Abstract
Aim
To investigate the predictive value of the systemic immune-inflammation index (SII) in patients with obstructive sleep apnea (OSA).
Material and methods
Patients diagnosed with OSA formed the patient group, and those with a normal polysomnography (PSG) result formed the control group. The neutrophil, thrombocyte, monocyte, and lymphocyte counts obtained from the hemogram were used to calculate the neutrophil-to-lymphocyte ratio (NLR), thrombocyte-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and the SII values. The two groups were compared with respect to the NLR, PLR, MLR, and SII values. Correlations were examined between the PSG parameters and the NLR, PLR, MLR, and SII values in the patient group.
Results
Evaluation included 146 subjects with 85 in the patient group and 61 in the control group. Statistically significantly higher SII and NLR values were found in the patient group (p = 0.037, p < 0.05; p = 0.015, p < 0.05). A statistically significant negative correlation was observed between the SII and the lowest O2 saturation measurements (r = − 0.246; p = 0.003; p < 0.01). A statistically significant negative correlation was found between the NLR and the lowest O2 saturation measurement (r = − 0.255; p = 0.002; p < 0.01). The cutoff value for SII was found to be 290, with 84.7% sensitivity and 29.5% specificity. A cutoff value of 1.71 for NLR was determined to have 61.2% sensitivity and 60.7% specificity.
Conclusion
SII may be a new, rapid, low-cost, and easy-to-measure biomarker for the prediction of obstructive sleep apnea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a chronic inflammatory disease, characterised by partial or complete obstruction of the upper airway during sleep, which affects 3–9% of the general population [1,2,3]. The two most important factors in the pathophysiology of OSA and associated comorbidities are systemic inflammation and oxidative stress. A potential mechanism that is thought to be involved is that intermittent nocturnal hypoxemia produces a decline in oxygen levels followed by re-oxygenation when breathing resumes. The cyclical episodes of hypoxia-re-oxygenation correspond to cardiac ischemia/re-oxygenation injury, cause depletion of adenosine triphosphate, and activate xanthine oxidase activation, leading to an increase in the generation of oxygen-derived free radicals, thereby resulting in local and systemic inflammation [4, 5].
Biological and haematological markers can be used to measure systemic inflammation. However, as these are generally expensive methods, it has been shown in recent years that cell counts and combinations of these such as the neutrophil–lymphocyte ratio (NLR) and thrombocyte-lymphocyte ratio (PLR) can reflect the inflammatory response with high sensitivity [6]. These ratios are easily obtained at no additional cost and have been shown to be as effective as classic haematological markers of systemic inflammation. More recently, the systemic immune-inflammation index (SII) has been formed as a biomarker calculated from neutrophils, lymphocytes, and thrombocytes from systemic inflammatory cells [7]. Research has shown a positive correlation between the SII and neutrophil and platelet counts and a negative correlation between the SII and lymphocyte count [8].
There are several recent studies that have examined the NLR and PLR in OSA [6, 9], but there have been very few investigations of the SII [10]. The aim of this study was to investigate the relationship between the inflammatory disease of OSA and the SII.
Material and methods
Study population
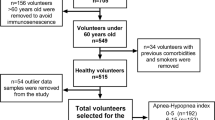
The data were retrospectively obtained from patients who underwent full-night polysomnography (PSG) in the sleep laboratory of Kocaeli Derince Training and Research Hospital between January 1, 2019, and September 30, 2022. Patients diagnosed with OSA formed the patient group, and those with a normal PSG result formed the control group. Patients were excluded from both groups if they had any haematological, gastrointestinal or gynaecological malignancy, chronic inflammatory disease, infection, congestive heart failure, severe renal or liver failure, or cerebrovascular disease, or if they were receiving anticoagulant treatment. Permission to use the patient records was obtained. Demographic data (age, gender, body mass index [BMI]), Epworth Sleepiness Scale (ESS) scores, and comorbidities were recorded for each patient.
Sleep study
All participants underwent full-night PSG (device brand and model: Embla N 7000) in the sleep disorder laboratory. The 2017 American Academy of Sleep Medicine criteria were applied in the scoring. OSA was defined as an apnea/hypopnea index (AHI) of ≥ 5/h. The severity of OSA was evaluated as mild for AHI ≥ 5/h and < 15/h, moderate for AHI ≥ 15/h and < 30/h, and severe for AHI ≥ 30/h. As a criterion for hypopnea, a decrease of 50% in the amplitude of the nasal cannula was accompanied by a decrease of at least 3% in desaturation or resulted in arousal. AHI, (non-REM stage 1) N-REM 1, N-REM 2, N-REM 3, REM percentages, minimum oxygen saturation, and nocturnal time spent with arterial oxgen saturation < 90% values were recorded from the PSG report.
Complete blood counts
Blood cell counts were retrieved retrospectively from the patient medical records. Blood samples were taken within the last month before undergoing the PSG test. Total leukocyte count and subtypes, including neutrophil, lymphocyte, and monocyte and platelet absolute counts, were analysed using an automated blood cell counter (Pentra Nexus(Horiba), Sysmex XN (Roche)). The NLR, PLR, MLR, and SII were calculated as follows: NLR, neutrophil count/lymphocyte count; PLR, platelet count/lymphocyte count; MLR, monocyte count/lymphocyte count; and SII, (neutrophil × platelet)/lymphocyte.
Ethical approval
The study procdures complied with the Helsinki Declaration. Approval for the study was granted by the Clinical Research Ethics Committee of SBU Kocaeli Derince Training and Research Hospital (protocol no: 119, dated: November 17, 2022).
Statistical analysis
Statistical analyses of the study data were made using Number Cruncher Statistical System (NCSS) 2020 Statistical Software (NCSS LLC, Kaysville, Utah, USA). Continuous variables were stated as mean ± standard deviation, median, minimum and maximum values, and categorical variables as number (n) and percentage (%). The Shapiro–Wilk test and boxplot graphs were used in the assessment of the data conformity to normal distribution. In the comparisons of two groups of quantitative variables, the Student’s t-test was applied to data showing normal distribution, and the Mann Whitney U-test was used for variables not showing normal distribution. When comparing more than two groups, the Kruskal Wallis test was used. In the evaluation of relationships between variables, Spearman correlation analysis was used. In the evaluation of the correlation coefficient (r), a value of 0–0.25 was accepted as very weak, 0.26–0.49 as weak, 0.50–0.69 as moderate, 0.70–0.89 as strong, and 0.90–1.00 as a very strong correlation [11]. In the comparisons of quantitative data, the chi-square test, Fisher’s Exact test, and the Fisher Freeman Halton test were used. Results were stated in a 95% confidence interval and a value of p < 0.05 was accepted as the level of statistical significance.
Results
The study included 146 subjects, comprising 102 (70%) males and 44 (30%) females with a mean age of 44.5 ± 9.2 years (range, 23–66 years), who underwent full-night PSG in the Neurology Clinic Sleep and Sleep Disorders Centre of SBU Kocaeli Derince Training and Research Hospital between January 1, 2019, and September 30, 2022. The patient characteristics are shown in Table 1. The groups were observed to be similar with respect to age, gender, BMI, smoking status, or the presence of DM, HT, COPD, thyroid disease, or ESS scores.
No statistically significant difference was found between the groups in respect of the platelet, neutrophil, lymphocyte, and monocyte counts and the MLR and PLR. The SII and NLR values were determined to be statistically significantly higher in the OSA group (p = 0.037, p < 0.05; p = 0.015, p < 0.05) (Table 2). When the cases were compared according to disease severity, the differences between the SII, MLR, PLR, and NLR values were not statistically significant (p > 0.05) (Table 3).
A statistically significant negative correlation at a weak level was determined between the SII and the lowest O2 saturation measurements (r = − 0.246; p = 0.003; p < 0.01). A statistically significant negative weak correlation was determined between the NLR and the lowest O2 saturation measurement (r = − 0.255; p = 0.002; p < 0.01). A statistically significant positive weak correlation was determined between the NLR and the desaturation periods (r = 0.252; p = 0.002; p < 0.01). No statistically significant relationship was determined between the SII, NLR, PLR, and MLR measurements of the cases and the N-REM 1, N-REM 2, N-REM 3, REM percentages, and ESS values (p > 0.05) (Table 4).
For a cutoff value of 290 for SII, sensitivity was 84.7%, specificity 29.5%, positive predictive value (PPV) 62.6%, and negative predictive value (NPV) 58.1%. In the ROC curve analysis, the area under the curve (AUC) was determined to be 60.1% with standard error 4.7% (Table 5).
The correlation between the SII cutoff value of 290 and the groups was determined to be statistically significant (p = 0.038; p < 0.05). The risk of developing OSA in cases with SII value of ≥ 290 was found to be 2.318-fold higher. The ODDS ratio for SII was 2.318 (95% CI: 1.034–5.197) (Fig. 1).
For the NLR, the cutoff value of 1.71 was determined to have 61.2% sensitivity, 60.7% specificity, PPV 68.4%, and NPV 52.9%. The ROC curve analysis results showed AUC of 61.8% with standard error 4.7%. A statistically significant relationship was determined between the NLR cutoff value of 1.71 and the groups (p = 0.015; p < 0.05). The risk of developing OSA in cases with NLR of ≥ 1.71 was found to be 2.429-fold higher. The ODDS ratio for NLR was 2.429 (95% CI: 1.238–4.766) (Fig. 2).
Discussion
The study results suggest that the SII and NLR values were higher for patients with OSA than for control group subjects. A significant negative correlation was determined between the SII and NLR values and the lowest O2 saturation measurement, and a significant positive correlation between the SII and NLR values and the duration of desaturation remaining < 90% [12].
OSA is a serious health problem, which is associated with cardiovascular diseases, neurological diseases, and various types of mortality [2, 13,14,15]. It has been shown in previous animal model studies that the changes induced in the leukocyte function by apnea and hypoxemia trigger systemic inflammation in OSA [16, 17]. With the increase in plasma norepinephrine level in OSA which leads to physical stress, the sympathetic system is activated and serum cortisol increases. Increased cortisol levels cause a decrease in lymphocyte concentration [18]. It has been reported in several studies that under physiological stress, neutrophils are increased and lymphocytes are decreased with the effect of endogenous cortisol and catecholamines [19, 20]. An increased neutrophil count has been shown in patients with OSA. However, in some publications, it has been stated that it is not fully clear by which mechanisms neutrophils are increased in OSA [21]. It has also been determined that platelet levels, which are a component of SII, are positively correlated with the degree of inflammatory response [22].
There has been reported to be a positive correlation between the SII and neutrophil and platelet counts, and a negative correlation between the SII and lymphocyte count [8]. Increased neutrophils in conditions of inflammation suppress natural killer and active T lymphocytes, thereby suppressing the immune system [23]. It is known that lymphocytes clear tumour cells through both humoral and cellular immune mechanisms [24]. Platelets, which are another parameter of the SII, have been reported to assist tumour cells escape from body immunity [25]. Therefore, this study was planned with the thought that the SII could reflect inflammation.
The current study results showed that the SII values were significantly higher in the patients with OSA than in the control group. In a 2021 study by Muhammet Fatih Topuz et al. of 194 patients, a significant correlation was determined between the SII and severe OSA. This correlation was reported to be stronger than the correlation with NLR and PLR, and thus it was stated that the SII could be used to show chronic inflammation in OSA [10]. That was the first and only previous study in literature. The current study is the second in literature that has aimed to compare OSA parameters with the SII. In the Topuz et al. study, the patient and control groups differed in respect of age, gender, and BMI whereas in the current study, there was no difference in age, gender, or BMI between the groups. No correlation was determined in the current study between ESS and SII, MLR, PLR, and NLR values, and no information about such a potential correlation could be found in the literature.
The current study results showed a significant negative relationship between the SII and the lowest oxygen saturation measurements. There was also determined to be a significant positive relationship between the SII value and the duration of desaturation remaining < 90%. OSA is a disease characterised by recurrent limitations in air flow or episodes of termination, and this results in nocturnal hypoxia and sleep fragmentation [25, 26]. Hypoxia re-oxygenation episodes, xanthine oxidase activation, and increased free radicals cause local and systemic inflammation [27, 28].
The results of this study showed that in patients with SII of ≥ 290, the risk of OSA was determined to be 2.318-fold higher. Therefore, patients with SII of ≥ 290 should be evaluated carefully with respect to OSA and should be questioned about OSA symptoms.
No significant difference was observed between the current study groups with respect to the MLR and PLR values. Köseoğlu et al. reported that the PLR value was significantly reduced in patients with severe OSA compared to control group subjects [6]. However, this correlation was not found in the current study. With further larger scale studies, it may be possible to determine this correlation.
The current study results showed that the NLR was higher in the OSA patient group than in the control group. In a meta-analysis by Min-Seok Rha et al., published in 2020, it was stated that NLR could be a reliable marker in the determination of systemic inflammation and the prediction of disease severity in OSA [29]. Although there was observed to be a difference between the patient and control groups in the current study, no correlation was determined between NLR and disease severity in the patient group. The NLR values were determined to be significantly positively correlated with the desaturation percentages in the current study patient group. Köseoğlu et al. reported that nocturnal time spent with arterial oxygen saturation < 90% increased with increasing NLR, and thus it was stated that NLR can be used as a marker to show chronic intermittent hypoxia in OSA [6]. The cutoff value for NLR was found to be 1.71 in the current study, whereas it was reported to be 1.85 in a study by Altıntaş et al. [9].
The main limitations of this study were the single-centre, retrospective design, and the fact that the number of patients included was relatively low. Further prospective studies with greater numbers of patients are required to strengthen the findings of the value of the SII in OSA.
Conclusion
The results of this study suggest that the SII may be an easily measured laboratory marker for the prediction of OSA.
Data availability
The data will be made available upon reasonable request.
References
Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. Lancet 383:736–747
Levy P et al (2015) Obstructive sleep apnoea syndrome. Nat Rev Dis Primers 1:15015
Young T, Peppard PE, Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165:1217–1239
Nadeem R, Molnar J, Madbouly EM, Nıda M, Aggarwal S, Sajıd H, Naseem J, Loomba R (2013) Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 9:1003–1012
Yoshıkawa M, Yamauchı M, Fujita Y, Koyama N, Fukuoka A, Tamakı S, Yamamoto Y, Tomoda K, Kımura H (2014) The impact of obstructive sleep apnea and nasal CPAP on circulating adiponectin levels. Lung 192:289–295
Koseoglu S, Özcan KM, Ikinciogullari A, Cetin MA, Yildirim E, Dere H (2015) Relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and obstructive sleep apnea syndrome 1 Ankara Numune Education and Research Hospital, ENT Clinic, Turkey 2 Kars State Hospital, ENT Clinic, Turkey). Adv Clin Exp Med 24(4):623–627
Hong X, Cui B, Wang M et al (2015) Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 236:297–304
Hu B, Yang XR, Xu Y et al (2014) Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 20:6212–6222
Altıntaş N, Çetinoğlu E, Yuceege M, Acet AN, Ursavas A, Fırat H, Karadağ M (2015) Neutrophil/lymphocyte ratio, obstructive sleep apnea. Neutrophil-to-lymphocyte ratio in obstructive sleep apnea; a multi center, retrospective study. Eur Rev Med Pharmacol Sci 19:3234–3240
Muhammet Fatih T, Nurullah T, Gönül A, Özlem A, Gülhan PY (2022) The importance of systemic immune-inflammation index in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-021-07227-0(YayınNo:7576182)
Akgül A, Çevik O (2003) “İstatistiksel Analiz Teknikleri”, Emek Ofset, Ankara, Dizin: s:45–456
Fava C, Montagnana M, Favaloro EJ, Guıdı GC, Lıppı G (2011) Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost 37:280–297
McNicholas WT, Bonsigore MR (2007) Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 29:156–178
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
Shamsuzzaman AS, Gersh BJ, Somers VK (2003) Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 290:1906–1914
Levy P, Pepın JL, Arnaud C, Tamısıer R, Borel JC, Dematteıs M, Godın-Rıbuot D, Rıbuout C (2008) Intermittent hypoxia and sleep-disordered breathing: current concepts and perspectives. Eur Respir J 32:1082–1095
Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM (2007) Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol 155:93–96
Ommen SR, Hodge DO, Rodeheffer RJ, Mcgregor CG, Thomson SP, Gıbbons RJ (1998) Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation 97:19–22
Zahorec R (2001) Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 102:5–14
Karakontantis S, Kalemaki D, Tzagkarakis E, Lyadakis C (2018) Pitfalls in studies of eosinpenia and neutrophil-to lymphocyte count ratio. Infect Dis ( Lond) 50:163–174
Korkmaz M, Korkmaz A, Küçüker F, Ayyıldız SN, Çankaya S (2015) Evaluatino of the associatiton of sleep apnea-related systemic inflamation with CRP, ESR and neutrophil-to- lymphocyte ratio. Med Sci Monit 21:477–481
Yan Q, Ertao Z, Zhimei Z, Weigang D, Jianjun P, Jianhui C, Chuangqi C (2020) Systemic immune-inflammation index (SII): a more promising ınflammation-based prognostic marker for patients with synchronic colorectal peritoneal carcinomatosis. J Cancer 11(18):5264–5272
Yilmaz A, Mirili C, Bilici M, Tekin SB (2019) A novel predictor in patients with gastrointestinal stromal tumors: systemic immune-inflammation index (SII). J BUON 24:2127–2135
Wang B, Huang Y, Lin T (2020) Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 99:e18571
Herman J, Kafoa B, Waınıqolo I, Robınson E, Mccaıg E, Connor J, Jackson R, Ameratunga S (2014) Driver sleepiness and risk of motor vehicle crash injuries: a population-based case control study in Fiji (TRIP 12). Injury 45:586–591
Guıllemınault C, Tılkıan A, Dement WC (1976) The sleep apnea syndromes. Ann Rev Med 27:465–484
Wang D, Yang JX, Cao DY (2013) Preoperative neutrophil-lymphcyte and platelet-lymphocyte ratios as independet predictors of cervical stromal involvement in surgecically treadet endometrioid adenocarsinoma. Onco Targets Ther 6:211–216
Gary T, Pichler M, Belaj K (2013) Platelet-to-lympocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occluzive disease patients. PLoS One 8:e67688
Rha M-S, Kim C-H, Yoon J-H, Cho H-J (2020) Association between the neutrophil-to-lymphocyte ratio and obstructive sleep apnea: a meta-analysis. Sci Rep 10:10862
Author information
Authors and Affiliations
Contributions
Concept: Z.Y.G; design: Z.Y.G; data collection or processing: Z.Y.G., F.M.G; analysis or interpretation: Z.Y.G., F.M.G; literature search: Z.Y.G, F.M.G; writing: Z.Y.G
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Disclosure
All authors have seen and approved the manuscript.
Informed consent
Informed consent was provided by all the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Güneş, Z.Y., Günaydın, F.M. The relationship between the systemic immune-inflammation index and obstructive sleep apnea. Sleep Breath 28, 311–317 (2024). https://doi.org/10.1007/s11325-023-02913-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02913-1