluted former Idrija mercury mine (Slovenia) area into the Gulf of Trieste where are several sources of industrial pollution (Covelli et al. 2008; Faganeli et al. 2003). The most obvious difference between measurements made during the Adriatic cruise in the autumn of 2004 and those during the summer of 2005 was the much greater variation in GOM concentrations, with numerous peaks observed in summer 2005. The relatively rough sea conditions, combined with the windy and cloudy meteorological conditions during the autumn cruise in the Adriatic, resulted the in situ oxidation of Hg0 producing observable peaks in the GOM concentration. Some GOM peaks observed during summer 2005 were however clearly related to some anthropogenic plumes encountered when the ship was in the path of air masses from nearby probable Hg emission sources. Back trajectory calculations were used to try to identify them; in addition, the high GOM values sometime recorded during the night time was an obvious indicator (Sprovieri et al. 2010a, b). The results obtained during these studies highlighted that there is the possibility that GOM originates from anthropogenic sources, or that the observed variation in GOM concentration could also be the result of changes in boundary layer height from night to day, rather than due to local photochemical oxidation of Hg0, as has been suggested in the case of MERCYMS measurements at coastal sites in the Mediterranean (Wängberg et al. 2008). Apart from a few exceptions, the summer 2005 GOM concentration peaked each day between noon and early afternoon, as was observed previously in the MBL, suggesting GOM in situ production. The oxidation processes of Hg0 occurring in the Mediterranean Sea imply that atmospheric deposition represents the major input of Hg to the Mediterranean (Hedgecock et al. 2006) estimated by the MECAWEx model (Pirrone et al. 2005) (20.4 t/year combining wet and dry Hg deposition), but the amount of Hg emitted from Mediterranean surface waters varied from 60 to 77 t year (Cossa et al. 1997; Ferrara et al. 2000; Gardfeldt et al. 2003; Hedgecock et al. 2006; Andersson et al. 2007) and thus far greater than that deposited, making the Mediterranean Basin a net source of Hg to the atmosphere instead of a sink, even if currently several of the processes involved remain poorly characterised.
Water
The highest concentrations of THg in the Mediterranean Sea can be found in the most northerly part of the Adriatic Sea (Table 3). The Adriatic Sea receives the inflow of heavily polluted rivers and other direct or indirect natural or anthropogenic Hg loads, especially in its northern and central parts. Elevated Hg levels were found on both the western and eastern coasts of the N Adriatic. Water concentrations are reflected in THg concentrations in the sediments and pore waters of the area. It is evident that Hg enrichment in coastal N Adriatic waters and sediments is limited to the near shore zone and continental shelf. The spatial distribution of THg in water and sediment strongly depends on the water circulation of the sea, but there are several biological and/or geological factors affecting its speciation. The vertical distribution of Hg species reflects well mixed water with low deviations from the average for each location. Some correlations between maximum DGM and RHg (so-called reactive Hg) peaks and the low oxygen zone were observed, which was not the case at the most polluted locations of the Gulf of Trieste and near Venice. Such an association is more evident in locations in the Central and Southern Adriatic where DGM at the surface is relatively low, reflecting the importance of evasion and photochemical oxidation due to the strong UV radiation and the presence of chlorine and bromine (Horvat et al. 2003) and hydroxyl radicals (Gardfeldt et al. 2001; Mason et al. 2001a, b) at the surface. In deeper water layers, the DGM distribution shows a correlation with the oxygen concentration and indicates the importance of redox processes because of microorganism activity, and another, usually sharp increase towards the bottom. This indicates microorganism production and diffusion from sediment and/or tectonic activity, especially at locations in the S Adriatic Pit, which is tectonically very active. RHg vertical profiles are mostly the opposite to those of DGM as HgII is a substrate for DGM production.
MMHg profiles in the Adriatic Sea are mostly related to Chl-a and oxygen concentrations. This underlines the role of planktonic production and regeneration in methylation/demethylation processes. The low MMHg concentrations found in shelf edge or coastal sediments and water over sediment indicate that coastal or shelf sediments are not a significant MMHg source for adjacent open sea waters. At some exceptional deep sea locations the increase of MMHg over the bottom suggests some bottom source (i.e. resuspension or diffusion from sediment), further supported by the estimated diffusive fluxes from sediments ranging from 0.15 to 16.6 pmol m−2 day−1. In the Adriatic Sea deposition and inflow from the Strait of Otranto are the most important sources of Hg, while evaporation and outflow to the Mediterranean are the most important sinks. The Adriatic is a net exporter of mercury species into the central Mediterranean.
THg concentrations in other Mediterranean regions are comparable in all measured water masses in the Basin (0.81–2.33 pM, av. 1.46 pM; p = 0.05) (Kotnik et al. 2007). However, Hg speciation data showed some significant differences among them. It was found that volatile Hg species were lower in surface MAW (0.06–0.53 pM, av. 0.017 pM for DGM; 0.017–2.6 fm, av. 1.19 fm for DMHg; p = 0.0001 to 0.002) than in deep water masses (0.16–0.47 pM, av.0.3 pM for DGM; 0.17–12.14 fm, av. 3.68 fm) (Kotnik et al. 2007), as a result of photoreduction and evaporation of DGM and photolytic degradation and evaporation of DMHg (Morel et al. 1998). Average DGM concentrations in water masses generally increased towards the bottom, especially in the tectonically most active areas, indicating its geotectonic origin. Higher values of RHg were found in WMDW (0.12–1.19 pM, av. 0.66 pM; p = 0.01) (Kotnik et al. 2007). Also on average a higher proportion of total Hg is available as RHg (52 ± 29 %) in WMDW. Differences in DGM concentrations in MAW were also found on comparing different sampling seasons. DGM exhibited higher values in summer than in spring because of higher water temperatures and consequently higher evaporation rates and because of oxidation of DGM under the influence of strong UV radiation during the summer months (Kotnik et al. 2007).
Despite the geological mercury anomaly in the Mediterranean Basin, the concentrations of various Hg compounds are generally lower than those found in Atlantic and Pacific waters (Mason et al. 1998, 2001a, b; Cossa et al. 2004; Mason and Sullivan 1999; Laurier et al. 2003; Kotnik et al. 2007). The relatively high concentrations and fractions of DGM and MMHg indicate the high reactivity of Hg in open marine waters. DGM was present in surface waters mainly as Hg0 as no DMHg was detected at the surface, while towards the bottom a noticeable, but relatively small fraction of DMHg was present. Vertical profiles show that DGM generally increased with depth, suggesting the presence of a source of volatile Hg in deeper waters. This is also confirmed by the average DGM concentration which was the highest in deep water masses (WMDW and EMDW). One possible source of DGM in deeper and bottom waters could be the intensive tectonic activity of the seafloor, which may be the main source of DGM in deep waters, as is indicated by the higher concentrations and fractions of DGM near the bottom at locations with strong tectonic activity (Alboran Sea, Strait of Sicily, Tyrrhenian Sea, Ionian Sea). It is well known that emissions from tectonic activity and geological anomalies could represent an important source of mercury (Ferrara et al. 2000; Gustin 2003). Rajar et al. (2007) calculated the total natural underwater emission of Hg in the Mediterranean Sea to be 11 to 20 t year−1 with a mean estimate of 15 t year−1. A second possible source could be bacterial activity that might produce DGM (Ferrara et al. 2003). From certain profiles it is possible to observe that the increase of DGM corresponds to a decrease in dissolved oxygen levels, suggesting biologically mediated processes as shown by previous studies in the Mediterranean Sea (Horvat et al. 2003; Ferrara et al. 2003; Kotnik et al. 2007). The latter found a significant negative correlation between DGM and oxygen levels in deeper marine waters. The observed decrease of DGM towards the surface is a result of the balance between production and loss processes. Photoproduction and bacterial activity is probably the main source of volatile Hg in surface waters, as was shown in several laboratory and field studies (Amyot et al. 1997; Costa and Liss 1999; 2000; Gardfeldt et al. 2001; Lanzillotta and Ferrara 2001; Lanzillotta et al. 2002).
Air–water exchange
The studies performed in the Mediterranean region during the last decades have highlighted the importance of gaseous Hg exchange processes between the atmosphere and surface waters (Sprovieri et al. 2003; Hedgecock and Pirrone 2004; Horvat et al. 2001, 2003; Gardfeldt et al. 2003; Kotnik et al. 2007; Sprovieri and Pirrone 2008). The lack of knowledge of the magnitude of these exchange mechanisms is one of the main factors affecting the overall uncertainty associated with the assessment of net Hg fluxes between the atmosphere and marine environments in the Mediterranean region. After the 2000, 2003 and 2004 cruise campaigns, further in-depth investigations were carried out in the Mediterranean Sea (Pirrone et al. 2003; Sprovieri et al. 2003; Hedgecock et al. 2003; Sprovieri and Pirrone 2008; Sprovieri et al. 2010a, b) to explain Hg-species behaviour and processes in the MBL, surface and deep water and sediment compartments, as well as gaseous Hg exchange rates at the air–water interface to calculate the annual Hg evasion from the sea (Sprovieri et al. 2003; Sprovieri and Pirrone 2008; Gardfeldt et al. 2003; Horvat et al. 2003; Hedgecock et al. 2006; Kotnik et al. 2007; Andersson et al. 2007). The cruise campaigns were performed during different seasons and covered both the western and eastern sectors of the Mediterranean Basin (Fig. 1). The evasion flux observed by Ferrara et al. (2000) at three selected sites (unpolluted, polluted and off-shore) in the Tyrrhenian Sea during 1998 showed a typical daily trend, with the mercury flux during night-time being 2- to 5-fold lower than that observed during the daytime, suggesting that solar radiation is one of the major driving factors affecting the release of Hg0 from surface waters (Ferrara et al. 2000). The daily average for Hg emission rates from surface water was in the range from approximately 50 ng m−2 day−1 (at unpolluted and off-shore sites) to 160 ng m−2 day−1 (at polluted sites). In addition, a seasonal trend was also observed, with minimum values during the winter period (28.7 ng m−2 day−1) and maximum values during the summer (138 ng m−2 day−1), probably because of the higher water temperature that may have facilitated biotic (i.e. bacteria and other aquatic organisms) and abiotic (i.e. solar radiation) processes that produce Hg in its elemental form and as DGM in the water column (Ferrara et al. 2000). Ferrara et al. (2000) calculated an annual mercury evasion from seawater of the entire basin of nearly 60 t lower than that (70 t) estimated by Hedgecock et al. (2006). Measurements of DGM, TGM, water temperature and wind speed were performed during the 2000 MedOceanor cruise campaign by Gardfeldt et al. (2003). Table 4 shows some physical parameters with corresponding Hg evasion estimations performed by Gardfeldt et al. (2003) using the gas exchange model developed by Wanninkhof (1992) in which the two layer ocean model is combined. The total Hg evasion estimated for the Mediterranean Sea during summer months was 66 t. It was lower in western than in the eastern sector, probably due to the higher mean degree of Hg0 saturation in the east compared with the west, highlighting an Hg evasion gradient from west to south-east (Gardfeldt et al. 2003; Horvat et al. 2001; 2003).
The first reported data by Gardfeldt et al. (2003) on mercury evasion from the eastern part of the Mediterranean Sea was again obtained during summer 2003, spring and autumn 2004 cruises to compare and confirm the previous results, and to calculate the annual Hg evasion from the Mediterranean water surface. The continuous measurements of DGM carried out during these three seasonal cruise campaigns highlighted a diurnal variation in concentration, at both coastal and offshore sites, with higher concentrations during daytime than night time (Andersson et al. 2007). The highest DGM concentrations were observed in summer 2003 and in autumn 2004. The degree of saturation was also calculated directly from the measurements and was found to vary between the different seasons. In particular, the highest average degree of saturation and the largest variation in saturation was observed during summer 2003, whereas spring 2004 showed the lowest variation and the lowest average degree of saturation. Autumn 2004 also showed a large variation in saturation but a lower average compared with the summer cruise. This might be explained by the temperature difference between the different seasons. The flux from the sea surface was calculated using the gas exchange model.
Table 4 shows the seasonal average Hg flux and wind speed observed in the different parts of the Mediterranean Sea during the cruise campaigns. It is possible to highlight that Hg evasion varied between the different seasons with the highest rate estimated during the autumn, probably due to the higher wind speed, which together with solar radiation is one of the major factors that affect Hg evasion, whereas the lowest value was estimated during the spring season. Using these data Andersson et al. (2007) estimated the yearly Hg0 evasion from the Mediterranean Sea surface to be 77 t, which is higher than that calculated by Gardfeldt et al. (2003) (66 t) but comparable to the yearly Hg0 evasion estimated by Hedgecock et al. (2006) (about 70 t) for the Mediterranean Basin over the 12 months of the modelling period. This emission estimate is comparable to the Hg emitted from stationary combustion facilities in Europe (Pirrone et al. 2010). Hedgecock et al. (2006) used the MECAWEx model (Pirrone et al. 2005) for estimation of wet and dry Hg deposition to the Mediterranean Sea surface. The results showed that the Hg deposition to the sea surface was 20.4 t making the Mediterranean Sea a net emitter of Hg. It should be noted that for evasion estimates different gas exchange models were used. Gardfeldt et al. (2003) used Wanninkhof (1992) model, while Andersson et al. (2007) used Nightingale et al. (2000) model. The difference between models is mainly in different approaches to calculate the gas transfer velocity which introduces different uncertainties into evasion calculation. For instance, Wanninkhof (1992) suggests an uncertainty of about 30 % in the result for his model.
Sediments
Settling of organic-rich particles of riverine or atmospheric origin is considered a major Hg delivery mechanism in coastal and open-ocean systems. As in other oceans also in the Mediterranean Sea most Hg research was focused on coastal deposits, which act as sites of inorganic Hg entrapment and MMHg production (Cossa et al. 1996; Mason and Sullivan 1999). Concentrations of total Hg in Mediterranean sediments vary more than 2,000-fold within and among locations (Table 5), depending on the source and natural or anthropogenic loadings. The lowest total Hg concentrations were found in regions that are remote from fluvial and point sources, and direct atmospheric Hg deposition presumably represents the principal source. The highest concentrations are associated with the transport of Hg because of mining activities (Gulf of Trieste) and direct industrial inputs (Kaštela Bay, Grado and Marano Lagoons). Total Hg concentration usually decreases with the distance from rivers and urbanised areas and recently, based on Hg stable isotopic analysis, it is also possible to determine the sources of Hg in coastal deposits. We were able to track Hg originating from the Idrija Hg mine transported by the Soča/Isonzo River in the surface sediments of the Gulf of Trieste (Foucher et al. 2009). Little variations of total Hg were observed in the upper few cm of marine sediments, mainly due to bioturbation and sediment mixing associated with tidal/fluvial currents.
While coastal areas have been extensively studied, data on Hg concentrations and its species in deep-sea cores are still lacking. Only a few studies were performed on deep-sea cores; two in the Mediterranean Sea during the EROS-2000, ADIOS and MERCYMS EU programmes (Cossa and Coquery 2005; Ogrinc et al. 2007) and one in the Arctic Ocean (Gobeil et al. 1999). The sediments of the Mediterranean are deposited on top of an extremely thick layer of evaporates, formed during the so-called Messinian Salinity Crisis whose origin was found to be dominantly tectonic. The sediments are characterised by a low organic content (<1 wt.%). It was found that total Hg concentrations in the off-shore marine sediments of the Mediterranean Sea, with an average of 0.5 nmol g−1 (Baldi et al. 1983), are twice as high as the supposed world-wide natural background. The observed enrichment is mainly the consequence of the world’s largest cinnabar deposits and surficial and submarine volcanic and geothermal activity. The concentrations of total Hg in these sediments ranged from 0.06 to 2.23 nmol g−1 and varied irregularly with depth. In the Adriatic Sea (without the Northern Adriatic) total Hg concentrations in sediments were lower, ranging from 0.26 to 1.28 nmol g−1. The results in the Adriatic Sea are in a good agreement with the concentrations of total Hg determined in the deep cores collected in the Eastern and Western Mediterranean (Cossa and Coquery 2005). The concentrations observed at the surface ranged from 0.39 to 0.45 nmol g−1 and from 0.04 to 0.2 nmol g−1 in the Western and Eastern Basin, respectively. In most cores the concentrations decreased from the surface and at a depth of 10 cm the concentrations varied between 0.08 and 0.2 nmol g−1, and were within the normal range of values of the earth’s crust. The irregular depth variation observed in the sediment profile may reflect changes or redistribution during diagenetic processes (Ogrinc et al. 2007) and/or climatic and depositional changes which may promote the formation of metal-rich layers (Pruysers et al. 1993). Thus, anthropogenic and diagenetic origins are both possible. A recent study related to deep-sea Tyrrhenian Basin sediments reported the Hg isotopic composition of sapropels and background sediment (Gehrke et al. 2009). No discrete differences were found between the Hg isotopic composition of sapropels and background sediments, despite the 6-fold difference in total Hg concentration. This might indicate that the isotopic composition of Hg in the oceans reflects that of Hg in the upper crust.
Total Hg is often correlated with organic carbon content (OC) in particular coastal marine sediments (Coquery et al. 1997; Mikac et al. 1999; Conaway et al. 2003; Hammerschmidt and Fitzgerald 2006; Canario et al. 2008; Hammerschmidt et al. 2008; Hollweg et al. 2009), as well as in open marine systems (Hammerschmidt and Fitzgerald 2006; Liu et al. 2009). In the Mediterranean Basin different relationships between OC content and total Hg were observed: sediments in which total Hg was strongly correlated with OC content (Piani et al. 2005; Monperrus et al. 2007a, b; Covelli et al. 2008), coastal and open sea sediments with a rather weak correlation between total Hg and OC content (Covelli et al. 2001; Berto et al. 2006; Ogrinc et al. 2007) and an unusual negative correlation between total Hg and OC content (Mikac et al. 2006). A low correlation between total Hg and OC content is usually observed in sediments with a low OC content of about 1 wt.%, suggesting that phases other than OC play an effective role in sequestration of Hg, such as complexation with dissolved and colloidal compounds involving Fe and Mg oxyhydroxides. This assumption is additionally supported by the low partitioning of HgII between pore waters and solid sediments determined in open sea sediments with log K D values ranging between 1.85 and 3.64 (Ogrinc et al. 2007). Furthermore, it appeared that not only the quantity but also the quality of organic matter affected partitioning of Hg in sediments (Hollweg et al. 2009).
The variation of MMHg within and among locations was high, reaching up to 700-fold, but lower compared with total Hg (Table 5). It appears that MMHg concentrations were lower in deep-sea sediments compared with coastal regions. Surface sediment MMHg concentrations range from 0.37 to 14.8 pmol g−1 with the highest found at the Strait of Otranto, probably due to the coastal down-welling waters from the Adriatic Sea transporting a relatively high proportion of MMHg on suspended material. The distribution of MMHg in the sediment profile was similar for all deep-sea sampling sites and more variable compared with total Hg with two clear characteristics: a MMHg maximum in the first few centimetres and decrease with depth in the core. A similar distribution in MMHg concentrations was also observed in other coastal sediment systems in the Mediterranean Basin (Covelli et al. 2001; Monperrus et al. 2007a, b). MMHg concentrations with an average of 5.37 ± 3.69 pmol g−1 were about twice as high in the Eastern Basin of the Mediterranean Sea compared with the Western Basin with an average MMHg concentration of 3.33 ± 2.09 pmol g−1. MMHg is often correlated with total Hg and this was observed even among different marine systems (Hammerschmidt and Fitzgerald 2006). Although levels of both total Hg and MMHg vary considerably within a particular system, it is evident from Table 5 that the range of the ratio of MMHg to total Hg in surface sediments among coastal marine systems is relatively narrow. As expected, the lowest ratios were observed in Hg contaminated areas (Table 6).
Sedimentary production is the primary source of MMHg in many coastal systems (Cossa et al. 1996; Mason et al. 1999; Hammerschmidt and Fitzgerald 2006), where it is apparent that biological methylation of HgII is more important than abiotic mechanisms. The net production of MMHg in marine sediments is influenced by a variety of factors affecting either the activity of methylating or demethylating bacteria, or the availability of Hg species for transformation. These factors can include loadings of HgII, partitioning of Hg species with organic matter, the effect of sulphide on the speciation of Hg complexes, the availability of labile organic substrates, temperature, redox conditions and sediment disturbance (Fitzgerald et al. 2007). For the Mediterranean Basin there are only a few studies where methylation rates were determined: the Gulf of Trieste, the Thau Lagoon and in deep-sea Mediterranean sediments (Hines et al. 2006; Monperrus et al. 2007a; Ogrinc et al. 2007). MMHg production was the highest close to the sediment-water interface, decreased with depth and correlated well with sulphate reduction, as was commonly found in many marine systems. Rates were generally the highest in summer in parallel to higher sediment temperature and redox status. The highest methylation rate constants (k meth) of up to 4 % day−1 were found in the Gulf of Trieste and increased with distance from the river Soča/Isonzo. As expected, the demethylation rate constants were higher compared with methylation constants, with the highest observed near the mouth of the river (Hines et al. 2006). Methylation rate constants in the Thau Lagoon ranged between 0.25 and 1.32 % day−1 (Monperrus et al. 2007b), while the methylation potential of deep-sea sediments was relatively low with methylation rate constants ranging from 0.16 to 0.71 % day−1 (Ogrinc et al. 2007). In addition, no genes that are involved in Hg resistance in sediment samples were detected suggesting that the microbial communities of these sediments have a low potential, if any, to reduce HgII and degrade MMHg (Barkay, personal communication). The quantity and quality of organic matter also affect MMHg production, primarily by controlling Hg concentrations in pore water. In the Mediterranean Sea, Hg methylation was found to vary inversely with OC content, indicating a possible inhibitory effect of organic matter. All these data indicate that there should be other sources of MMHg in the Mediterranean Sea. Both abiotic and biotic Hg methylation are possible. Recent data suggests that MMHg occurs in Mediterranean waters (Monperrus et al. 2007b), and that methylation could be linked to organic matter regeneration and the presence of small sized nano and pico phytoplankton that dominate in oligotrophic conditions (Heimbürger et al. 2010). Abiotic methylation is another possible source of MMHg. However, laboratory experiments demonstrating that HgII is methylated in the presence of methyltin in seawater at conditions typical of Mediterranean waters used high methyltin concentrations which are not present in surface Mediterranean waters (Celo et al. 2006), therefore this hypothesis has to be further justified.
Water–sediment exchange
Production in sediment seems to be an important source of MMHg to the Mediterranean, which is also evident from the observed higher concentrations of MMHg found at the bottom in water profiles at all sampling locations. Estimation of water/sediment exchange was made based on accumulation rates and diffusive fluxes of total Hg and MMHg in coastal and open marine systems. Sediment profiles of total Hg and MMHg were used for calculation of accumulation rates. Using sedimentation rates of 0.012 and 0.024 cm year−1 (Zuo et al. 1997; Cossa and Coquery 2005) determined in the Western and 0.003 cm year−1 in the Eastern Basin (Van Santvoort et al. 1996), the total Hg accumulation rates were estimated to be between 5 and 141 kmol year−1. In the Adriatic Sea total Hg accumulation rates ranging from 8 to 37 kmol year−1 were calculated from the sedimentation rate of 0.03 cm year−1 determined in the central Adriatic (Maselli et al. 2010). The MMHg accumulation rates in the Mediterranean Sea varied between 0.1 and 2.2 kmol year−1. Furthermore, fluxes of total Hg and MMHg from coastal marine sediments have been measured using in situ flux chambers (Covelli et al. 1999; Point et al. 2007; Covelli et al. 2008; Emili et al. 2011), while in the open Mediterranean fluxes were estimated from the gradients of total Hg and MMHg in filtered pore water (Ogrinc et al. 2007). The fluxes estimated differ widely among the systems, with the highest observed in the Grado Lagoon. Diffusive fluxes estimated by Ogrinc et al. 2007, ranged between 3 and 32 pmol m−2 day−1 in deep-sea sediments and are comparable to those estimated for the southern New England continental shelf (Hammerschmidt and Fitzgerald 2006) and the mid-Atlantic continental margin (Hollweg et al. 2009) using the same approach. The estimated average accumulation rates were considerably lower than MMHg diffusion from the sediment at all sampling locations. Thus, much of the MMHg produced in the surface sediment of the Mediterranean is not accumulated by the solid phase, but is lost to the overlying water and could constitute an important source of MMHg in marine biota. The emission of MMHg from sediments was estimated to be 14 kmol year−1 (Ogrinc et al. 2007). In addition also larger quantities of total Hg are released from deep-sea sediments. Higher total Hg fluxes were observed in the Eastern Basin than in the Western Basin, showing that submarine tectonic activity could represent an important source of Hg to the Mediterranean. The integrated flux of total Hg was calculated from measurements to be 109 kmol year−1 and agrees well with the mass balance calculation preformed for total underwater emissions of total Hg in the Mediterranean Sea of 80 kmol year−1 by Rajar et al. (2007) and of 174 kmol year−1 from sediment and geotectonic sources modelled by Zagar et al. (2013).
Conclusions
One of the major findings of these studies is the omnipresence of oxidised Hg compounds over the water surface in the Mediterranean Sea, with concentrations significantly higher than those observed in the northern Europe (Pirrone et al. 2003; Sprovieri et al. 2003; Wängberg et al. 2001), probably due to a combination of the contribution of natural emissions with more active atmospheric transformation processes driven primarily by the higher solar radiation, humidity and temperature characteristic of this semi-enclosed basin (Ferrara et al. 2000; Sprovieri et al. 2003; Pirrone et al. 2001). The higher Hg0 concentrations observed over open waters compared with those observed at coastal sites are related to Hg0 re-emission fluxes from seawater because of reduction of oxidised mercury in the water column, suggesting that surface seawater is an important source of Hg released to the global atmosphere. Moreover, although atmospheric deposition represents the major input of Hg to the Mediterranean, the amount of Hg emitted from the surface waters is actually far greater than that deposited, making it a net Hg source to the atmosphere (Hedgecock et al. 2006). The highest concentrations of THg in waters of the Mediterranean Sea were found in the most northerly part of the Adriatic Sea, where it receives the inflow of heavily polluted rivers and other direct or indirect natural or anthropogenic Hg loads, especially in its northern and central parts. Elevated Hg levels were found on both the western and eastern coasts of the N Adriatic. It is evident that the Hg enrichment in coastal N Adriatic waters and sediments is limited to the near shore zone and continental shelf. The spatial distribution of Hg in water and sediment strongly depends on the water circulation of the sea, though there are several biological and/or geological factors affecting its speciation.
THg concentrations in other Mediterranean regions are comparable in all measured water masses in the Basin. However, Hg speciation data showed some significant differences among them. It was found that the volatile Hg species (DGM and DMHg) were lower in surface MAW than in deeper water masses, because of photoreduction and evaporation of DGM and photolytic degradation and evaporation of DMHg (Morel et al. 1998).
It was found that total Hg concentrations in the off-shore marine sediments of the Mediterranean are twice as high as the supposed world-wide natural background. The observed enrichment is mainly the consequence of the world’s largest cinnabar deposits and surficial and submarine volcanic and geothermal activity. The lowest total Hg concentrations in sediments were found in regions that are remote from fluvial and point sources, and direct atmospheric Hg deposition presumably represents the principal source. The highest concentrations are associated with the transport of Hg because of mining activities (Gulf of Trieste) and direct industrial inputs (Kaštela Bay, Grado, Marano Lagoons). It appears that deep-sea Mediterranean sediments are the source of both MMHg and THg to the water column, while coastal regions are sources only of MMHg. In coastal regions accumulation rates of THg are higher than estimated diffusive fluxes of THg. The integrated flux of THg in the Mediterranean Sea was estimated to be 109 kmol year−1, while the emissions of MMHg were estimated to be 14 kmol year−1.
The results of studies in the Mediterranean Sea indicate that although much research has already been performed, there are still gaps in our knowledge which require to be filled to fully understand the past and present dynamics of Hg in this marine environment, and thus in urgent need of further investigation.
References
Amyot M, Gill GA, Morel FMM (1997) Production and loss of dissolved gaseous mercury in coastal sea water. Environ Sci Technol 31:3606–3611
Andersson M, Gårdfeldt K, Wängberg I, Sprovieri F, Pirrone N, Lindqvist O (2007) Seasonal and daily variation of mercury evasion at coastal and offshore sites from the Mediterranean Sea. Mar Chem 104(3–4):214–226
Ariya PA, Khalizov A, Gidas A (2002) Reactions of gaseous mercury with atomic and molecular halogens. J Phys Chem A 106:7310–7320
Baldi F, Bargagli R, Focardi S, Fossi C (1983) Mercury and chlorinated hydrocarbons in sediments from the bay of Naples and adjacent marine areas. Mar Pollut Bull 14:108–111
Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL (2003) Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems biogeochemistry of environmentally important trace elements. Book Series: ACS Symposium Series, 835, pp. 262–297
Berto D, Giani M, Covelli S, Boscolo R, Cornello M, Macchia S, Massironi M (2006) Mercury in sediments and Nassarius reticulatus (Gastropoda Prosobranchia) in the southern Venice Lagoon. Sci Total Environ 368(1):298–305
Canario J, Poissant L et al (2008) Mercury partitioning in surface sediments of the upper St. Lawrence River (Canada): evidence of the importance of the sulphur chemistry. Water Air Soil Pollut 187(1–4):219–231
Celo V, Lean DR, Scott SL (2006) Abiotic methylation of mercury in the aquatic environment. Sci Total Environ 368(1):126–137
Conaway CH, Squire S, Mason RP, Flegal AR (2003) Mercury speciation in the San Francisco Bay estuary. Mar Chem 80:199–225
Coquery M, Cossa D, Sanjuan J (1997) Speciation and sorption of mercury in two macro-tidal estuaries. Mar Chem 58(1–2):213–227
Cossa D, Coquery M (2005) The Mediterranean mercury anomaly, a geochemical or a biologocal issue. In: The handbook of environmental chemistry, vol. 5: the Mediterranean Sea. Springer, Berlin
Cossa D, Martin J-M, Sanjuan J (1994) Dimethylmercury formation in the Alboran Sea. Mar Pollut Bull 28:381–384
Cossa D, Coquery M, Gobeil C, Martin J-M (1996) Mercury fluxes at the ocean margins. In: Baeyens W, Ebinghaus R, Vasiliev O (eds) Regional and global cycles of mercury: sources, fluxes, and mass balances. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 229–247
Cossa D, Martin J, Takayanagi K, Sanjuan J (1997) The distribution and cycling of mercury species in the western Mediterranean. Deep-sea Res Ii Top Stud Oceanogr 44(3–4):721–740
Cossa D, Cotte Krief M-H, Mason RP, Bretaudeau-Sanjuan J (2004) Total mercury in the water column near the shelf edge of the European continental margin. Mar Chem 90(1–4):21–29
Costa M, Liss P (1999) Photoreduction of mercury in sea water and its possible implications for Hg0 air–sea fluxes. Mar Chem 68:87–95
Costa M, Liss P (2000) Photoreduction and evaluation of mercury from sea water. Sci Total Environ 261:125–135
Covelli S, Faganeli J, Horvat M, Brambati A (1999) Porewater distribution and benthic flux measurements of mercury and methylmercury in the Gulf of Trieste (Northern Adriatic Sea). Estuar Coast Shelf Sci 48(4):415–428
Covelli S, Horvat M, Faganeli J, Brambati A (2001) Mercury contamination in coastal sediment as a result of long-term cinabar minig activity (Gulf of Trieste, Northern Adriatic). Appl Geochem 541(16/5)
Covelli S, Faganeli J, De Vittor C, Predonzani S, Acquavita A, Horvat M (2008) Benthic fluxes of mercury species in a lagoon environment (Grado Lagoon, northern Adriatic Sea, Italy). Appl Geochem 23(3):529–546, str
Dommergue A, Sprovieri F, Pirrone N, Ebinghaus R, Brooks S, Courteaud J, Ferrari C (2010) Overview of mercury measurements in the Antarctic troposphere. Atmos Chem Phys 10:3309–3319
Emili A, Koron N, Covelli S, Faganeli J, Acquavita A, Predonzani S, De Vittor C (2011) Does anoxia affect mercury cycling at the sediment-water interface in the Gulf of Trieste (northern Adriatic Sea)? Incubation experiments using benthic flux chambers. Appl Geochem 26:194–204
Faganeli J, Horvat M, Covelli S, Fajon V, Logar M, Lipej L, Čermelj B (2003) Mercury and methylmercury in the Gulf of Trieste (nothern Adriatic Sea). Sci Total Environ 304:315–326
Fantozzi L, Ferrara R, Frontini FP, Dini F (2007) Factors influencing the daily behaviour of dissolved gaseous mercury concentration in the Mediterranean Sea. Mar Chem 107:4–12
Fantozzi L, Manca G, Ammoscato I, Pirrone N, Sprovieri F (2013) The cycling and sea-air exchange of mercury in the waters of the Eastern Mediterranean during the 2010 MED-OCEANOR cruise campaign. Sci Total Environ. doi:10.1016/j.scitotenv.2012.09.062, STOTEN14005
Ferrara R, Mazzolai B, Lanzillotta E, Nucaro E, Pirrone N (2000) Temporal trend in gaseous mercury evasion from the Mediterranean Sea Waters. Sci Total Environ 259:183–190
Ferrara R, Ceccarini C, Lanzillotta E, Gardfeldt K, Sommar J, Horvat M, Logar M, Fajon V, Kotnik J (2003) Profiles of dissolved gaseous mercury concentration in the Mediterranean Seawater. Atmos Environ 37(1):S85–S92
Fitzgerald WF, Mason RP (1997) Biogeochemical cycling of mercury in the marine environment. In: Sigel A, Sigel H (eds) Mercury and its effects on environment biology. Marcel Dekker, New York, pp 53–111
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107(2):641–662
Foucher D, Ogrinc N, Hintelmann H (2009) Tracing mercury contamination from the Idrija mining region (Slovenia) to the Gulf of Trieste using Hg isotope ratio measurements. Env Sci Technol 43:33–39
Gardfeldt K, Feng X, Sommar J, Lindqvist O (2001) Total gaseous mercury exchange between air and water over lake and sea surfaces. Atmos Environ 35:3027–3038
Gardfeldt K, Sommar J, Ferrara R, Ceccarini C, Lanzillotta E, Munthe J, Wangberg I, Lindqvist O, Pirrone N, Sprovieri F, Pesenti E, Strömberg D (2003) Evasion of mercury from coastal and open waters of the Atlantic ocean and the Mediterranean Sea. Atmos Environ 37(S1):73–84
Gehrke GE, Blum JD, Meyers PA (2009) The geochemical behavior and isotopic composition of Hg in a mid-Pleistocene western Mediterranean sapropel. Geochim Cosmochim Acta 73(6):1651–1665
Gobeil C, Macdonald RW, Smith JN (1999) Mercury profiles insediments of the Arctic Ocean Basins. Environ Sci Technol 33:4194–4198
Gustin MS (2003) Are mercury emissions from geologic sources significant? A status report. Sci Total Environ 304(1–3):153–167
Hammerschmidt CR, Fitzgerald WF (2006) Methylmercury cyclingb in sediments on the continental shelf of southern New England. Geochim Cosmochim Acta 70:918–930
Hammerschmidt CR, Fitzgerald WF, Balcom PH, Visscher PT (2008) Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Harbor. Mar Chem 109:165–182
Hedgecock IM, Pirrone N (2004) Chasing Quicksilver: modeling the atmospheric lifetime of Hg0 (g) in the marine boundary layer at various latitudes. Environ Sci Technol 38:69–76
Hedgecock IM, Pirrone N, Sprovieri F, Pesenti E (2003) Reactive gaseous mercury in the marine boundary layer: modelling and experimental evidence of its formation in the Mediterranean region. Atmos Environ 37(Supplement 1):41–49
Hedgecock IM, Pirrone N, Trunfio GA, Sprovieri F (2006) Integrated mercury cycling, transport, and air–water exchange (MECAWEx) model. Journal of Geophysical Research—Atmospheres 111(D20302), doi:10.1029/2006JD007117
Heimbürger LE, Cossa D, Marty JC, Migon C, Averty B, Dufour A, Ras J (2010) Methyl mercury distributions in relation to the presence of nano- and picophytoplankton in an oceanic water column (Ligurian Sea, North-western Mediterranean). Geochim Cosmochim Acta 74(19):5549–5559
Hines ME, Horvat M, Faganeli J, Bonzongo JC-J, Barkay T, Major EB, Scott KJ, Bailey EA, Warwick JJ (2000) Mercury biogeochemistry in the Idrija River, Slovenia, from above the Mine into the Gulf of Trieste. Environ Res 83:129–139
Hines ME, Faganeli J, Adatto I, Horvat M (2006) Microbial mercury transformations in marine, estuarine and freshwater sediment downstream of the Idrija mercury mine, Slovenia. Appl Geochem 21(11):1924–1939
Hollweg TA, Gilmour CC, Mason RP (2009) Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental marin. Mar Chem 114:86–101
Horvat M, Covelli S, Faganeli J, Logar M, Mandić V, Rajar R, Širca D, Žagar D (1999) Mercury in contaminated coastal environments; a case study: the Gulf of Trieste. Sci Total Environ 237(238):43–56
Horvat M, Kotnik J, Fajon V, Logar M, Zvonaric T, Pirrone N (2001) Speciation of mercury in waters of the Mediterranean Sea. Mater Geoenviron 48:24–252
Horvat M, Kotnik J, Fajon V, Logar M, Zvonaric T, Pirrone N (2003) Speciation of mercury in surface and deep seawater in the Mediterranean Sea. Special issue of atmospheric environment. Atmos Environ 37(Supplement 1):93–108
Kim JP, Fitzgerald WF (1988) Gaseous mercury profiles in the Tropical Pacific Oceans. Geophys Res Lett 15:40–43
Kinder TH, Parrilla G (1987) Some of the Mediterranean outflow does come from great depth. J Geophys Res 92(c3):2901–2906
Kock HH, Bieber E, Ebinghaus R, Spain TG, Thees B (2005) Comparison of long-term trends and seasonal variations of atmospheric mercury concentrations at the two European coastal monitoring stations Mace Head, Ireland, and Zingst, Germany. Atmos Environ 39(39):7549–7556
Kontas A (2006) Mercury in the Izmir Bay: an assessment of contamination. J Marine Syst 61:67–78
Kotnik J, Horvat M, Tessier E, Ogrinc N, Monperrus M, Amouroux D, Fajon V, Gibicar D, Žižek S, Sprovieri F, Pirrone N (2007) Mercury speciation in surface and deep waters of the Mediterranean Sea. Mar Chem 107:13–30
Lamborg CH, Von Damm KL, Fitzgerald WF, Hammerschmidt CR, Zierenberg R (2006) Mercury and monomethylmercury in fluids from Sea Cliff submarine hydrothermal field, Gorda Ridge. Geophys Res Lett 33, L17606
Landis M, Stevens R, Schaedlich F, Prestbo E (2002) Development and characterization of an annular denuder methodology for the measurement of divalent inorganic reactive gaseous mercury in ambient air. Environ Sci Technol 36:3000–3009
Lanzillotta E, Ferrara R (2001) Daily trend of dissolved gaseous mercury concentration in coastal seawater of the Mediterranean Basin. Chemosphere 45:935–940
Lanzillotta E, Ceccarini C, Ferrara R (2002) Photo-induced formation of dissolved gaseous mercury in coastal and offshore seawater of the Mediterranean Basin. Sci Total Environ 300:179–187
Laurier, FJG, Mason, RP, Whalin, L and Kato S (2003) Reactive gaseous mercury formation in the North Pacific Ocean’s marine boundary layer: a potential role of halogen chemistry. Journal of Geophysical Research 108: doi:10.1029/2003JD003625. ISSN: 0148-0227
Lindberg S, Bullock R, Ebinghaus R, Engstrom D, Feng XB, Fitzgerald W, Pirrone N, Prestbo E, Seigneur C (2007) A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 36(1):19–32
Liu, B, Schaider, LA, Mason, RP, Bank, MS, Rabalais, NN, Swarzenski, PW, Shine, JP, Hollweg, TA, Senn DB (2009) Disturbance impacts on mercury dynamics in northern Gulf of Mexico sediments. Journal of Geophysical Research—Biogeosciences 114, G00C07
Maselli V, Trincardi F, Cattaneo A, Ridente D, Asioli A (2010) Subsidence pattern in the central Adriatic and its influence on sediment architecture during the last 400 kyr. J Geophys Res 115, B12106. doi:10.1029/2010JB007687
Mason RP (2009) Mercury emissions from natural processes and their importance in the global mercury cycle. Chap. 7. Springer, New York, pp. 173–191, 4723, 4724
Mason RP, Fitzgerald WF (1990) Alkylmercury in the equatorial Pacific. Nature 374:457–459
Mason RP, Fitzgerald WF (1993) The distribution and biogeochemical cycling of mercury in the equatorial Pacific Ocean. Deep-Sea Res 40:1897–1907
Mason RP, Gill GA (2005) Mercury in the marine environment. In: Parsons MB, Percival JB (eds) Mercury: sources, measurements, cycles and effects. Short Course Series 34. Mineralogical Association of Canada, Québec, pp. 179–216
Mason, RP, Sheu GR (2002) Role of the ocean in the global mercury cycle. Global Biogeochemical Cycles 16 (4), 40–1–40–14
Mason R, Sullivan K (1999) The distribution and speciation of mercury in the south and equatorial Atlantic. Deep Sea Res II Top Stud Oceanogr 46(5):937–956
Mason RP, Fitzgerald WF, Morel FMM (1994) The biogeochemical cycling of elemental mercury: anthropogenic influences. Geochim Cosmochim Acta 58:3191–3198
Mason RP, Morel FMM, Hemond HF (1995a) The role of microorganisms in elemental mercury formation in natural waters. Water Air Soil Pollut 80:775–789
Mason RP, Reinfelder JR, Morel FMM (1995b) Methylated and elemental mercury cycling in surface and deep ocean waters of the North Atlantic. Air Soil Pollut 80:665–677
Mason R, Rolfhus K, Fitzgerald W (1998) Mercury in the North Atlantic. Mar Chem 61(1–2):37–53
Mason RP, Lawson NM, Lawrence AL, Leaner JJ, Lee JG, Sheu GR (1999) Mercury in the Chesapeake Bay. Mar Chem 65(1–2):77–96
Mason R, Lawson N, Sheu GR (2001a) Mercury in the Atlantic ocean: factors controlling air–sea exchange of mercury and its distribution in the upper waters. Deep-Sea Res II Top Stud Oceanogr 48(13):2829–2853
Mason PR, Lawson NM, Sheu G-R (2001b) Mercury in the Atlantic Ocean: factors controlling air–sea exchange of mercuryand its distribution in the upper waters. Deep-Sea Res II 48:2829–2853
Mikac N, Niessen S, Oudane B, Wartel M (1999) Speciation of mercury in sediments of the Seine estuary (France). Appl Organomet Chem 13:1–11
Mikac N, Roje V, Cukrov N, Foucher D (2006) Mercury in aquatic sediments and soils from Croatia. Arch Ind Hyg Toxicol (Arh Hig Rada Toksikol) 57(3):325–332
Monperrus M, Tessier E, Amouroux D, Leynaert A, Huonnic P, Donard O (2007a) Mercury methylation, demethylation and reduction rates in coastal and marine surface waters of the Mediterranean Sea. Mar Chem 107(1):49–63
Monperrus M, Tessier E, Point D, Vidimova K, Amouroux D, Guyoneaud R, Leynaert A, Grall J, Chauvaud L, Thouzeau G, Donard O (2007b) The biogeochemistry of mercury at the sediment-water interface in the Thau Lagoon. 2. Evaluation of mercury methylation potential in both surface sediment and the water column. Estuar Coast Shelf Sci 72(3):485–496
Morel FMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566
Munthe J, Wangberg I, Pirrone N, Iverfeld A, Ferrara R, Ebinghaus R, Feng R, Gerdfelt K, Keeler GJ, Lanzillotta E, Lindberg SE, Lu J, Mamane Y, Prestbo E, Schmolke S, Schroder WH, Sommar J, Sprovieri F, Stevens RK, Stratton W, Tuncel G, Urba A (2001) Intercomparison of methods for sampling and analysis of atmospheric mercury species. Atmos Environ 35:3007–3017
Mzoughi N, Stoichev T, Dachraoui M, El Abed A, Amouroux D, Donard OFX (2002) Inorganic mercury and methylmercury in surface sediments and mussel tissues from a microtidal lagoon (Bizerte, Tunisia). J Coast Conservat 8(2):141–145
Nightingale PD, Malin G, Law CS, Watson AJ, Liss PS, Liddicoat MI, Boutin J, Upstill-Goddard RC (2000) In situ evaluation of air–sea gas exchange parameterization using novel conservative and volatile tracers. Glob Biogeochem Cycles 14(1):373–387
Ogrinc N, Monperrus M, Kotnik J, Vidimova K, Horvat M, Fajon V, Amouroux D, Kocman D, Tessier E, Žižek S (2007) Distribution of mercury and methylmercury in deep-sea surficial sediments of the Mediterranean Sea. Mar Chem 107:31–48
Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S (2006) Global anthropogenic mercury emission inventory for 2000. Atmos Environ 40:4048–4063
Pacyna EG, Pacyna JM, Sundseth K, Munthe J, Kindbom K, Wilson S et al (2010) Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmos Environ 44(20):2487–2499
Piani R, Covelli S, Biester H (2005) Mercury contamination in Marano Lagoon (Northern Adriatic sea, Italy): source identification by analyses of Hg phases. Appl Geochem 20:1546–1559
Pirrone, N and Mason R (2009) In: Pirrone, N., and Mason, R. (eds) Mercury fate and transport in the global atmosphere: emissions, measurements and models. Springer, New York: ISBN: 978-0-387-93958-2, p. 637
Pirrone N, Keeler GJ, Nriagu JO, Warner PO (1996) Historical trends of airborne trace metals in Detroit from 1971 to 1992. Water air Soil Pollut 88:145–165
Pirrone N, Pacyna JM, Barth H (2001) Preface to the special issue of atmospheric environment mercury research in Europe. Atmos Environ 35:2977
Pirrone N, Ferrara R, Hedgecock IM, Kallos G, Mamane Y, Munthe J, Pacyna JM, Pytharoulis I, Sprovieri F, Voudouri A, Wangberg I (2003) Dynamic processes of atmospheric mercury over the Mediterranean Region. Atmos Environ 37(S1):21–40
Pirrone N, Sprovieri F, Hedgecock IM, Trunfio A, Cinnirella S (2005) Dynamic processes of atmospheric mercury and its species in the Mediterranean region. In: Pirrone N, Mahaffey K (eds) Dynamics of mercury pollution on regional and global scales: atmospheric processes, human exposure around the world. Chapter-14. Springer, New York, pp. 541–579
Pirrone N, Hedgecock IM, Sprovieri F (2008) New directions: atmospheric mercury, easy to spot and hard to pin down: impasse? Atmos Environ. doi:10.1016/j.atmosenv.2008.09.004
Pirrone N, Cinnirella S, Feng X, Finkelman R, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher G, Streets DG, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10:1–33
Point D, Monperrus M, Tessier E, Amouroux D, Chauvaud L, Thouzeau G, Jean F, Amice E, Grall J, Leynaert A, Clavier J, Donard O (2007) Biological control of trace metal and organometal benthic fluxes in a eutrophic lagoon (Thau Lagoon, Mediterranean Sea, France). Estuar Coast Shelf Sci 72(3):457–471
Pruysers PA, de Lange GJ, Middelburg JJ, Hydes DJ (1993) The diagenetic formation of metal-rich layers in sapropel-containing sediments in the eastern Mediterranean. Geochim Cosmochim Acta 57:527–536
Rajar R, Cetina M, Horvat M, Zagar D (2007) Mass balance of mercury in the Mediterranean Sea. Mar Chem 107:89–102
Ribera d’Alcalá, M, Civitarese, G, Conversano, F, and Lavezza R (2003) Nutrient fluxes and ratios hint at overlooked processes in the Mediterranean Sea, J. Geophys. Res., 108, 8106, doi:10.1029/2002JC001250
Roth I, Hornung H (1977) Heavy metal concentrations in water, sediments, and fish from Mediterranean coastal area, Israel. Environ Sci Technol 11(3):265–269
Schroeder W, Munthe J (1998) Atmospheric mercury—an overview. Atmos Environ 32(5):809–822
Sheu GR, Mason RP, Lawson NM (2001) Speciation and distribution of atmospheric mercury over the Northern Chesapeake Bay Chemicals in the Environment. ACS Books, Washington, DC
Slemr F, Brunke E-G, Ebinghaus R, Kuss J (2011) Worldwide trend of atmospheric mercury since 1995. Atmos Chem Phys 11:4779–4787. doi:10.5194/acp-11-4779-2011
Sprovieri F, Pirrone N (2008) Spatial and temporal distribution of atmospheric mercury species over the Adriatic Sea. Environ Fluid Mech 8(2):117–128
Sprovieri F, Pirrone N, Hedgecock IM, Landis M, Stevens R,K (2002) Intensive atmospheric mercury measurements at Terra Nova Bay in Antarctica during November and December 2000. J Geophys Res 107(D23):4722–4729
Sprovieri F, Pirrone N, Gardfeldt K, Sommar J (2003) Atmospheric mercury speciation in the marine boundary layer along 6000 km cruise path over the Mediterranean Sea. Atmos Environ 37(S1):63–72
Sprovieri F, Pirrone N, Landis M, Stevens RK (2005a) Oxidation of Gaseous elemental mercury to gaseous divalent mercury during 2003 Polar Sunrise at Ny Alesund. Environ Sci Technol 39(23):9156–9165
Sprovieri F, Pirrone N, Landis MS, Stevens RK (2005b) Atmospheric mercury behavior at different altitudes at Ny Alesund during Spring 2003. Atmos Environ 39:7646–7656
Sprovieri F, Hedgecock IM, Pirrone N (2010a) An investigation of the origins of reactive gaseous mercury in the Mediterranean marine boundary layer. Atmos Chem Phys 10:3985–3997
Sprovieri F, Pirrone N, Ebinghaus R, Kock H, Dommergue A (2010b) A review of worldwide atmospheric mercury measurements. Atmos Chem Phys 10:8245–8265
Storelli M, Stuffler R, Marcotrigiano G (2002) Total and methylmercury residues in tuna-fish from the Mediterranean Sea. Food Addit Contam 19(8):715–720
Storelli M, Giacominelli-Stuffler R, Storelli A, Marcotrigiano G (2005) Accumulation of mercury, cadmium, lead and arsenic in swordfish and bluefin tuna from the Mediterranean Sea: a comparative study. Mar Pollut Bull 50(9):1004–1007
Strode SA, Jaeglé L, Selin NE, Jacob DJ, Park RJ, Yantosca RM, Mason RP, Slemr F (2007) Air-sea exchange in the global mercury cycle, Global Biogeochem. Cycles, 21, GB1017. doi:10.1029/2006GB002766
Van Santvoort PJM, de Lange GJ, Thomson J, Cussen H, Wilson TRS, Krom MD, Ströhle K (1996) Active post-depositional oxidation of the most recent sapropel (S1) in sediments of the Eastern Mediterranean. Geochim Cosmochim Acta 60:4007–4024
Wängberg I, Munthe J, Pirrone N, Iverfeldt Å, Bahlman E, Costa P, Ebinghaus R, Feng X, Ferrara R, Gårdfeldt K, Kock H, Lanzillotta E, Mamane Y, Mas F, Melamed E, Osnat Y, Prestbo E, Sommar J, Schmolke S, Spain G, Sprovieri F, Tuncel G (2001) Atmospheric mercury distributions in North Europe and in the Mediterranean Region. Atmos Environ 35:3019–3025
Wängberg I, Munthe J, Amouroux D, Andersson ME, Fajon V, Ferrara R, Gårdfeldt K, Horvat M, Mamane Y, Melamed E, Monperrus M, Ogrinc N, Yossef O, Pirrone N, Sommar J, Sprovieri F (2008) Atmospheric mercury at Mediterranean coastal stations. Environ Fluid Mech 8(2):101–116
Wanninkhof R (1992) Relationship between windspeed and gas exchange over the ocean. J Geophys Res 97:7373–7382
Zagar D, Sirnik N, Cetina M, Horvat M, Kotnik J, Ogrinc N, Hedgecock IM, Cinnirella S, De Simone F, Gencarelli CN, Pirrone N (2013) Mercury in the Mediterranean. Part 2: processes and mass balance. Environ Sci Pollut Res Int. doi:10.1007/s11356-013-2055-5
Zavatarelli M, Mellor GL (1995) A numerical study of the Mediterranean Sea circulation. J Phys Oceanogr 25:1384–1414
Zuo Z, Eisma D, Gieles R, Beks J (1997) Accumulation rates and sediment deposition in the northwestern Mediterranean. Deep-Sea Res II 44(3–4):560–597
Acknowledegments
This work was done within the framework of different EU founded projects (MAMCS/MOE, MERCYMS and on-going GMOS project) and National Italian (i.e. MedOceanor) and Slovenian founded research programmes and projects (P1-0143). The authors would like to thank the captains and crew of the R/V Urania for their help during the cruises.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vera Slaveykova
Rights and permissions
About this article
Cite this article
Kotnik, J., Sprovieri, F., Ogrinc, N. et al. Mercury in the Mediterranean, part I: spatial and temporal trends. Environ Sci Pollut Res 21, 4063–4080 (2014). https://doi.org/10.1007/s11356-013-2378-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-2378-2