Abstract
Purpose
The present study aimed to evaluate the lamina cribrosa thickness (LCT), lamina cribrosa depth (LCD), subfoveal and parafoveal choroidal thicknesses, peripapillary choroidal thickness (PCT), and retina nerve fiber layer (RNFL) thickness in patients with obstructive sleep apnea syndrome (OSAS) using spectral domain optical coherence tomography (SD-OCT).
Methods
This single-center, case-control study included 45 OSAS patients and 43 age-and sex-matched healthy controls. Only the right eyes of the patients and controls were included. Each participant underwent a comprehensive ophthalmic assessment including slit lamp examination (biomicroscopy), stereoscopic fundus examination, and intraocular pressure (IOP) measurement. The SD-OCT measurements were also performed in both patients and controls.
Results
The mean ages of the patients (females, 55.6%) and controls (females, 51.2%) were 50.09 ± 9.7 years and 50.30 ± 4.2 years, respectively. The groups were similar in terms of age and gender. Evaluation of the study parameters revealed that there were no significant differences between the OSAS patients and controls regarding IOP, RNFL thickness, subfoveal and parafoveal choroidal thicknesses, and PCT. A significant difference was found between the OSAS patients and controls regarding LCT but not regarding LCD. The mean LCT values were 213.38 ± 30.7 μm and 300.49 ± 42.6 μm for the OSAS patients and controls, respectively (p ˂ 0.001).
Conclusions
The results of the present study indicated that the lamina cribrosa was significantly thinner in the OSAS patients than in the controls. In our opinion, this finding should be supported by large-scale studies and the reason underlying the thinning of the lamina cribrosa in OSAS patients should be investigated physiopathologically.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a severe sleep disorder with apnea-hypopnea episodes, during which air flow is intermittently interrupted despite respiratory effort due to repetitive complete or partial collapse of the upper airways during sleep [1]. In OSAS, sleep breaks result in insomnia, hypoxemia due to upper respiratory tract obstruction, and discharge of sympathetic nervous system which in turn lead to awakening reactions and consequently cause both sleep disturbances and cardiovascular problems. OSAS is diagnosed based on the total number of apneas and hypopneas experienced per hour of sleep (apnea-hypopnea index [AHI]), being 5 or more and on the presence of insomnia-related symptoms [2].
OSAS has been shown to be associated with neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [3,4,5]. In OSAS, primary open–angle glaucoma, normotensive glaucoma, thinning of the retinal nerve fiber layer (RNFL), and visual field defects have been reported more frequently [6, 7]. In addition, optic nerve involvement can also be seen in OSAS. Optic nerve involvement in OSAS is explained by vascular and mechanical factors. Vascular factors include increased vascular resistance due to repetitive hypoxia, autonomic regulatory impairment, oxidative stress, inflammation and subsequent reperfusion, decreased cerebral perfusion pressure, and optic nerve direct hypoxic damage. Mechanical factors include increased intraocular pressure (IOP) due to obesity and supine position, increased intracranial pressure, and loss of elastic fibers in lamina cribrosa and/or trabeculae [8]. Impairment of perfusion of the optic nerve in OSAS patients is also important in the development of glaucoma [9]. In addition, an optical coherence tomography angiography study showed that vessel densities in the peripapillary regions in OSAS patients decreased in relation to disease severity [10]. All these data have suggested that lamina cribrosa might be affected in OSAS patients.
The lamina cribrosa is the optic nerve region through which optic nerve fibers pass through to the upper centers. There is increasing evidence that the lamina cribrosa of the optic nerve head (ONH) is the first affected area in the pathologies with axonal damage such as glaucomatous optic neuropathy. In vitro studies have shown that thinning of the lamina cribrosa occurs due to glaucomatous optic nerve damage [11, 12].
Spectral domain optical coherence tomography (SD-OCT) is a non-invasive, non-contact, high-sensitivity ophthalmologic diagnostic imaging technique that allows the examination of the thickness and depth of lamina cribrosa in addition to RNFL thickness and choroidal thickness of the subfoveal, parafoveal, and peripapillary regions. According to our hypothesis, lamina cribrosa thickness and depth may be affected in OSAS patients because of vascular and mechanical stresses on optic nerve. These findings may be an important assessment for the high risk of developing glaucoma in OSAS patients. The present study aimed to evaluate the thickness and depth of lamina cribrosa in OSAS patients using spectral domain optical coherence tomography (SD-OCT).
Methods
Study design and subjects
The present single-center, case-control study included OSAS patients who underwent polysomnography in the Neurology Clinic of Kayseri Training and Research Hospital between April 2018 and October 2018 and had an AHI score of ≥ 5. Only newly diagnosed OSAS patients with no prior treatment for OSAS were included. An age- and sex-matched control group was formed from healthy individuals who were suspected of having OSAS, underwent polysomnography, had an AHI score of < 5, and thereby did not diagnose with OSAS. The OSAS patients were divided into 3 subgroups with regard to OSA severity based on AHI scores as follows: mild (5 < AHI ≤ 15), moderate (15 < AHI ≤ 30), and severe (AHI > 30) [13]. The results were analyzed according to the data of the American Academy of Sleep Medicine [13]. Also, each patient was evaluated according to the positional status. Patients were deemed to be position-dependent when they met the following criteria: an overall AHI of more than 5 events per hour with a supine AHI of at least twice as high as non-supine AHI [14]. In the present study, only the right eyes of the patients and controls were included in the study. All participants underwent a complete ophthalmologic examination including refraction examination (TONOREF II autorefractor/tonometer; Nidek Co. Ltd., Japan), slit lamp examination (biomicroscopy), fundus examination, and gonioscopy. Additionally, IOP (Goldmann applanation tonometry), central corneal thickness (CCT), and axial length (AL) were measured using the IOLMaster device (Carl Zeiss Meditec Inc., Dublin, CA, USA).
Participants who had a spherical refractive error between − 3 and + 3 diopter (D), a cylindrical fracture of ≤ 3, a best corrected visual acuity of at least 10/10, and an IOP of ≤ 20 mmHg were included. Patients with ocular problems such as retinal diseases and optic disc diseases (including glaucoma), those with previous ocular surgery (including cataract surgery), and those with additional systemic diseases such as diabetes mellitus and systemic hypertension were excluded. In addition, patients with an IOP of > 21 mmHg based on Goldmann applanation tonometry measurement and patients who were determined to have glaucoma based on standard automated perimetry visual field examination were also excluded from the study because the excluding of structural damage caused by glaucoma or high IOP was aimed. The present study was approved by the Clinical Research Ethics Committee of Bozok University (Protocol No: 2018-03-71; date: March 28, 2018) Local Ethics Committee and all procedures were conducted in accordance with the Helsinki Declaration. Informed consents were obtained both from patients and from controls for their participation in the study.
Spectral domain optical coherence tomography measurements
The Heidelberg Spectralis SD-OCT imaging platform (Heidelberg Engineering, Heidelberg, Germany) with an enhanced depth imaging program (HEYEX software 6.0) was used after full pupil dilation with cyclopentolate for the measurements of choroidal thickness, RNFL thickness, lamina cribrosa depth (LCD), and lamina cribrosa thickness (LCT).
Choroidal thickness was defined as the vertical distance between the retinal pigment epithelium (RPE) highly reflective line and the internal scleral border. This technique was described by Margolis et al. in previous study [15]. The choroidal thickness was measured in the subfoveal area and at 500, 1000, and 1500 μm both temporal and nasal to the center of the fovea; the central foveal thickness, which was determined automatically and analyzed by OCT software, was also recorded. All SD-OCT scans were performed in the morning (09.00–11.00 am).
The method of measuring peripapillary choroidal thickness (PCT) has been previously reported as the distance between the outer border of the RPE line and the inner border of the choroidal-scleral junction [16]. Lines used for the measurements of RNFL thickness were placed on the borders of the choroidal tissue. The algorithm function of the RNFL thickness measurement was used to obtain the choroidal thickness in each region.
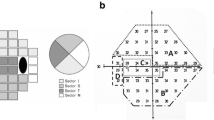
The RNFL circle scans were obtained as a circle (3.46 mm in diameter) centered on the ONH, comprising a total of 1536 A-scans. The obtained images were then used for the measurements of PCT. The circular scan pattern (Spectralis software version 4.0) of the Spectralis SD-OCT was used for all peripapillary RNFL thickness measurements. The average RNFL thickness using the Spectralis SD-OCT software is calculated for the overall global (360°), for 4 quadrants (superior [S], inferior [I], nasal [N], and temporal [T], 90° for each), and for 4 sectors (superior-temporal [TS, 45°–90°], superior-nasal [NS, 90°–135°], inferior-nasal [NI, 225°–270°], and inferior-temporal [TI, 270°–315°]).
Enhanced depth imaging with SD-OCT of the ONH was performed as previously described in the literature [17]. Briefly, the SD-OCT device was set to obtain a 15° × 10° rectangular image centered on the optic disc. This rectangle was divided into nearly 65 sections, each containing an average of 100 OCT frames. Measurements were performed using these horizontal B scans by selecting the frame passing through the vertical center of the ONH. The line connecting both ends of Bruch’s membrane was defined as Bruch’s membrane opening. LCT was defined as the distance between the LCT borders, which were the anterior and posterior borders of the highly reflective region at the vertical center of the ONH in the horizontal SD-OCT cross-section. Contrast settings were adjusted to identify the images that provided the clearest vision of the LCT. LCD was defined as the distance between Bruch’s membrane opening and the anterior border of the LCT.
Two ophthalmologists (B.K. and E.S.) were independently analyzed all obtained images. The LCT and LCD measurements were performed twice by each of the ophthalmologist; thus, 4 measurements were obtained for each of the LCT and LCD and their mean was used in the primary analysis. The reproducibility of the LCT and LCD measurements were tested before the primary analysis; interexaminer and intraexaminer intraclass correlation coefficients were calculated using 15 randomly selected images and comparison revealed a Spearman correlation coefficient of > 0.90.
Statistical analysis
Data analysis was performed using the IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). The normality of data was tested using the Kolmogorov-Smirnov test. Two group comparisons were performed using Student’s t test for parametric data and using the Mann-Whitney U test for non-parametric data. The Pearson correlation coefficient was used to investigate the relationship between 2 variables and the Spearman correlation coefficient was used to investigate the relationship between non-parametric variables. Categorical data were analyzed using the Chi-square test. A one-way ANOVA test was used to compare variation across the three groups. A p value of < 0.05 was considered statistically significant.
Results
The present study included 45 eyes of 45 patients with OSAS and 43 eyes of 43 healthy subjects. General characteristics and ocular findings of the OSAS patients and controls are presented in Table 1. No significant difference was determined between the OSAS patients and controls and between the OSAS subgroups according to age, gender, and body mass index (BMI). Each patient had a BMI of greater than 30. Moreover, the IOP values did not significantly differ between the OSAS patients and controls. There was a significant difference between the OSAS patients and controls and between the OSAS subgroups in the AHI scores (p < 0.001 and p < 0.001). Furthermore, all OSAS patients had non-positional OSAS.
The findings of the measurements of the RNFL thickness are summarized in Table 2. No significant differences were found between the OSAS patients and controls in terms of RNFL thicknesses. Furthermore, there was not a statistically significant difference in the average and all quadrants RNFL thicknesses between the OSAS subgroups (mild, moderate, and severe). The measurement of the RNFL thickness is demonstrated in Fig. 1.
Measurement of the retina nerve fiber layer (RNFL) thickness. a RNFL thinning in average (G) and temporal (T), temporoinferior (TI), temporosuperior (TS), and nasal (N) quadrants in a 45-year-old male patient with obstructive sleep apnea syndrome (OSAS) (Right eye). b Normal RNFL thickness in a 44-year-old male healthy subjects. The layer between red and green line shows RNFL. Numbers indicate individual mean RNFL thickness (right eye). Numbers in parenthesis indicate the mean thickness in the spectralis normative database
Table 3 shows the results of the between-groups comparison of subfoveal and parafoveal choroidal thicknesses measurements. The subfoveal and parafoveal choroidal thicknesses of the patients and controls revealed no significant difference. Also, there was not a statistically significant difference in the mean subfoveal and perifoveal choroidal thicknesses values among the three OSAS subgroups. The measurement of the subfoveal and parafoveal choroidal thickness is demonstrated in Fig. 2.
Mean values of PCT measurements in the different groups are summarized in Table 4. Evaluation of the PCT revealed no significant difference between the OSAS patients and controls. Additionally, the differences in mean PCT measurements among the OSAS subgroups were not statistically significant.
Intraexaminer intraclass correlation coefficient values for LCT and LCD were 0.884 (95% confidence interval [CI], 0.768–0.969) and 0.972 (95% CI, 0.924–0.992), respectively. Interexaminer intraclass correlation coefficients for LCT and LCD were 0.856 (95% CI, 0.764–0.985) and 0.934 (95% CI, 0.801–0.962), respectively. A significant difference was found between the OSAS patients and controls in terms of LCT but not in terms of LCD (Table 5). The mean LCT values were 213.38 ± 30.7 μm and 300.49 ± 42.654 μm for the OSAS patients and controls, respectively, indicating that the lamina cribrosa was significantly thinner in the OSAS patients than in the controls (p ˂ 0.001). Although, significant differences among the 3 subgroups were not found with respect to the LCT, LCT values were lower in severe OSAS patients than the other subgroups (mild and moderate). The measurements of the LCT, LCD, and BMO in an OSAS patient are shown in Fig. 3.
No significant correlation was found between the AHI scores and LCT values (p = 0.424, r = 0.124) and between the AHI scores and LCD values (p = 0.164, r = 0.223).
Discussion
In the present study, in which posterior segment assessment was performed in patients with OSAS as compared with healthy control subjects, there was no significant difference between the OSAS patients and controls in terms of the depth of lamina cribrosa, whereas the lamina cribrosa was found to be significantly thinner in the OSAS patients. Moreover, subfoveal, parafoveal, and peripapillary choroidal thicknesses and RNFL thickness did not significantly differ between the OSAS patients and controls. Furthermore, there were not any significant differences in choroidal thicknesses, RNFL thicknesses, and LC measurements between the OSAS subgroups.
Choroidal thickness in OSAS patients has been investigated in several studies. In their meta-analysis including 7 case-control studies (558 eyes in the OSAS group and 226 eyes in normal controls), He et al. [18] reported that subfoveal choroidal thickness tended to decrease with increasing disease severity in OSAS patients [18]. In the case-control study on patients with OSAS performed by Yazgan et al. [19], the PCT of the moderate and severe patient groups was reported to be decreased as compared with the controls [19]. It has been suggested that there may occur changes in the retinal and choroidal blood flow in OSAS patients due to intermittent hypoxia [20]. It has been also emphasized that choroidal thinning in OSAS patients may be associated with autonomic dysregulation [21]. Unlike the results of the above-mentioned studies, in the present study, no significant differences were determined between the OSAS patients and controls in terms of the choroidal thicknesses of the subfoveal, parafoveal, and peripapillary regions. Also, subfoveal, parafoveal, and peripapillary choroidal thickness measurements were not significantly different among the OSAS subgroups. This finding could be attributed to the lower AHI scores of our patients as compared with those reported in the literature.
The RNFL thickness in OSAS patients has also been studied in many studies. In the case-control study by Yazgan et al. [19], the OSAS patients were classified as those with mild, moderate, or severe OSAS according to their AHI scores and the RNFL was investigated in all quadrants using the SD-OCT with enhanced depth imaging technique. Accordingly, they reported that, as compared with the controls, the RNFL was thinner in all quadrants in the patients with moderate and severe OSAS and only in the nasal and inferotemporal quadrants in the patients with mild OSAS. In another study, the RNFL was also found to be thinner in the patients with moderate/severe OSAS than in the controls [22]. In one study, thinning of the RNFL was detected in the superior and nasal quadrants only in the patients with severe OSAS [23]. On the contrary, Adam et al. reported that the average and all quadrants RNFL thicknesses were not significantly different between OSAS patients and healthy subjects [24]. There is also a study which indicated no change in the RNFL thickness in OSAS patients as compared with controls [25]. In the present study, thinning of the RNFL was not observed in any of the quadrants in the OSAS patients as compared with controls. In addition, RNFL thicknesses were similar in the OSAS subgroups. This finding may be attributed to excluding patients with glaucoma and high IOP in this study.
Lamina cribrosa is a sieve-like structure that fills the posterior sclera foramen and from which the myelin-free retinal ganglion cell axons pass through. The lamina cribrosa should have a porous structure for passage of the retinal ganglion cell axons and concurrently it should resist stress and tension adequately. Studies have suggested that lamina cribrosa displaces posteriorly in the glaucoma [26]. There are also studies suggesting that structural differences in the lamina cribrosa may lead individuals to be more susceptible to IOP-mediated damage [27]. The prevalence of glaucoma is high in OSAS patients and all of the glaucoma patients have been reported to be in the severe OSAS group [28]. In the present study, the thinning of the lamina cribrosa observed in the OSAS patients as compared with controls might explain the susceptibility of these patients to glaucoma. From a different point of view, a hypothesis can be established that, in OSAS patients, the lamina cribrosa layer is affected before any defect is observed in the RNFL. Lamina cribrosa has a role in preserving the structural integrity of the nerve fibers and in the transmission of neurotrophic factors from the lateral geniculate nucleus to the retinal ganglion cells. A defect in the lamina cribrosa in OSAS patients causes disruption of transmission of neurotrophic material from the lateral geniculate nucleus to the retinal ganglion cells which in turn may result in thinning of the RNFL. The present study revealed that the lamina cribrosa was thinner but RNFL thickness did not change in the OSAS patients as compared with controls; this is a finding which supports the above-mentioned hypothesis.
The lamina cribrosa is located between two different pressure compartments, to which the ONH is exposed. While the pressure in front of the lamina cribrosa is exerted by the IOP, the pressure behind the lamina cribrosa is exerted by the cerebrospinal fluid (CSF). Additionally, the IOP creates a pressure load on the inner surface of the eye wall and thereby leads to a circumferential hoop tension on it. These pressures affect the depth and thickness of the lamina cribrosa [29, 30]. It has been stated that not taking the CSF pressure into account during examination of the lamina cribrosa in glaucoma would cause an underestimation. Various studies have also emphasized the importance of CSF pressure in glaucoma [31, 32]. IOP is more likely to cause posterior displacement of the lamina cribrosa; however, anterior displacement of the lamina cribrosa is also possible due to the pressure applied to the posterior sclera foramina circumferentially through application of a pressure to the inner surface of the ocular wall by IOP [33]. The increase in the CSF pressure leads the lamina cribrosa to shift anteriorly [34]. In the present study, the lamina cribrosa displaced forward in the OSAS patients as compared with the controls; although not statistically significant, this might be associated with an increase in the CSF pressure due to hypercapnia in OSAS patients.
The present study has some limitations. The study being a single-center study and its small sample size could be considered as the limitations of the present study. In addition, we could not create other subgroups according to the positional status (positional OSAS vs non-positional OSAS) and BMI (high BMI vs low BMI) due to the small sample size.
In conclusion, the present study demonstrated that the lamina cribrosa of the OSAS patients was thinner than that of the control group. Many studies demonstrate that OSAS is an important risk factor for the development of glaucoma. The thinning of lamina cribrosa may be an early finding of glaucoma in OSAS patients. For these reasons, complete ophthalmic examination should be performed at every follow-up for OSAS patients. Lamina cribrosa must be evaluated at every ophthalmic visit for early detection of glaucoma. This finding should be supported by large-scale studies. Furthermore, the reason underlying the thinning of the lamina cribrosa in OSAS patients should be investigated physiopathologically.
References
Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR (2010) Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 11:441–446
The report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults (1999) Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22:667–689
Cochen De Cock V, Benard-Serre N, Driss V et al (2015) Supine sleep and obstructive sleep apnea syndrome in Parkinson’s disease. Sleep Med 16:1497–1501
Buratti L, Viticchi G, Falsetti L, Cagnetti C, Luzzi S, Bartolini M, Provinciali L, Silvestrini M (2014) Vascular impairment in Alzheimer’s disease: the role of obstructive sleep apnea. J Alzheimers Dis 38:445–453
Braley TJ, Segal BM, Chervin RD (2012) Sleep-disordered breathing in multiple sclerosis. Neurology 79:929–936
Liu S, Lin Y, Liu X (2016) Meta-analysis of association of obstructive sleep apnea with glaucoma. J Glaucoma 25:1–7
Zhao XJ, Yang CC, Zhang JC, Zheng H, Liu PP, Li Q (2016) Obstructive sleep apnea and retinal nerve fiber layer thickness: a meta-analysis. J Glaucoma 25:e413–e418
Pérez-Rico C, Gutiérrez-Díaz E, Mencía-Gutiérrez E, Díaz-de-Atauri MJ, Blanco R (2014) Obstructive sleep apnea-hypopnea syndrome (OSAHS) and glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol 252:1345–1357
Karakucuk S, Goktas S, Aksu M, Erdogan N, Demirci S, Oner A, Arda H, Gumus K (2008) Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS). Graefes Arch Clin Exp Ophthalmol 246:129–134
Yu J, Xiao K, Huang J et al (2017) Reduced retinal vessel density in obstructive sleep apnea syndrome patients: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci 58:3506–3512
Bellezza AJ, Rintalan CJ, Thompson HW, Downs JC, Hart RT, Burgoyne CF (2003) Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci 44:623–637
Hernandez MR, Ye HG (1993) Changes in extracellular matrix in the optic nerve head. Ann Med 25:309–315
American Academy of Sleep Medicine (2014) The International Classification of Sleep Disorders. Diagnostic and Coding Manual, 3rd edn. AASM, Darien
Cartwright RD (1984) Effect of sleep position on sleep apnea severity. Sleep. 7:110–114
Margolis R, Spaide RF (2009) A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 147:811–815
Li L, Bian A, Zhou Q et al (2013) Peripapillary choroidal thickness in both eyes of glaucoma patients with unilateral visual field loss. Am J Ophthalmol 156:1277–1284
Spaide RF, Koizumi H, Pozonni MC (2008) Enhanced depth imaging spectral domain optical coherence tomography. Am J Ophthalmol 146:496–500
He M, Han X, Wu H, Huang W (2016) Choroidal thickness changes in obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Breath 20:369–378
Yazgan S, Erboy F, Celik HU, Ornek T, Ugurbas SH, Kokturk F, Ayar O, Akdemir MO, Celik E (2017) Peripapillary choroidal thickness and retinal nerve fiber layer in untreated patients with obstructive sleep apnea-hypopnea syndrome: a case-control study. Curr Eye Res 42:1552–1560
Xin C, Wang J, Zhang W et al (2014) Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS). Eye (Lond) 28:415–421
Karalezli A, Eroglu FC, Kivanc T, Dogan R (2014) Evaluation of choroidal thickness using spectral-domain optical coherence tomography in patients with severe obstructive sleep apnea syndrome: a comparative study. Int J Ophthalmol 7:1030–1034
Lin PW, Friedman M, Lin HC, Chang HW, Pulver TM, Chin CH (2011) Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch Clin Exp Ophthalmol 249:585–593
Bayhan HA, Aslan Bayhan S, İntepe YS, Muhafiz E, Gürdal C (2015) Evaluation of the macular choroidal thickness using spectral optical coherence tomography in patients with obstructive sleep apnoea syndrome. Clin Exp Ophthalmol 43:139–144
Adam M, Okka M, Yosunkaya S et al (2013) The evaluation of retinal nerve fiber layer thickness in patients with obstructive sleep apnea syndrome. J Ophthalmol 2013:292158
Ferrandez B, Ferreras A, Calvo P et al (2016) Assessment of the retinal nerve fiber layer in individuals with obstructive sleep apnea. BMC Ophthalmol 16:40
Park SC, Brumm J, Furlanetto RL, Netto C, Liu Y, Tello C, Liebmann JM, Ritch R (2015) Lamina cribrosa depth in different stages of glaucoma. Invest Ophthalmol Vis Sci 56:2059–2064
Burgoyne CF, Downs JC, Bellezza AJ, Hart RT (2004) Three-dimensional reconstruction of normal and early glaucoma monkey optic nerve head connective tissues. Invest Ophthalmol Vis Sci 45:4388–4399
Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, Mathis J (1999) High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology 106:1009–1012
Morgan WH, Yu DY, Cooper RL, Alder VA, Cringle SJ, Constable IJ (1995) The influence of cerebrospinal fluid pressure on the lamina cribrosa tissue pressure gradient. Invest Ophthalmol Vis Sci 36:1163–1172
Jonas JB, Berenshtein E, Holbach L (2003) Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 44:5189–5195
Jóhannesson G, Eklund A, Lindén C (2018) Intracranial and intraocular pressure at the lamina cribrosa: gradient effects. Curr Neurol Neurosci Rep 18:25
Promelle V, Daouk J, Bouzerar R et al (2016) Ocular blood flow and cerebrospinal fluid pressure in glaucoma. Acta Radiol Open 5:2058460115624275
Fazio MA, Johnstone JK, Smith B, Wang L, Girkin CA (2016) Displacement of the lamina cribrosa in response to acute intraocular pressure elevation in normal individuals of African and European descent. Invest Ophthalmol Vis Sci 57:3331–3339
Villarruel JM, Li XQ, Bach-Holm D, Hamann S (2017) Anterior lamina cribrosa surface position in idiopathic intracranial hypertension and glaucoma. Eur J Ophthalmol 27:55–61
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Küçük, B., Sırakaya, E. & Delibaş, Ş. Posterior segment assessment in patients with obstructive sleep apnea syndrome. Sleep Breath 23, 997–1005 (2019). https://doi.org/10.1007/s11325-019-01837-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01837-z