Abstract
Purpose
To investigate the impact of obstructive sleep apnea (OSA) on postoperative delirium (PD), and evaluate the effectiveness of positive airway pressure (PAP) therapy on PD among OSA patients.
Methods
We systematically searched Embase, Cochrane Library and PubMed databases from their establishment to November 27, 2022. A random-effects approach was employed to determine aggregated results. Subgroup and sensitivity analyses were carried out to investigate heterogeneity.
Results
Sixteen eligible studies were included in the analysis. Thirteen studies revealed that OSA significantly elevated the likelihood of developing PD (OR = 1.71; 95%CI = 1.17 to 2.49; p = 0.005). Subgroup analysis according to delirium assessment scales showed that OSA did not exhibit an association with the incidence of PD assessed by the Confusion Assessment Method-Intensive Care Unit (OR = 1.14; 95%CI = 0.77 to 1.67; p = 0.51) but enhanced the likelihood of developing PD evaluated with other measurement scales (OR = 2.15; 95%CI = 1.44 to 3.19; p = 0.0002). Three additional studies explored the impact of PAP treatment on PD among OSA individuals, indicating no significant reduction in PD incidence with PAP use (OR = 0.58; 95%CI = 0.13 to 2.47; p = 0.46).
Conclusions
OSA may not be a risk factor for PD in critically ill patients in the intensive care unit, but may increase the likelihood of developing PD among individuals receiving regular care in the ward postoperatively. The efficacy of PAP therapy in decreasing PD incidence among OSA patients remains debatable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Delirium manifests as an acute neuropsychiatric symptom marked by attention deficits, disruptions in consciousness, and impairments in perception. Delirium is observed at a substantial rate in postoperative patients, estimated between 11% and 51% [1, 2]. Postoperative delirium (PD) is linked to long-term cognitive decline, prolonged hospitalization, elevated mortality, and increased healthcare costs [2,3,4]. Therefore, identifying risk factors is essential for preventing the occurrence of PD.
Obstructive sleep apnea (OSA) is a common respiratory disease, with prevalence rates estimated between 9% and 38% in adults aged 18 and above [5]. Due to the potential for OSA to cause hypoxia and sleep structure disorders, there has been growing interest in exploring its association with delirium [6, 7]. However, the correlation between OSA and PD has still been controversial. Some observational studies suggested that OSA was predisposed to PD [8, 9]. A meta-analysis by Sun X and colleagues suggested that OSA increased the incidence of PD [10]. In contrast, He E and colleagues showed no link between OSA and an elevated risk of PD [11]. Previous reviews and meta-analyses may have limitations in their inclusion criteria, such as not specifically targeting PD as a postoperative complication [10] or not focusing on OSA-related sleep disturbances [11], potentially leading to incomplete literature inclusion. Moreover, recent large-sample and high-quality studies, such as King CR et al., presented conflicting results to previous major findings, indicating that OSA did not demonstrate an elevated risk for PD [10, 12, 13].
Therefore, we updated the meta-analysis by Sun X and colleagues to quantitatively explore the role of OSA on PD [10]. In addition, positive air pressure (PAP) is the preferred and efficacious treatment for OSA patients [14, 15]. Some randomized controlled trials (RCTs) have explored the role of PAP treatment on decreasing the likelihood of PD in OSA patients, but the conclusion has not been well established [16,17,18]. Thus, we also analyzed current evidence on the efficacy of PAP therapy in preventing PD among OSA individuals.
Methods
Search strategy
The current meta-analysis was conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Table S1 in supplementary file) [19]. Two authors (YX and PJL)independently performed a thorough search of relevant literature from the inception of Embase, PubMed, and Cochrane Library databases up to November 27, 2022. Furthermore, the authors examined the citation lists of the encompassed studies to discover any additional potentially eligible studies. Any inconsistencies were addressed by consulting the third contributor (ZAL). The search terms were: (“Sleep Apnea Syndromes” OR “Sleep Apnea, Obstructive” OR “Obstructive Sleep Apnea Syndrome” OR “Obstructive Sleep Apnea Hypopnea Syndrome” OR “Sleep Apnea, Sleep Disordered Breathing”) AND (“Postoperative Complications” OR “Confusion” OR “Delirium”), with no language restrictions.The detailed retrieval strategy for PubMed database was listed in Table S2 in supplementary file.
Inclusion and exclusion criteria
The inclusion criteria for evaluating the impact of OSA on postoperative complications were: (1) patients undergoing operation; (2) cohort or case-control studies which were divided into OSA and non-OSA groups to compare the effects of OSA on complications after surgery; and (3) postoperative complications involving delirium at least. Additionally, the criteria for assessing the effects of PAP therapy on complications following surgery were: (1) individuals diagnosed with OSA receiving surgical procedures; (2) RCTs which were divided into PAP and routine care groups to compare the effects of PAP on postoperative complications; and (3) postoperative complications involving delirium at least.
The exclusion criteria were: (1) non-adult patients (age < 18 years old); (2) studies not available in English; (3) incomplete data or unable to be extracted; or (4) no full article.
OSA was diagnosed by polysomnography (PSG), overnight oximetry, apnea-hypopnea index (AHI), or screening questionnaire, etc. Delirium was diagnosed by some delirium assessment tools or medical records. The routine care group was defined as receiving standard care equivalent to the PAP group, with the exception of not utilizing PAP therapy.
Data extraction
Data collection and extraction from the identified literature were conducted by two researchers (YX and PJL) in accordance with the predefined criteria. The third contributor (ZAL) was consulted to address any disagreements. The extracted data included study design, author’s name, sample size, publication date, population characteristics (gender, mean age, and body mass index [BMI]), anesthesia type, operation type, criteria for OSA, criteria for delirium, interventions for OSA, number of PD patients, and hospital length.
Study outcomes
The main result was the rate of PD between OSA and non-OSA groups. The secondary outcomes included the hospital length between OSA and non-OSA groups, and the PD rate in PAP and routine care groups in OSA patients.
Qualitative evaluation and bias assessment
The quality and potential bias of enrolled studies were independently evaluated by two researchers (YX and PJL), with any discrepancies being resolved by consulting the third author (ZAL). The Newcastle-Ottawa scale (NOS) was used to assess the quality of the included case-control and cohort studies [20]. The NOS has a total score of 10, with a score of 7 or higher considered indicative of high quality. The quality of enrolled RCTs was assessed by the Cochrane Collaboration’s Risk of Bias tool and categorized as low, high, or unclear risk [21].
Statistical analysis
We conducted statistical analysis using Review Manager 5.3 and Stata 15 software. Categorical variables were analyzed by using odds ratio (OR) and corresponding 95% confidence interval (CI) for the assessment of outcome effects. Continuous variables were assessed by using mean difference (MD) and its 95%CI [22]. When I2 > 50% and p < 0.05, it was considered as significant heterogeneity, and the random effects model was employed to analyze data. Exploration of heterogeneity involved sensitivity and subgroup analyses. The funnel plot and Begg’s test were used to explore publication bias. A statistically significant threshold was established at p < 0.05.
Results
Study selection
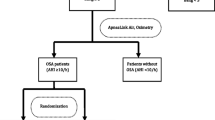
A flowchart outlining the selection process is displayed in Fig. 1. Following the database search, 8098 articles were initially enrolled, with an additional 4 articles included after screening the reference lists. 2988 articles were removed due to duplication checking. Subsequently, 5029 articles were removed after abstract assessment. 85 studies were remained for entire text assessment. Further exclusions were made based on the following criteria: PD not related to OSA (n = 1), postoperative complications not involving PD (n = 35), no control groups (n = 29), or not available in English (n = 4). Finally, 16 studies were enrolled in our meta-analysis.
Characteristics of included studies
The basic characteristics of the 16 enrolled studies with 12,806 patients [8, 13, 16,17,18, 23,24,25,26,27,28,29,30,31,32,33] are shown in Table 1. Of them, 13 studies [8, 13, 23,24,25,26,27,28,29,30,31,32,33] enrolling 12,386 patients described PD rates between OSA and non-OSA groups, including 6 retrospective studies and 7 prospective studies. The remaining 3 studies [16,17,18] involving 420 patients were all randomized controlled trails and illustrated the rate of PD in PAP and routine care groups. Patients in 6 studies [16,17,18, 23, 8, 30] underwent orthopedic surgery, while patients in the other studies [13, 24,25,26,27,28,29, 31,32,33] underwent other major surgeries. OSA diagnosis criteria varied across the studies, with 7 studies using PSG [23,24,25, 27, 8, 30, 33], while others based on the STOP-Bang questionnaire or other assessments [13, 16,17,18, 26, 28, 29, 31, 32]. For the delirium assessment tools, 5 studies [13, 25,26,27,28] used the Confusion Assessment Method-Intensive Care Unit (CAM-ICU), while the remaining studies [16,17,18, 23, 24, 29,30,31,32,33] adopted other measurement scales, such as Confusion Assessment Method (CAM), Delirium Rating Scale-Revised-98 (DRS.R-98), or Nursing Delirium Screening Scale (Nu-DESC). There are some differences between those delirium assessment tools. On the one hand, these tools are designed for different users. CAM is designed for non-psychiatrists [34], while DRs.R-98 is designed for psychiatrists [35], and Nu-DESC is designed for caregivers [36]. Most importantly, on the other hand, CAM-ICU aims to evaluate delirium in ICU patients, especially those undergoing intubation and not able to communicate [37].
Methodological quality and bias
The quality evaluation and risk of bias for the enrolled articles are detailed in Table S3 in supplementary file. The assessment scores of 13 included studies using the NOS [8, 13, 23,24,25,26,27,28,29,30,31,32,33] were equal to or greater than 7 points, suggesting that each research was of high quality. All of enrolled RCTs adopted random assignment and allocation concealment with low risk [16,17,18]. The intervention of using PAP therapy made it challenging to implement blinding on participants, resulting in high risk of performance bias. Totally, the quality of enrolled trials was moderate to high.
Pooled analysis of effect of OSA on PD
A total of 13 studies [8, 13, 23,24,25,26,27,28,29,30,31,32,33] with 12,386 patients investigated the impact of OSA on PD, revealing a significant association between OSA and an elevated rate of PD (OR = 1.71; 95%CI = 1.17 to 2.49; p = 0.005) with high heterogeneity (I2 = 69%; p = 0.0001). One study by Kaw R et al. [24] was considered potential bias due to the absence of PD among patients without OSA and was therefore excluded.After removing the research of Kaw R et al. [24], the results indicated that OSA still had an impact on the incidence of PD (OR = 1.64; 95%CI = 1.14 to 2.38; p = 0.008), but the heterogeneity was not reduced (I2 = 69%; p = 0.0002). We conducted subgroup analysis according to the delirium assessment tools to explore heterogeneity. The statistical results of the studies evaluated with the CAM-ICU indicated that OSA showed no correlation with the risk of PD (OR = 1.14; 95%CI = 0.77 to 1.67; p = 0.51) with heterogeneity (I2 = 62%; p = 0.03), while the results of studies evaluated by other delirium assessment tools indicated that OSA was linked to an elevated rate of PD (OR = 2.15; 95%CI = 1.44 to 3.19; p = 0.0002) without statistical heterogeneity (I2 = 8%; p = 0.37) (Fig. 2). In addition, to address potential selection bias, only studies using PSG as diagnostic tools for OSA detection were retained [23,24,25, 27, 8, 30, 33], yielding consistent results. OSA diagnosed by PSG was not associated with PD rates evaluated with CAM-ICU (OR = 1.15; 95%CI = 0.47 to 2.81; p = 0.77) without heterogeneity (I2 = 0%; p = 0.36), while it raised PD rates evaluated with other delirium assessment scales (OR = 2.74; 95%CI = 1.63 to 4.60; p = 0.0001) without statistical heterogeneity (I2 = 10%; p = 0.34) (Fig. S1 in the supplemental material).
Length of hospital stay
In the 13 included studies [8, 13, 23,24,25,26,27,28,29,30,31,32,33], 4 studies [24, 28, 30, 32] provided data on the hospitalization duration. Pooled results suggested that OSA increased the hospitalization duration (MD = 1.10; 95%CI = 0.53 to 1.66; p = 0.0001) without statistical heterogeneity in postoperative patients (I2 = 44%; p = 0.15) (Fig. S2 in the supplemental material).
Pooled analysis of impact of PAP treatment on PD among OSA individuals
Of the 16 studies included [8, 13, 16,17,18, 23,24,25,26,27,28,29,30,31,32,33], 3 RCTs [16,17,18] comprising 420 OSA individuals explored the efficacy of PAP therapy on PD. As indicated in Fig. 3, PAP therapy failed to decrease the likelihood of PD among OSA individuals (OR = 0.58; 95%CI = 0.13 to 2.47; p = 0.46) without statistical heterogeneity (I2 = 44%; p = 0.17).
Publication bias
There were various sources of heterogeneity in our subgroup analysis, including criteria for OSA and PD, operation type, and anesthesia type. Egger’s test indicated no notable bias in either the assessment of OSA and PD using the CAM-ICU (p = 0.806) or in evaluating OSA and PD by other delirium assessment tools (p = 0.764) (see in Fig. S3 in the supplemental materials).
Discussion
Our meta-analysis found that OSA patients faced over a 1.5 times higher likelihood of developing PD compared to those without OSA. Subgroup analyses showed that OSA did not exhibit an association with the incidence of PD diagnosed by the CAM-ICU, while OSA elevated PD rates diagnosed by other delirium assessment scales. Besides, OSA was related to a prolonged hospital stay. Moreover, PAP therapy did not demonstrate efficacy in decreasing the prevalence of PD among OSA patients.
According to our findings, OSA increased the likelihood of PD, consistent with previous meta-analyses [10, 12]. However, the heterogeneity of this result in our study was significant, which might be caused by study design, and diagnostic criteria for OSA and PD. The onset of PD can be influenced by the patients’ critical condition, ICU stay duration, and postoperative sedation length [38]. Not all the included studies specified whether patients after surgery were either hospitalized in the ICU or on a general ward, therefore we were unable to conduct subgroup analysis based on the postoperative treatment location.Some studies have shown that the tools for assessing PD are different in patients with different conditions [34,35,36,37, 39]. The CAM-ICU is designed for patients in ICU, especially for intubated patients [37], while other delirium assessment tools such as CAM, DRs.R-98, and Nu-DESC are available to different specialists or caregivers to assess PD in non-ICU settings [34,35,36]. According to the applicable population and characteristics of delirium assessment tools, we could indirectly speculate that patients with PD diagnosed by the CAM-ICU were in serious condition and needed intensive care in ICU, while patients with PD assessed by other delirium assessment tools were in stable condition and could be cared for in the ward. Therefore, we performed a subgroup analysis according to the delirium assessment tools to indirectly reflect the effect of OSA on PD in different locations (ICU or ward). Our subgroup analysis suggested that OSA did not exhibit an association with the incidence of PD in patients requiring intensive ICU care after operations, while OSA individuals had a higher incidence of developing PD if they only required regular ward care following surgery.
It is still not clear how OSA contributes to a higher incidence of PD. Reduced oxygen metabolism in OSA might play a role [22]. Patients with OSA suffer from airway collapse, disrupted sleep, and repetitive decreases in oxygen levels, which may damage the brain and result in the cognitive dysfunction [10, 40]. A study by Zimmerman ME et al. suggested that the main mechanism by which sleep-disordered breathing affected cognition was chronic intermittent hypoxemia [41]. This study presented a microvascular model proposing that chronic intermittent hypoxemia triggered vasculopathy by chemoreflex activation, pressor effect, and endothelial dysfunction. Ultimately, these vascular changes manifested as cognitive impairment in elderly people [41]. Other possible mechanisms linking OSA and delirium might involve elevated levels of proinflammatory cytokines due to OSA or chronic oxidative stress. OSA has been shown to be an inflammatory process [42, 43]. Many studies provide evidence for the significant involvement of inflammatory cytokines during the progression of delirium [44, 45]. Thus OSA may have an association with an increased likelihood of developing PD. While in ICU some measures taken to protect the airway may attenuate the impact of OSA on delirium, such as endotracheal intubation and tracheostomy, which could keep the airway open and may prevent cerebral hypoxia and delirium caused by airway collapse [40].
In our study, patients assessed by the CAM-ICU had higher delirium rates compared to those evaluated by other delirium assessment tools. In the CAM-ICU subgroup, King CR et al. reported a 47% incidence of PD among 7792 patients [13]. Roggenbach J et al. indicated that 48% of 92 patients undergoing elective cardiac surgery developed delirium after surgery [25]. In another subgroup, Chan MTV and colleagues indicated that the rate of PD among patients with major noncardiac operation was only 5% [33]. Wu WQ et al. observed a 11% incidence of PD among 130 patients [8]. Patients who are in serious condition and need intensive care in ICU after operations may have longer intraoperative anesthesia and postoperative sedation, which can increase the occurrence of PD [38, 46, 47].
PAP is considered as the preferred therapy among OSA patients [48]. Theoretically, PAP should effectively reduce the incidence of hypoxemia and complications following surgery among OSA individuals [49]. However, it is debatable whether PAP therapy can affect postoperative complications in OSA patients [10, 50, 51]. There are currently no specific meta-analyses investigating the role of PAP on PD among OSA. Our meta-analysis data indicated no notable distinction in PD rates between the PAP and routine therapy groups, which may be due to low adherence. Potential factors contributing to the limited adherence to PAP therapy following surgery include vomiting, nausea, and discomfort in the postoperative phase [52]. Besides, there is no consensus on whether PAP therapy should be used already before or only after surgery. The timing of PAP initiation varied across studies enrolled in our meta-analysis. The studies conducted by O’Gorman SM and Wong J colleagues administered PAP therapy to patients after surgery [17, 18], whereas in the study by Nadler JW et al., PAP therapy was given around the time of surgery [16]. The practice guideline issued by the American Society of Anesthesiologists suggested the commencement of PAP therapy before surgery whenever feasible [53]. Liao and colleagues found that perioperative application of PAP therapy led to a pronounced reduction in postoperative AHI among OSA individuals [52]. In a meta-analysis of perioperative PAP for OSA patients by Nagappa M et al., patients in the PAP group had a shorter hospital stay compared to those in the non-PAP group [50]. The clinical heterogeneity on the timing of PAP therapy may have an important role on the incidence of PD among OSA individuals. Therefore, further RCTs are required to explore the timing of PAP therapy in perioperative individuals with OSA.
Compared to the previous meta-analysis by Sun and colleagues [10], our study reduced the interference of the severity of the disease itself on PD. As mentioned above, the CAM-ICU is generally used to assess PD in ICU patients with serious condition [37], while other delirium assessment tools are commonly designed to assess delirium in non-ICU patients only needing regular care [34,35,36]. Therefore, our subgroup analysis based on delirium assessment tools could indirectly reduce the effect of disease severity on PD. In addition, our study included more comprehensive literature [13, 17, 18, 30, 31]. Especially, a new retrospective single-center cohort study by King CR et al. [13] enrolling 7792 patients, found contrary results compared to the previous literature [10], suggesting that OSA did not exhibit an association with an elevated rate of PD.
Our study also had a few limitations. First of all, the non-randomization design of the included studies may introduce residual confounding due to unmeasured variables. Factors such as different OSA diagnostic criteria, variations in anesthesia types, and differences in perioperative pain management were not considered. Further RCTs involving different unmeasured variables are needed in this field. Secondly, the Intensive Care Delirium Screening Checklist (ICDSC) is one of the most well-known questionnaires to assess delirium [34], but no included studies used the ICDSC, possibly due to challenges in its administration for ventilated patients. Those who use this scale need professional training to interpret the results correctly. Besides ICDSC requires a specific timeframe for assessment, which can cause an increase of false positives [34, 54]. Thirdly, heterogeneity was found in some results. Because of the restricted number of studies, a comprehensive examination of diversity was not always achievable. Some measures were taken to address heterogeneity, including the use of a random-effects model when I2 > 50% and conducting sensitivity and subgroup analyses. We investigated the variability of outcomes derived from sufficient studies and verified the credibility. Finally, the timing of PAP initiation was not well recorded, resulting in poor interpretation of results. Further trials are essential to address these gaps in knowledge.
In conclusion, OSA may not be a risk factor for PD in critically ill patients in the intensive care unit, but may increase the likelihood of developing PD among individuals receiving regular care in the ward postoperatively. The efficacy of PAP therapy in decreasing PD incidence among OSA patients remains debatable. Future well-designed trials especially RCTs are required to further evaluate the role of OSA on PD and the efficacy of PAP therapy in decreasing the occurrence of PD among OSA patients.
Data availability
The paper and its supplementary information contain all the data that support the findings of this study.
Abbreviations
- OSA:
-
Obstructive Sleep Apnea
- PD:
-
Postoperative Delirium
- PAP:
-
Positive Air Pressure
- CAM-ICU:
-
Confusion Assessment Method-Intensive Care Unit
- CAM:
-
Confusion Assessment Method
- DRS-R98:
-
Delirium Rating Scale-Revised-98
- 3D-CAM:
-
3-minute Diagnostic interview for Confusion Assessment Method
- Nu-DESC:
-
Nursing Delirium Screening Scale
- ICU:
-
Intensive Care Unit
- CI:
-
Confidence Interval
- MD:
-
Mean Difference
- OR:
-
Odds ratio
- NOS:
-
Newcastle-Ottawa scale
- BMI:
-
Body Mass Index
- PSG:
-
Polysomnography
- AHI:
-
Apnea-Hypopnea Index
- GSDS:
-
General Sleep Disturbance Scale
- PSQI:
-
Pittsburgh Sleep Quality Index
- HSAT:
-
Home Sleep Apnea Test
- DSM-IV:
-
Diagnostic and Statistical Manual of Mental Disorders-IV
- MMSE:
-
Mini-Mental State Examination
- MDAS:
-
Memorial Delirium Assessment Scale
- RCTs:
-
Randomized Controlled Trials
References
Avidan MS, Maybrier HR, Abdallah AB et al (2017) Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 390(10091):267–275
Lu Y, Li YW, Wang L et al (2019) Promoting sleep and circadian health may prevent postoperative delirium: a systematic review and meta-analysis of randomized clinical trials. Sleep Med Rev 48:101207
Saczynski JS, Marcantonio ER, Quach L et al (2012) Cognitive trajectories after postoperative delirium. N Engl J Med 367(1):30–39
Leslie DL, Marcantonio ER, Zhang Y et al (2008) One-year health care costs associated with delirium in the elderly population. Arch Intern Med 168(1):27–32
Labarca G, Schmidt A, Dreyse J et al (2021) Efficacy of continuous positive airway pressure (CPAP) in patients with obstructive sleep apnea (OSA) and resistant hypertension (RH): systematic review and meta-analysis. Sleep Med Rev 58:101446
Abboud F, Kumar R (2014) Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 124(4):1454–1457
Maldonado JR (2013) Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 21(12):1190–1222
Wu WQ, Zheng WB, Wang HB et al (2022) Influence of obstructive sleep apnea on postoperative cognitive dysfunction in elderly patients undergoing joint replacement. Am J Transl Res 14(6):4050–4057
Cho MR, Song SK, Ryu CH (2020) Sleep disturbance strongly related to the development of postoperative delirium in proximal femoral fracture patients aged 60 or older. Hip Pelvis 32(2):93–98
Sun X, Yu J, Luo J et al (2022) Meta-analysis of the association between obstructive sleep apnea and postoperative complications. Sleep Med 91:1–11
He E, Dong Y, Jia H et al (2022) Relationship of sleep disturbance and postoperative delirium: a systematic review and meta-analysis. Gland Surg 11(7):1192–1203
Rong X, Ding ZC, Yu HD et al (2021) Risk factors of postoperative delirium in the knee and hip replacement patients: a systematic review and meta-analysis. J Orthop Surg Res 16(1):76 Published 2021 Jan 22
King CR, Fritz BA, Escallier K et al (2020) Association between Preoperative Obstructive Sleep Apnea and Preoperative positive airway pressure with postoperative Intensive Care Unit Delirium. JAMA Netw Open 3(4):e203125
Giles TL, Lasserson TJ, Smith BJ et al (2006) Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. ;(1):CD001106
Fava C, Dorigoni S, Dalle Vedove F et al (2014) Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 145(4):762–771
Nadler JW, Evans JL, Fang E et al (2017) A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia 72(6):729–736
O’Gorman SM, Gay PC, Morgenthaler TI (2013) Does autotitrating positive airway pressure therapy improve postoperative outcome in patients at risk for obstructive sleep apnea syndrome? A randomized controlled clinical trial. Chest 144(1):72–78
Wong J, Doherty HR, Singh M et al (2022) The prevention of delirium in elderly surgical patients with obstructive sleep apnea (PODESA): a randomized controlled trial. BMC Anesthesiol 22(1):290
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Wells G, Shea B, O’Connell D et al (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Higgins JPT, Altman DG, Sterne JAC (2011) Assessing risk of bias in included studies. In: Higgins, J. P. T., & Green, S. (ed.), Cochrane handbook for systematic reviews of interventions, version 5.1.0. http://handbook.cochrane.org
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Flink BJ, Rivelli SK, Cox EA et al (2012) Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 116(4):788–796
Kaw R, Pasupuleti V, Walker E et al (2012) Postoperative complications in patients with obstructive sleep apnea. Chest 141(2):436–441
Roggenbach J, Klamann M, von Haken R et al (2014) Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Crit Care 18(5):477
Strutz PK, Kronzer V, Tzeng W et al (2019) The relationship between obstructive sleep apnoea and postoperative delirium and pain: an observational study of a surgical cohort. Anaesthesia 74(12):1542–1550
Tafelmeier M, Knapp M, Lebek S et al (2019) Predictors of delirium after cardiac surgery in patients with sleep disordered breathing. Eur Respir J 54(2):1900354
Wang S, Sigua NL, Manchanda S et al (2018) Preoperative STOP-BANG scores and postoperative delirium and coma in thoracic surgery patients. Ann Thorac Surg 106(4):966–972
Wong AM, Wang M, Garner DJ et al (2020) Obstructive sleep apnoea predicted by the STOP-BANG questionnaire is not associated with higher rates of post-operative complications among a high-risk surgical cohort. Sleep Breath 24(1):135–142
Gupta RM, Parvizi J, Hanssen AD et al (2001) Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. ;76(9):897–905
Leung JM, Sands LP, Newman S et al (2015) Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med 11(8):907–913
Pereira H, Xará D, Mendonça J et al (2013) Patients with a high risk for obstructive sleep apnea syndrome: postoperative respiratory complications. Rev Port Pneumol 19(4):144–151
Chan MTV, Wang CY, Seet E et al (2019) Association of Unrecognized Obstructive Sleep Apnea with Postoperative Cardiovascular events in patients undergoing major noncardiac surgery. JAMA 321(18):1788–1798
Inouye SK, van Dyck CH, Alessi CA et al (1990) Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 113(12):941–948
Trzepacz PT, Mittal D, Torres R et al (2001) Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 13(2):229–242
Gaudreau JD, Gagnon P, Harel F et al (2005) Fast, systematic, and continuous delirium assessment in hospitalized patients: the nursing delirium screening scale. J Pain Symptom Manage 29(4):368–375
Miranda F, Arevalo-Rodriguez I, Díaz G et al (2018) Confusion Assessment Method for the intensive care unit (CAM-ICU) for the diagnosis of delirium in adults in critical care settings. Cochrane Database Syst Reviews Issue 9. Art. No.: CD013126.
Oh ST, Park JY (2019) Postoperative delirium. Korean J Anesthesiol 72(1):4–12
Marcantonio ER, Ngo LH, O’Connor M et al (2014) 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med 161(8):554–561
Isono S (2009) Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology 110(4):908–921
Zimmerman ME, Aloia MS (2012) Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep 12(5):537–546
Dyugovskaya L, Lavie P, Lavie L (2002) Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165(7):934–939
Entzian P, Linnemann K, Schlaak M et al (1996) Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med 153(3):1080–1086
Cerejeira J, Firmino H, Vaz-Serra A et al (2010) The neuroinflammatory hypothesis of delirium. Acta Neuropathol 119(6):737–754
Adamis D, Lunn M, Martin FC et al (2009) Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing 38(3):326–251
Bilotta F, Lauretta MP, Borozdina A et al (2013) Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol 79(9):1066–1076
Iamaroon A, Wongviriyawong T, Sura-Arunsumrit P et al (2020) Incidence of and risk factors for postoperative delirium in older adult patients undergoing noncardiac surgery: a prospective study. BMC Geriatr 20(1):40
Malhotra A, White DP (2002) Obstructive sleep apnoea. Lancet 360(9328):237–245
Rennotte MT, Baele P, Aubert G et al (1995) Nasal continuous positive airway pressure in the perioperative management of patients with obstructive sleep apnea submitted to surgery. Chest 107(2):367–374
Nagappa M, Mokhlesi B, Wong J et al (2015) The effects of continuous positive Airway pressure on postoperative outcomes in obstructive sleep apnea patients undergoing surgery: a systematic review and Meta-analysis. Anesth Analg 120(5):1013–1023
Abdelsattar ZM, Hendren S, Wong SL et al (2015) The impact of untreated obstructive sleep apnea on cardiopulmonary complications in General and vascular surgery: a Cohort Study. Sleep 38(8):1205–1210
Liao P, Luo Q, Elsaid H et al (2013) Perioperative auto-titrated continuous positive airway pressure treatment in surgical patients with obstructive sleep apnea: a randomized controlled trial. Anesthesiology 119(4):837–847
American Society of Anesthesiologists Task Force on Perioperative Management of patients with (2014) Obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 120(2):268–286
Brummel NE, Vasilevskis EE, Han JH et al (2013) Implementing delirium screening in the ICU: secrets to success. Crit Care Med 41(9):2196–2208
Funding
This study did not receive dedicated funding from public, private, or non-profit organizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
None of the authors conducted studies involving human participants or animals for this article.
Informed consent
Not applicable.
Conflict of interest
The authors have no conflicts of interest to declare.
Reference for the abstract
Li PJ, Xiao Y, Wang MY, Liang ZA. Effect of obstructive sleep apnea on postoperative delirium: A system review and meta-analysis.The 8th Chinese National Conference on Sleep Breathing Disorders. https://tsb2023.sciconf.cn.(Meeting Abstract).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Li, PJ., Guo, MY. et al. Effect of obstructive sleep apnea on postoperative delirium: a system review and meta-analysis. Sleep Breath (2024). https://doi.org/10.1007/s11325-024-03073-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11325-024-03073-6