Abstract
Purpose
The Haemoglobin E (HbE) trait is a common inherited haemoglobinopathy. HbE carriers have mild hypochromic microcytic erythrocytes results in susceptibility to oxidative damage. Acute exhaustive exercise effects on metabolism elevation contribute to more reactive oxygen species (ROS) formation. This study was to determine and compare the levels of total antioxidant capacity (TAC) and oxidative markers, such as malondialdehyde (MDA), protein carbonyl (PC), and 8-hydroxy-2’-deoxyguanosine (8-OHdG) after an acute single bout of treadmill exercise in HbE-trait subjects and healthy controls.
Methods
Fourteen subjects with the HbE trait and 14 healthy subjects performed the treadmill exercise at 80% maximum heart rate using the Bruce protocol. Blood samples were collected for oxidative stress analysis at pre-exercise, post-exercise, and 60 min post-exercise.
Results
At baseline, the TAC levels in HbE traits were significantly higher than that of the control group (P = 0.021). The TAC levels in HbE subjects were elevated after exercise and subsequently reduced significantly at recovery (P = 0.012 when compared with pre-exercise). Furthermore, TAC in the control group at different time points displayed no significant differences. Moreover, the levels of MDA and PC in both HbE subjects and healthy controls tended to increase after exercise and returned to baseline 60 min post-exercise. Furthermore, the 8-OHdG level tended to continuously increase over time in both groups.
Conclusion
This study suggested that HbE-trait subjects displayed loss of oxidative stress homeostasis after acute exhaustive exercise. Therefore, oxidative stress imbalance in HbE carriers could be considered when performing the strenuous activity in daily life.
Thai clinical trials registry number
TCTR20201103005, date of registration: 3 November 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
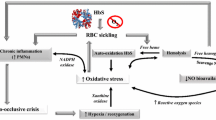
Hemoglobin E (HbE) is a hemoglobinopathy commonly found in Southeast Asia including Thailand [1]. It is classified into the β globin chain abnormality resulting from a substitution of glutamic acid by lysine at codon 26 of the β globin gene. This mutation causes β globin chain reduction and contributes to a thalassemia [2]. Moreover, HbE is able to combine with other forms of beta-globin, such as in βE-thalassemia, which has been identified as a significant health problem in Thailand [3, 4]. Beta-thalassemia patients’ have unstable and abnormal red blood cells and also have increased iron accumulation, as well as platelet and vascular stimulation, in their tissues due to haemolysis [3, 4]. Iron accumulation in red blood cells generates free radicals, which causes diverse clinical complications and multiple organ dysfunction [5]. Although HbE carriers are usually asymptomatic, and their RBCs are characterised by minimal morphological disorder, their RBCs still display genetic and hemoglobin level abnormalities, which may result in high free-radical production [1].
The potential harmful effects of free radicals are cellular disorganisation and destruction, affecting DNA, proteins, and lipids. Lipid damage produces malondialdehyde (MDA) as a by-product. Furthermore, protein carbonyl (PC) is a product of protein oxidation or protein carbonylation [6, 7]. Additionally, DNA deterioration is usually performed via 8-hydroxy-2’-deoxyguanine (8-OHdG) [8]. To correct an oxidative imbalance, various antioxidant mechanisms are activated, including vitamin E, glutathione (GSH), and superoxide dismutase (SOD; a superoxide radical scavenger) [9]. Previous oxidative stress reports have demonstrated that β-thal/HbE patients have elevated oxidant levels of MDA, reactive oxygen species (ROS), PC, and SOD enzymatic antioxidants, whereas GSH is decreased [10, 11]. In β-thalassemia major and β-thalassemia carriers, the concentration of total antioxidant status (TAS) and MDA are higher than that of healthy controls [12, 13]. However, the minimal amount of information available related to oxidative stress in HbE patients remains unclear and inconsistent. Some reports have claimed that glutathione peroxidase (GPx) is no difference between HbE carriers and control, whereas TAS is low [14]. However, Chakraborty et al. reported the reduction of GPx in HbE patients [10]. Thus, oxidative stress and iron overload may play key roles in the pathophysiology of thalassemia and its associated complications.
Regular exercise is considered beneficial and is able to reduce the risk of disorders of the cardiovascular, endocrine, and osteomuscular systems, as well as immune system diseases and the onset of neoplasms. Intense physical exercise results in an elevated energy requirement, increasing oxidative phosphorylation considerably leading to reactive oxygen species (ROS) overproduction and subsequent oxidative damage in biological molecules [15, 16]. Exercise-associated physiological pathways, including those involved in muscle injury, enlarged phagocytic activity, disruption to iron-containing proteins, and the production of xanthine oxidase can also result in altering the balance between pro-oxidants and antioxidants [17]. There is convincing evidence of the risks of oxidative stress induced by intense exercise is not only healthy individuals but also those with various acute and chronic diseases, including thalassemia [18,19,20]. The concentration of oxidative stress is generally high and antioxidant levels low after a single bout of exercise among patients with hemoglobinopathy, such as sickle cell anemia and β thalassemia/HbE [15, 20]. Several studies have evaluated the antioxidant and oxidant status of patients with thalassemia. Most of them have concentrated on severe and intermediate states and, thus, minimal information is provided for heterozygote profiles.
To summarize, evidence of the oxidative stress status in the HbE trait is inconsistent. Strangely, to our knowledge, only one study has considered exhaustive exercise as a factor affecting antioxidant levels. Palasuwan [14] reported that decreased glutathione peroxidase levels in HbE subjects fail to recover to baseline levels 45 min post-exercise; however, antioxidant levels were improved in trained subjects. These data do not include the subjects’ oxidant status. Patients with HbE traits have characteristics similar to healthy individuals. Therefore, they might engage in activities without considering potential health complications such as muscle pain and damage, oxidative stress imbalance leading to tissue injury, or hemolysis anemia. In this study, we attempt to elucidate whether a single bout of exhaustive exercise has an effect on the antioxidant and oxidant status of HbE carries. We hypothesise that the oxidant/antioxidant status of individuals with HbE traits is similar to healthy controls. We expect a poorer oxidative balance in individuals with HbE traits after exercise than in healthy controls. We believe that this study has the potential to provide useful information for HbE carriers regarding strenuous activities or even daily life.
Methods
Participants
The case–control study was conducted from January 2015 to June 2016. Fourteen HbE carriers and 14 healthy subjects were recruited for the study. Healthy participants and HbE-trait subjects who reported no regular exercise and who did not take any antioxidant supplements were assigned to perform a single bout of intense physical exercises. The groups of HbE carriers and controls were determined by HbE screening using a dichlorophenol indophenol precipitation (DCIP) test (KKU-DCIP Reagent Kit, Thailand) and complete blood count (mean corpuscular volume [MCV] < 80 fL), and confirmed by Hb typing with capillary electrophoresis (Capillarys 2 Flex Piercing, Sebia, France). The protocol was approved by the Human Research Ethics Committee (REC 57-333-19–9) and complied with guidelines set by the Declaration of Helsinki. The subjects were volunteers and gave their written informed consent and agreed before participating in the study (Fig. 1). This research was registered Thai clinical trials registry with the registration number TCTR20201103005.
Physical exercise programme
The exercise programme was conducted at the department of Physical therapy, Faculty of medicine in Songklanagarind hospital. All participants performed an experiment under safety circumstances, under ethical human trials and with competent cardiorespiratory physiotherapist and rehabilitation and the best and most effective exercise equipment.
Gender- and age-matched HbE carriers (n = 14) and healthy controls (n = 14) completed a medical examination, including height, weight, blood pressure, heart rate (HR), and respiratory rate (RR) measurements. Moreover, maximal oxygen consumption (VO2 max), which is the maximal consumption of oxygen in 1 min per kilogram of body weight and indicates the physical fitness and endurance an individual, was estimated using the Bruce formula: VO2 max (mL/kg/min) = 14.76–(1.379 × T) + (0.451 × T2)–(0.012 × T3) for males and VO2 max (mL/kg/min) = (4.38 × T)–3.9 for female [21]. The subjects exercised on a standard treadmill (T9250S, Vision Fitness, UK) according to the Bruce protocol [22]. After a 1-min warm-up at a speed of 2.74 km/hr, the treadmill procedures, which increased progressively in 3-min stages, were followed until 80% of the participant’s submaximal heart rate was reached, and running was maintained at this stage for 5–10 min, until the onset of fatigue, as indicated by volitional exhaustion [23]. Each participant’s heart rate was measured throughout the exercise using a chest belt monitor (Polar FT1, Polar Electro, Kempele, Finland). The Bruce protocol included a combination of increasing the treadmill speed and incline (Table 1).
Blood sample collection
Before beginning the treadmill exercise, 3 ml blood samples were collected in EDTA tubes (pre-exercise). Then, blood samples were obtained immediately after the end of exercise (post-exercise) and 1 h into recovery (recovery). Plasma was obtained by centrifugation at 4000 rpm for 10 min and kept at – 20 °C until antioxidant capacity and oxidative marker (MDA, PC, and 8-OHdG) analyses were conducted.
Total antioxidant capacity (TAC) by Trolox equivalent antioxidant capacity (TEAC) [24, 25]
The total antioxidant capacity (TAC) of biomolecules was determined based on the conversion of oxidised ABTS⋅+ (2, 2-azinobic-[3-ethybenzothaiazoline-6-sulphonic acid] diammonium salt; Sigma-Aldrich, USA) radicals to ABTS. Antioxidants neutralise radicals in a concentration-dependent manner, which is associated with a proportional decrease in color intensity (from blue to colourless). Antioxidant capacity in the sample was compared to known concentrations of Trolox standard, a vitamin E analogue, which varied from 0.0625 to 2.5 mM in a 96-well microtiter plate. One ml of ABTS⋅+ was mixed with 10 μl of plasma; then, it was incubated at room temperature for 6 min, and the absorbance was measured at 734 nm. Results were expressed in mM.
Malondialdehyde (MDA) by Thiobarbituric acid (TBA) [24, 25]
MDA levels were used as a marker for lipid peroxidation, as they are a widely used marker of oxidative stress. The concentration of plasma MDA was determined using thiobarbituric acid reactive substances (TBAR) according to a previous method, with minor modifications. The 160 μl plasma samples were mixed with 160 μl of 10% tricholoacetic acid (TCA), shaken vigorously, and then centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatants were incubated with 0.67% TBA at 95 °C for 10 min. After cooling, the level of TBARs (pink chromogen) was measured at 532 nm using a nanodrop spectrophotometer (ThermoScientific, USA), with 1,1,3,3-tetraethoxypropane as the standard. Results were expressed in μM.
Protein carbonyls (PC) by 2,4-dinitrophenylhydrazine (DNPH) [26]
PCs were determined using the 2,4-dinitrophenolhydrazine (DNPH) spectrophotometric method according to the modified Levine method. Briefly, 200 μl of plasma was incubated with 800 μl of DNPH at room temperature for 1 h in the dark. Proteins were precipitated by 1 ml of 20% TCA for 5 min on ice and then centrifuged at 10,000 rpm for 10 min at 4 °C. The pellets were suspended with 10% TCA on ice for 5 min, and centrifugation was repeated. The protein pellets were washed three times with 1 ml of 1:1 ethanol/ethyl acetate and resuspended with 500 μl of 6 M guanidine hydrochloride; next, they were centrifuged at 10,000 rpm for 10 min. The carbonyl content was measured at a wavelength of 375 nm with an ELISA microplate spectophotometer (PowerWave XS2, Biotex, USA). PC levels were calculated using the molar absorption coefficient, 22,000 M− 1 cm− 1. The results were expressed in nmol/L.
8-hydroxy-2’-deoxyguanosine (8-OHdG) as a measure of DNA/RNA oxidative damage.
The level of oxidative-damaged guanine, such as 8-OHdG, in the plasma was determined using an ELISA kit (Cayman Chemical, USA) according to the manufacturer’s recommended procedure. This assay is based on competition between 8-OHdG in the plasma sample and 8-OHdG acetylcholinesterase conjugate (an 8-OHdG tracer) for a limited number of DNA/RNA oxidative damage monoclonal antibodies. The amount of tracer able to bind with monoclonal antibodies is inversely proportional to the concentration of 8-OHdG. The results were reported as ng/ml.
Statistical analysis
Statistical analyses were performed using IBM SPSS version 23 software (SPSS Inc., Chicago, USA). All data were presented as the mean ± SD. The distribution of the continuous variables was checked for normality before the analysis. Then the student’s t-test was used to compare the results between HbE-trait subjects and normal controls. Moreover, the difference between mean values of the antioxidant and oxidant markers across the pre-exercise, post-exercise, and recovery were compared within groups using one-way repeated measure analysis of covariance (repeated measure ANCOVA). The oxidative stress markers: TAC, MDA, PC, and 8-OHdG levels at pre-exercise were separately used as a covariate in the ANCOVA. Pairwise post hoc hypotheses were tested the significant association within the group using the Bonferroni correction for multiple comparisons. For all statistical data, P < 0.05 was considered significant.
Results
HbE trait screening
The participants were screened for HbE using positive DCIP and complete blood count (CBC) results, and their status was confirmed using Hb typing. Haematological parameters demonstrated that MCV and mean corpuscular hemoglobin (MCH) were significantly lower in HbE-trait subjects than in controls. However, the number of RBCs and red cell distribution widths (RDWs) were significantly higher in HbE subjects than in healthy controls. Thus, RBCs were hypochromic microcytic in HbE carriers. According to hemoglobin typing, the amounts of HbE in HbE carriers ranged from approximately 23% to 26% (data not shown).
Anthropometric and fitness characteristics
Sex- and age-matched participants performed an exertional treadmill exercise, and their physical fitness was measured (Table 2). The results revealed no significant differences between the two groups in various parameters. However, we found lower heart rates in HbE individuals post-exercise when compared with controls. The baseline heart rate in HbE carriers was lower than in controls; furthermore, rapidly increasing heart rate values appeared to be smaller in HbE carriers than in controls. However, their heart rate was still within the normal range. Thus, both groups displayed similar physical fitness. This result indicates that participants with HbE traits who displayed certain weaknesses in terms of RBCs did not demonstrate poor fitness. Both HbE carriers and controls had similar strength; therefore, no confounding factors affected the results of the oxidative status examination.
Total antioxidant capacity (TAC) at baseline and after submaximal exercise
At pre-exercise, the level of total antioxidants was significantly higher in HbE carriers than in the control group (HbE 4.348 ± 0.140 mM, control 4.114 ± 0.315 mM,P = 0.021) (Fig. 2). There is a time effect to the level of TAC, therefore, ANCOVA testing considering TAC at baseline as covariate was performed. The TAC level at post-exercise (HbE 4.365 ± 0.090 mM and control 4.174 ± 0.379 mM) and recovery (HbE 3.998 ± 0.384 mM and control 4.178 ± 0.412 mM) did not differ significantly between both groups (adjusted P-value 0.096 and 0.220, respectively). Time difference analysis within-group revealed that the level of TAC in HbE carriers at recovery was statistically significant lower than pre-exercise (P = 0.012). Furthermore, the level of total antioxidants did not reach statistical significance at any time point in controls (Fig. 2).
Total antioxidant capacity (TAC) in HbE carriers and controls at pre-exercise, post-exercise, and recovery. Values are presented as means ± SD. #Significant difference between HbE trait and control group using student’s t-test. *Significant difference between categories within the group using the Bonferroni multiple comparison post-hoc test
The concentrations of oxidative stress markers
The levels of MDA, PC, and 8-OHdG were determined in both E-trait and healthy control groups. There were no significant differences between HbE and normal subjects in MDA, PC, and 8-OHdG concentrations (Fig. 3). When the results were analysed according to the time difference, we found no statistical difference for MDA in both the HbE and normal control groups at baseline and submaximal exercise. In addition, at the recovery state, PC levels were significantly lower than at the post-exercise state (P = 0.001) in the normal group. However, PC concentrations tended to increase immediately post-exercise and declined progressively toward baseline values within 1 h of recovery in both groups. On the other hand, the 8-OHdG levels tended to continuously increase over time in either HbE-trait subjects or normal controls (Fig. 3).
Malondialdehyde (MDA), Protein carbonyl (PC), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in HbE carriers and controls at pre-exercise, post-exercise, and recovery. Values are presented as means ± SD. *Significant difference between categories within the group using the Bonferroni multiple comparison post-hoc test
Discussion
HbE trait carriers’ physical fitness was identical to that of healthy controls, indicating their oxidant/ antioxidant status might not have been confounded by strength or physical ability. The main results of this study demonstrate that the TAC level at baseline was significantly higher in HbE carriers than in healthy controls. These results are similar to those of previous studies. Some studies have documented that TAC levels are significantly higher in thalassemic patients, including β-thalassemia major [14]. Kalpavidh’s [11] study revealed increased SOD and GPx in thalassemia/HbE patients, while Palasuwan [27] reported that GPx activity is higher in individuals with the HbE trait than in controls. The increased antioxidant status of individuals with HbE traits is due to the compensatory antioxidant response arising from an excessive oxidative stress-dependent elevation in the proportion of younger RBCs which were increasing to compensate for the low Hb level. This explanation suggests erythrocytes may play a role as a protective mechanism in reducing pro-oxidants.
Oxidant markers in this study, including MDA, PC, and 8-OHdG, displayed no significant differences in individuals when compared between HbE traits and healthy controls. The oxidant status results at baseline levels were in line with Palasuwan et al. [27] study, suggesting MDA concentrations in HbE carriers and controls did not differ significantly. However, Kalpravidh et al. [11] found that in β-thalassemia subjects with HbE, MDA levels increased along with antioxidant concentrations when compared with healthy controls. Previous studies have supported the association between elevated MDA plasma levels and antioxidant capacity and thalassemia [12, 13, 28]. Our baseline oxidative marker results suggest that in daily life, HbE carriers may have good physical fitness and an oxidative balance similar to healthy controls.
This study produced findings regarding the balance in oxidation after vigorous activity in HbE carriers. All participants engaged in an acute exhaustive exercise as an extensive activity model. The important findings are the significant TAC reduction in the recovery state in HbE-trait subjects when compared with pre-exercise, whereas no effects are observed in the control group. This finding demonstrates that HbE carriers have an impaired physiological adaptation that counterbalances the increase in pro-oxidants following exercise. Data on the impact of exercise on serum TAC and oxidative markers for the HbE trait are not available in the literature. Previous studies have investigated the effect of exercise on GPx. The data showed failure in antioxidant turnover at 45 min after exercise to the baseline [18]. In addition, Faes C. depicted results on anti-oxidant status that the levels of SOD and GPX were higher at postexercise, 1 and 24 h into recovery, while the catalase activities were lower at 2 and 24 h after exercise when compared with baseline [29]. In contrast to the present study, Chaki [23] reported elevated SOD levels in pubertal males immediately after exercise. Moreover, Bogdanis [19] found that TAC, GPx, lipid peroxidation, and PC were elevated above baseline 24 h post-exercise in healthy men. We proposed that the impaired antioxidant mechanism in HbE carriers could be caused by two mechanisms. First, the assessment of antioxidant only in the plasma might not represent the whole activity of TAC because the RBCs also play a crucial role in antioxidant capacity [29]. Second mechanism was the haemolysis of unstable RBCs. Haemolysis could not only increase iron molecules or ROS production but also decrease the level of antioxidant molecules in the body, leading to a loss of antioxidant capacity. Furthermore, exercise-induced haemolysis contributed to various mechanisms including physical and metabolic mechanisms. Direct mechanical injuries, such as repeated muscle contractions, can cause erythrocyte rupture. Moreover, metabolic factors (e.g., hyperthermia, hypoxia, shear stress, dehydration, and oxidative damage) can contribute to RBC membrane impairment. Therefore, patients with certain erythrocyte disorders, such as the HbE trait, may be vulnerable to increased exercise-induced haemolysis [30].
Oxidant markers, the MDA and PC concentrations, tended to increase immediately after exercise and decline toward baseline values in the hour post-exercise, while the expression of 8-OHdG was not significantly different. The exercise-induced free radical production, resulting in increased levels of oxidative markers. In line with our study, Bloomer et al.[16] demonstrated that MDA and PC levels were elevated after exercise in healthy subjects and gradually decreased at 1, 6, and 24 h post-exercise. However, 8-OHdG levels were not significantly different. The increase in PC concentrations immediately post-exercise may be due to an increased oxygen influx in mitochondria, leading to an increased leakage of free radicals. A slight elevation in MDA levels immediately after exercise may result from not only the short half-life of MDA contributing to a rapid return to the baseline but also exercise intensity. The 8-OHdG levels might have remained unchanged because the short duration and level of exercise were insufficient to cause DNA damage. Additionally, 8-OHdG measurements derived from blood samples are not a good representation of damage because the concentration of 8-OHdG might be diluted when compared with concentrations in injured muscles or organs affected by exercise [16]. Contrary to our findings, MDA level remained increased in sickle cell trait during the whole recovery phase (1, 2, and 24 h), whereas, PC increased at postexercise and it return to baseline during recovery [29]. The different types of thalassemia could describe this inconsistency. Our data reveal the turnover of oxidative substances to the baseline in individuals with HbE traits, indicating the antioxidant defence system was stimulated to balance oxidant production. Overall, the results support our hypothesis that a poorer oxidative balance occurs in individuals with HbE traits after exercise than in healthy controls. However, the supplement with vitamin E, which is an essential antioxidant food, in HbE traits were able to improve antioxidant capacity and reduce lipid peroxidation [22, 27].
Despite the potential usefulness of the findings, there are some limitations that should be acknowledged. First, the sample size was rather small, some participants exercised less vigorously than others due to differences in endurance, which may have affected the level of oxidative stress. Second, we did not measure hemoglobin levels or iron status such as ferritin, which could have contributed to oxidative stress accumulation, to determine red blood cell lysis post-exercise. Third, it is possible that unassessed latent variables might have had an influence, and oxidative stress measures at 6 or 24 h post-recovery may be different from earlier measures. Accordingly, future studies might assess other time points to track changes in the oxidative balance in HbE trait carriers and evaluate other possible associated blood markers, such as muscle markers, iron status and antioxidant markers (for example SOD, GPx, and CAT). Indeed, although the HbE trait carriers were apparently normal and had a similar level of physical fitness as the healthy controls, the possibility of some degree of metabolic disturbance cannot be excluded.
Conclusions
Our results demonstrate that a bout of progressive acute exercise has no deleterious effects on oxidative stress in healthy persons. However, this type of exercise may impact oxidative stress in HbE carriers by reducing their antioxidant capacity. This data could be useful in cautioning individuals with HbE traits to consider the types or intensity of activities they engage in to prevent tissue or organ such as erythrocyte and muscle damage from oxidative imbalance. In addition, HbE carriers should consume more antioxidant supplements, including antioxidant-rich foods, for increased antioxidant efficiency.
Availability of data and material
The data used to support the findings of this study are included within the article.
Code availability
Not applicable.
References
Fucharoen S, Weatherall DJ (2012) The hemoglobin E thalassemias. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a011734
Vichinsky E (2007) Hemoglobin e syndromes. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2007.1.79
Kohne E (2011) Hemoglobinopathies: clinical manifestations, diagnosis, and treatment. Dtsch Arztebl Int 108(31–32):532–540. https://doi.org/10.3238/arztebl.2011.0532
Olivieri NF, Pakbaz Z, Vichinsky E (2011) Hb E/beta-thalassaemia: a common & clinically diverse disorder. Indian J Med Res 134(4):522–531
Chaichompoo P, Qillah A, Sirankapracha P, Kaewchuchuen J, Rimthong P, Paiboonsukwong K, Fucharoen S, Svasti S, Worawichawong S (2019) Abnormal red blood cell morphological changes in thalassaemia associated with iron overload and oxidative stress. J Clin Pathol 72(8):520–524. https://doi.org/10.1136/jclinpath-2019-205775
Ahmad R, Tripathi AK, Tripathi P, Singh S, Singh R, Singh RK (2008) Malondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemia. In Vivo 22(4):525–528
Ramana KV, Srivastava S, Singhal SS (2017) Lipid Peroxidation Products in Human Health and Disease 2016. Oxid Med Cell Longev 2017:2163285. https://doi.org/10.1155/2017/2163285
Sertan Copoglu U, Virit O, Hanifi Kokacya M, Orkmez M, Bulbul F, Binnur Erbagci A et al (2015) Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res 229(1–2):200–205. https://doi.org/10.1016/j.psychres.2015.07.036
Fibach E, Rachmilewitz EA (2010) The role of antioxidants and iron chelators in the treatment of oxidative stress in thalassemia. Ann N Y Acad Sci 1202:10–16. https://doi.org/10.1111/j.1749-6632.2010.05577.x
Chakraborty D, Bhattacharyya M (2001) Antioxidant defense status of red blood cells of patients with beta-thalassemia and Ebeta-thalassemia. Clin Chim Acta 305(1–2):123–129. https://doi.org/10.1016/s0009-8981(00)00428-9
Kalpravidh RW, Siritanaratkul N, Insain P, Charoensakdi R, Panichkul N, Hatairaktham S et al (2010) Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin Biochem 43(4–5):424–429. https://doi.org/10.1016/j.clinbiochem.2009.10.057
Bazvand F, Shams S, Borji Esfahani M, Koochakzadeh L, Monajemzadeh M, Ashtiani MT et al (2011) Total antioxidant status in patients with major beta-thalassemia. Iran J Pediatr 21(2):159–165
Ondei Lde S, Estevao Ida F, Rocha MI, Percario S, Souza DR, Pinhel MA et al (2013) Oxidative stress and antioxidant status in beta-thalassemia heterozygotes. Rev Bras Hematol Hemoter 35(6):409–413. https://doi.org/10.5581/1516-8484.20130122
Palasuwan A, Soogarun S, Suksom D, Pitaksathienkul C, Rousseau AS (2015) Antioxidant status in Hemoglobin E carriers after acute and chronic strenuous exercises. Res Sports Med 23(4):351–366. https://doi.org/10.1080/15438627.2015.1076412
Chirico EN, Martin C, Faes C, Feasson L, Oyono-Enguelle S, Aufradet E et al (2012) Exercise training blunts oxidative stress in sickle cell trait carriers. J Appl Physiol 112(9):1445–1453. https://doi.org/10.1152/japplphysiol.01452.2011
Bloomer RJ, Goldfarb AH, Wideman L, McKenzie MJ, Consitt LA (2005) Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J Strength Cond Res 19(2):276–285. https://doi.org/10.1519/14823.1
Filaire E, Toumi H (2012) Reactive oxygen species and exercise on bone metabolism: friend or enemy? Joint Bone Spine 79(4):341–346. https://doi.org/10.1016/j.jbspin.2012.03.007
Meijer EP, Coolen SA, Bast A, Westerterp KR (2001) Exercise-induced oxidative stress in older adults as measured by antipyrine oxidation. Metabolism 50(12):1484–1488. https://doi.org/10.1053/meta.2001.28086
Bogdanis GC, Stavrinou P, Fatouros IG, Philippou A, Chatzinikolaou A, Draganidis D et al (2013) Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem Toxicol 61:171–177. https://doi.org/10.1016/j.fct.2013.05.046
Fasmall RC, Martin C, Chirico EN, Feasson L, Oyonno-Enguelle S, Dubouchaud H et al (2012) Effect of alpha-thalassaemia on exercise-induced oxidative stress in sickle cell trait. Acta Physiol 205(4):541–550. https://doi.org/10.1111/j.1748-1716.2012.02434.x
Tapking C, Popp D, Herndon DN, Branski LK, Mlcak RP, Suman OE (2018) Estimated versus achieved maximal oxygen consumption in severely burned children maximal oxygen consumption in burned children. Burns 44(8):2026–2033. https://doi.org/10.1016/j.burns.2018.06.004
Powers SK, Radak Z, Ji LL (2016) Exercise-induced oxidative stress: past, present and future. J Physiol 594(18):5081–5092. https://doi.org/10.1113/JP270646
Chaki B, Pal S, Chattopadhyay S, Bandyopadhyay A (2019) High-intensity exercise-induced oxidative stress in sedentary pre-pubertal & post-pubertal boys: A comparative study. Indian J Med Res 150(2):167–174. https://doi.org/10.4103/ijmr.IJMR_2094_17
Chusak C, Thilavech T, Adisakwattana S (2014) Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects. Am J Chin Med 42(2):315–336. https://doi.org/10.1142/S0192415X14500219
Sompong W, Cheng H, Adisakwattana S (2015) Protective Effects of Ferulic Acid on High Glucose-Induced Protein Glycation, Lipid Peroxidation, and Membrane Ion Pump Activity in Human Erythrocytes. PLoS ONE 10(6):e0129495. https://doi.org/10.1371/journal.pone.0129495
Darvishi-Khezri H, Salehifar E, Kosaryan M, Karami H, Alipour A, Shaki F et al (2017) The impact of silymarin on antioxidant and oxidative status in patients with beta-thalassemia major: A crossover, randomized controlled trial. Complement Ther Med 35:25–32. https://doi.org/10.1016/j.ctim.2017.08.007
Palasuwan A, Sriprapun M, Dahlan W, Luechapudiporn R, Soogarun S (2008) Antioxidant protection in hemoglobin E trait subjects after vitamin E supplementation. Age (yrs) 22(4):25–10
Asif M, Manzoor Z, Farooq MS, Munawar SH, Aziz A, Khan IA (2015) Status of oxidant, antioxidantand serum enzymes in thalassaemic children receiving multiple blood transfusions. J Pak Med Assoc 65(8):838–843
Faёs C, Martin C, Chirico EN, Féasson L, Oyonno-Enguelle S, Dubouchaud H, Francina A, Thiriet P, Pialoux V, Messonnier L (2012) Effect of α-thalassaemia on exercise-induced oxidative stress in sickle cell trait. Acta Physiol (Oxf) 205(4):541–550. https://doi.org/10.1111/j.1748-1716.2012.02434.x
Lippi G, Sanchis-Gomar F Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann Transl Med. 2019;7(12):270. https://doi.org/10.21037/atm.2019.05.41
Acknowledgements
We also would like to express our gratitude to the staff, Pokpon Keereelak, Wesuni Jareemit, Haruthai Kamsuwanpojjana, and Krisak Sirimak, for their dedication in the study.
Funding
This work was supported by the Research Fund, Prince of Songkla University [MET590375S].
Author information
Authors and Affiliations
Contributions
Natthaphon Nanakorn: Conceptualization, Methodology, Investigation, Data analysis, Resources, Writing-Original Draft and Editing, and Visualization. Saikaew Chuechan: Methodology, Investigation, and Writing-Original editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Human and animal rights
The study is in agreement with the Declaration of Helsinki.
Ethical approval
The study received ethical approval from Human Research Ethics Committee (REC 57-333-19-9).
Informed consent
Participants were informed about the research at the beginning of the study that participation was voluntary and provided with further information about the study. Informed consent was implied by completing the questionnaire.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nanakorn, N., Chuechan, S. Impaired oxidative stress in Thalassemia-Hemoglobin E traits after acute exhaustive exercise. Sport Sci Health 18, 789–797 (2022). https://doi.org/10.1007/s11332-021-00857-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11332-021-00857-1