Abstract
Purpose
NO and NO synthase (NOS) are known to play key roles in the development of myocardial apoptosis induced by ischemia/hypoxia. Current evidence suggests that angiotensin II type 1 receptor blockers, such as telmisartan, lower blood pressure and produce beneficial regulatory effects on NO and NOS. Here, we examined the protective role of telmisartan in myocardial apoptosis induced by chronic intermittent hypoxia (CIH).
Methods
Adult male Sprague–Dawley rats were subjected to 8 h of intermittent hypoxia/day, with/without telmisartan for 8 weeks. Myocardial apoptosis, NO and NOS activity, and levels of inflammatory mediators and radical oxygen species were determined.
Results
Treatment with telmisartan preserved endothelial NOS expression and inhibited inducible NOS and excessive NO generation, while reducing oxidation/nitration stress and inflammatory responses. Administration of telmisartan before CIH significantly ameliorated the CIH-induced myocardial apoptosis.
Conclusions
This study show that pre-CIH telmisartan administration ameliorated myocardial injury following CIH by attenuating CIH-induced myocardial apoptosis via regulation of NOS activity and inhibition of excessive NO generation, oxidation/nitration stress, and inflammatory responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS), which is characterized by chronic, repetitive short cycles of oxygen desaturation followed by rapid reoxygenation (chronic intermittent hypoxia, CIH), has become a public health concern because it leads to poor sleep quality and clinical complications. Among the complications associated with OSAS, cardiovascular issues are the most severe [1]. Patients with OSAS who do not accept effective treatment have a higher rate of cardiovascular mortality [2]. Myocardial apoptosis, which can lead to cardiovascular dysfunction, can also be induced by chronic intermittent hypoxia [3, 4]. Therefore, inhibition of myocardial apoptosis could improve cardiovascular complications caused by OSAS.
NO is an important signaling molecule in the human body that participates in several physiological functions including sleep regulation [5]. NO and NO synthases (the neuronal, endothelial, and inducible isoforms of nitric oxide synthase, known as nNOS, eNOS, and iNOS, respectively) are known to be important regulators of the cardiovascular system [6]. After ischemia or hypoxia, normal metabolism of NO and NOS activity is disrupted, leading to a loss of redox equilibrium, which is associated with pathological damage in the cardiovascular system [7]. These results, together with the fact that chronic hypoxia is the most characteristic pathophysiological change associated with OSAS, suggest that the NO metabolic pathways might be involved in CIH-induced cardiovascular damage, and that restoration of normal NO metabolism may be a potential treatment for cardiovascular complications induced by CIH.
Telmisartan, an angiotensin II type 1 receptor blocker (ARB), is used to treat high blood pressure. Recently, pleiotropic effects of telmisartan were reported in several preclinical and clinical studies, which showed beneficial effects of telmisartan for conditions other than hypertension [8, 9]. Compared to other ARB antihypertensive drugs, telmisartan has some unique biological activities, including regulation of NO metabolites [10] and attenuation of ischemic myocardial injury [11]. However, the therapeutic potential of telmisartan in OSAS remains unknown. Here, we report the results of our investigation into the protective effect of telmisartan in a rodent model of intermittent hypoxia-induced myocardial apoptosis.
Methods
Animal model and experimental design
Forty male Sprague–Dawley (SD) rats (220–250 g) were purchased from the experimental animal center of Wuhan University (Wuhan, China). Animals were kept in a departmental animal facility on a 12:12-h light–dark cycle under standard laboratory conditions (temperature 25 ± 2 °C, humidity 60 ± 5 %). Rats were provided with standard rodent chow and allowed free access to water. The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Tongji Medical College at Huazhong University of Science and Technology. Rats were randomly divided into four groups (n = 10 each): normoxia + vehicle, normoxia + telmisartan, CIH + vehicle, and CIH + telmisartan. Rats were administered either telmisartan (10 mg/kg dissolved in double-distilled water) or vehicle by oral gavage prior to exposure to intermittent hypoxia on every day of the 8-week experimental period.
Intermittent hypoxia exposure
CIH was performed using custom-built chambers (OxyCycler A84, BioSpherix, Redfield, NY, USA) connected to a supply of O2 and N2 gas. The CIH protocol was as follows: O2 level was reduced from 21 to 8 % over a period of 120 s, held at 8 % for 120 s, returned to 21 % over a period of 50 s, and held at 21 % for 300 s. The rats were exposed to intermittent hypoxia for 8 h/day (during the day) on 7 days/week over 8 weeks. For the normoxic group, rats were placed in similar chambers under normoxic conditions. Within 24 h after the last exposure, rats were killed with pentobarbital sodium (40 mg/kg administered by intraperitoneal injection). The left ventricular free wall was excised from each rat, perfused with cold PBS, and preserved in liquid nitrogen or 10 % formalin for in vitro analyses.
Western blotting
Protein abundance of nNOS, eNOS, iNOS, and 3-nitrotyrosine (3-NT) were determined by Western blotting, which was performed according to routine procedures. Briefly, after tissue homogenization, proteins were extracted from the left ventricular free wall sample using RIPA lysis buffer (Beyotime, Jiangsu, China) containing a protease inhibitor cocktail to prevent protein degradation. Protein concentration was determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA). Proteins (30 μg/band) were separated via 10 % SDS-PAGE and transferred to 0.45-μm nitrocellulose membranes (Bio-Rad). Membranes were blocked in 5 % non-fat dry milk in TBST (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05 % Tween-20) for 1 h at room temperature. Membranes were incubated with rat monoclonal antibodies (Abgent, San Diego, CA, USA) against nNOS(1:500), eNOS(1:500), iNOS(1:500), and anti-3-NT(1:500) overnight at 4 °C, after which the membranes were incubated with a secondary antibody conjugated to horseradish peroxidase at room temperature for 2 h. Reactive proteins were analyzed with an ECL Western blotting detection system. All experiments were performed three or more times.
Measurement of NO production
NO content in myocardial tissue was detected using a commercially available NO assay kit (Keming Bioengineer Company, Suzhou, China) according to the manufacturer’s instructions. Because NO is very active and NO monomers have a very short existence within tissues, indirect methods were adopted to determine NO content in cardiac muscle tissue. Within tissue, NO is easily oxidized to NO2−, which reacts under acidic conditions with diazonium salts to form diazo compounds, which further couple with naphthyl-based ethylene diamine to form specific products with characteristic absorption peaks at 550 nm, through which NO content can be determined. Briefly, fresh tissue of the rat left ventricular wall (0.05 g) was homogenized with the extraction solution (0.5 mL) by centrifugation (12,000 rpm for 15 min at 4 °C), and 100 μL of the supernatant was collected and mixed with the specific reaction reagent. The resulting mixture was allowed to rest for 15 min at room temperature, after which its absorbance at 550 nm was measured using a microplate reader.
Lipid peroxidation assay
Intermittent hypoxia often leads to increased lipid peroxidation that causes tissue damage. Malondialdehyde (MDA) is commonly used to measure the effect of lipid peroxidation. In this study, oxidative stress was measured using a commercially available kit to detect the level of MDA production in myocardial tissue according to the manufacturer’s instructions (Keming Bioengineer Company, Suzhou, China).
Detection of plasma levels of C-reactive protein and interleukin 6
Changes in plasma levels of inflammatory cytokines were detected. We used ELISA kits (Neobioscience Technology Company, Beijing, China) to measure C-reactive protein (CRP) and interleukin 6 (IL-6) in the plasma according to the manufacturer’s instructions.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining for apoptotic cells in the left ventricle was used to evaluate myocardial injury. As described previously [12], a commercially available TUNEL staining kit (Boster Biological Technology, Ltd., China) was used to detect apoptosis according to the manufacturer’s instructions. At least three apoptotic cells were selected from each section of each group (three hearts per group) for photographing in the visual field (×400 magnification). The numbers of apoptotic cells and total cardiomyocytes were determined. The results are expressed as the percentage of apoptotic cells among the total cell population.
Statistical analysis
All data were expressed as mean ± SD. Data were statistically analyzed using one-way analysis of variance (ANOVA) for group comparisons. Student–Newman–Keuls post hoc tests were used when appropriate. A value of P < 0.05 was considered statistically significant.
Results
Treatment with telmisartan regulated NOS expression and limited excessive NO production
Immunoblot analysis confirmed that eNOS expression in the left ventricle was significantly decreased after CIH (P < 0.05; Fig. 1a), while iNOS expression was significantly increased (P < 0.05; Fig. 1b). There was no significant difference in nNOS abundance between the control and hypoxia groups (P > 0.05; Fig. 1c). Compared to the control group, NO synthesis was significantly elevated in the left ventricle after exposure to CIH (P < 0.05; Fig. 1d). Treatment with telmisartan abolished CIH-induced changes in eNOS and iNOS expression and inhibited excessive NO synthesis in the left ventricle after exposure to CIH, but had no effect on the expression of nNOS.
Chronic intermittent hypoxia (CIH) disrupted NOS/NO. After male SD rats were exposed to CIH for 8 weeks, the expression of eNOS was inhibited (a), while iNOS was activated (b) and NO was overproduced (d), but there was no significant difference in nNOS expression between the control group and the hypoxia group (c). Pre-treatment with telmisartan effectively inhibited CIH-induced iNOS activation and excessive production of NO, and preserved eNOS levels, but had no effect on nNOS. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; (I) normoxic, (II) normoxic + telmisartan, (III) hypoxia, and (IV) hypoxia + telmisartan. Results represent mean ± SD with means compared using one-way ANOVA. A value of P < 0.05 is considered statistically significant. ★P < 0.05 vs normoxia; ★★P < 0.05 vs hypoxia; &P > 0.05 vs normoxia; &&P > 0.05 vs hypoxia
Telmisartan inhibited CIH-induced oxidation/nitration stress in the left ventricle
Oxidation/nitration stress is considered to play a key role in the pathophysiological process of CIH-induced tissue injury. We measured MDA production in the left ventricle after exposure to CIH as an indicator of oxidation stress. MDA levels were significantly elevated in the left ventricle of rats exposed to CIH as compared to the control group (P < 0.05; Fig. 2a). Furthermore, we found a significant increase in 3-NT protein expression in the left ventricle after CIH exposure (P < 0.05; Fig. 2b). The effects of CIH on MDA levels and 3-NT protein expression were suppressed by the treatment with telmisartan.
MDA levels and 3-NT protein expression are increased in the left ventricular (LV) myocardium by chronic intermittent hypoxia (CIH). Intermittent hypoxia significantly increased expression of MDA (a) and 3-NT protein (b) in the LV myocardium. Pre-treatment with telmisartan significantly inhibited CIH-induced MDA and 3-NT expression in the LV myocardium. (I) Normoxic, (II) normoxic + telmisartan, (III) hypoxia, and (IV) hypoxia + telmisartan. Results represent mean ± SD with means compared using one-way ANOVA. A value of P < 0.05 is considered statistically significant. ★P < 0.05 vs normoxia; ★★P < 0.05 vs hypoxia
Treatment with telmisartan suppressed CIH-induced overexpression of inflammatory mediators
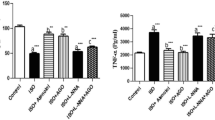
CIH can activate inflammation involved in the pathophysiological processes of CIH-induced tissue injury. In this study, we examined the plasma levels of two typical inflammatory cytokines, CRP and IL-6, using the ELISA method. Plasma levels of CRP (P < 0.05; Fig. 3a) and IL-6 (P < 0.05; Fig. 3b) were increased in rats exposed to CIH as compared to the control group. Treatment with telmisartan suppressed plasma CIH-induced overexpression of CRP and IL-6.
Inflammatory cytokines in the circulating blood. Chronic intermittent hypoxia (CIH) significantly increased expression of C-reactive protein (CRP) (a) and interleukin 6 (IL-6) (b) in the circulating blood. Pre-treatment with telmisartan significantly inhibited CIH-induced expression of these cytokines in the circulating blood. Results represent mean ± SD with means compared using one-way ANOVA. A value of P < 0.05 is considered statistically significant. ★P < 0.05 vs normoxia; ★★P < 0.05 vs hypoxia
Telmisartan significantly reduced CIH-induced myocardial apoptosis in the left ventricle of rats
Little apoptosis were found in the left ventricle of control rats, but apoptosis was significantly increased in the left ventricle of rats exposed to CIH (P < 0.05; Fig. 4). Treatment with telmisartan effectively attenuated CIH-induced myocardial apoptosis in the left ventricle of rats exposed to CIH.
Chronic intermittent hypoxia (CIH) caused myocardial apoptosis. After 8 weeks of CIH, male SD rats showed clear evidence of myocardial apoptosis as compared to animals exposed to normal oxygen concentrations. Pre-treatment with telmisartan effectively inhibited CIH-induced myocardial apoptosis. Representative photomicrographs of TUNEL assay (×400). Apoptotic cardiomyocyte nuclei appear brown stained (red arrows), whereas TUNEL-negative nuclei appear blue. a Normoxic. b Normoxic + telmisartan. c Hypoxia. d Hypoxia + telmisartan. Results represent mean ± SD with means compared using one-way ANOVA. A value of P < 0.05 is considered statistically significant. ★P < 0.05 vs normoxia; ★★P < 0.05 vs hypoxia
Discussion
Cardiovascular damage is the most common complication of OSAS. The precise pathophysiological mechanisms involved in OSAS-induced cardiovascular damage are poorly understood. There is no doubt that the pathogenesis of OSAS-related cardiovascular damage is multifactorial. In the current study, we used a rodent model of CIH to demonstrate that inflammatory processes and oxidative/nitration stress were involved in the pathophysiological myocardial apoptosis induced by CIH. More importantly, we found that CIH altered the activity of NO synthase and disrupted NO metabolism. Because treatment with telmisartan attenuated these pathological changes, these results support the potential of telmisartan in the treatment of OSAS-induced cardiovascular complications as an alternative to continuous positive airway pressure (CPAP). In addition, these results serve as a valuable reference for OSAS patients who need to take antihypertensive drugs.
Inflammatory processes and oxidative stress are considered to play key roles in the development of CIH-induced cardiovascular complications [13]. Similarly, in our current study, we found that CIH induced inflammation and expression of oxidative stress products in circulating blood and myocardial tissue. In addition, NO is believed to play a key role in the pathological mechanisms underlying myocardial damage induced by hypoxia/ischemia [7]. NO is an important signaling molecule that is involved in the regulation of almost all aspects of cellular function, including gene transcription [14] and apoptosis [15]. In humans and other mammals, NO is produced from l-arginine by the three enzymes of the NOS family: eNOS, nNOS, and iNOS. NO signaling also acts to maintain normal functioning of the cardiovascular system. Under normal physiological conditions, the level of NO in the cardiovascular system is stable, and it is mainly produced by eNOS and nNOS, which are constitutively expressed in cardiac myocytes [16] and vascular endothelial cells [17], and are known as constitutive NOSs. However, in pathological conditions such as ischemia and hypoxia, constitutive NOS activity is disrupted, NO homeostasis is disturbed, and, more importantly, iNOS is activated, which plays a significant role in the manifestation of harmful effects.
NO/NOS have been studied extensively in the context of cardiovascular diseases, but the precise role of NO/NOS in cardiovascular diseases is still unclear. The source of NO and the NO content in local tissue are of decisive significance for the ultimate effect of NO. eNOS, which is considered to be the most important NO synthase in myocardial tissue, maintains vascular tone and produces anti-thrombotic and anti-inflammatory effects [18]. When myocardial tissue is exposed to ischemia/hypoxia, eNOS activity is inhibited and tissue damage occurs [19]. Correspondingly, preservation of eNOS activity has become a focus of studies aimed at protecting myocardial tissue against hypoxic/ischemic damage [20–22]. In our study, we found that CIH significantly inhibited eNOS expression in myocardial tissue, and that telmisartan preserved the activity of eNOS and attenuated CIH-induced myocardial apoptosis. Therefore, we speculate that preservation of eNOS activity is a crucial mechanism by which telmisartan ameliorates myocardial injury induced by CIH.
nNOS is considered to mediate hypoxic/ischemic myocardial injury [7]. However, in our study, we found that there was no significant change in nNOS. In our test model, various reasons may explain why intermittent hypoxia did not cause any change in nNOS expression in myocardial tissue. However, in our study, intermittent hypoxia lasted for 8 weeks, which was longer than in most similar studies concerned with tissue injuries caused by intermittent hypoxia. Therefore, we hypothesize that in our study, nNOS in the myocardial tissue of the rat might have adapted to the intermittent hypoxic environment; therefore, there was no change in nNOS expression.
Low levels of NO are necessary to maintain normal cardiovascular function, and excess NO is harmful to cardiovascular tissue. When myocardial tissue was exposed to ischemia/hypoxia, NO levels significantly increased, and this excess NO was mainly produced by the activated iNOS [23, 24]. Excess NO can damage cardiac tissue through a variety of mechanisms such as the induction of apoptosis by changing the balance between the apoptosis mediators Bak and BCL-2 [25]. Most importantly, excess NO reacts with superoxide anion (O−· 2) to form the potent oxidant peroxynitrite (ONOO2 −), which causes oxidative damage, nitration, and S-nitrosylation of biomolecules such as proteins, lipids, and DNA [26]. Similarly, in our studies, high levels of NO were found in myocardial tissue exposed to CIH, which were accompanied by increased expression of iNOS, while eNOS expression decreased and nNOS expression remained constant. These results showed that elevated NO was a consequence of activated iNOS. In addition, the level of 3-NT was increased in the left ventricle after exposure to CIH. We speculate that 3-NT was produced because of reactions between the excessive NO and products of CIH-induced oxidative stress. Our results suggest that together, all these factors, namely inflammatory processes, high levels of NO, and oxidative/nitration stress, lead to the CIH-induced myocardial injury.
Telmisartan, an angiotensin II type 1 receptor blocker, is mainly used to reduce blood pressure. Many studies have demonstrated the therapeutic potential of ARB in the treatment of hypoxic/ischemic organ damage [27, 28]. In comparison with other ARBs, telmisartan has unique advantages, including better fat solubility that allows it to more easily penetrate cell membranes [29]. The protective effect of telmisartan against ischemic myocardial injury has been confirmed [11]. In our study, the protective effect of telmisartan on CIH-induced myocardial injury was demonstrated, and this effect was found to be mediated by regulation of NOS activity, inhibition of excessive NO synthesis, and suppression of oxidation/nitration stress and the inflammatory response.
In summary, this study provides the first evidence that telmisartan attenuates CIH-induced myocardial apoptosis, in part by preserving eNOS levels, inhibiting iNOS expression and excessive NO generation, and suppressing oxidation/nitration stress and the inflammatory response. The present findings provide novel insight into the mechanisms through which OSAS induces cardiovascular complications. Meanwhile, considering the extensive use of telmisartan in the clinic and the high incidence of OSAS, there will likely be more meticulous and in-depth studies performed in the future. Further research into the relationship between the dosage of telmisartan and its cardiovascular protective effect, and the relationship between the protective effect of telmisartan and the severity of OSAS, is required. However, it should be noted that our study had some limitations. The use of NOS or specific NOS inhibitors would help to clarify the role of NO/NOS in protective effects in this setting, and the relationship between NO/NOS, oxidative stress, and the inflammatory response should be demonstrated in future studies.
References
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF et al (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122:352–360
Chen L, Zhang J, Hu X, Philipson KD, Scharf SM (2010) The Na+/Ca2+ exchanger-1 mediates left ventricular dysfunction in mice with chronic intermittent hypoxia. J Appl Physiol 109:1675–1685, 1985
Ding W, Zhang X, Huang H, Ding N, Zhang S, Hutchinson SZ et al (2014) Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One 9:e94545
Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigne S (2012) Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev 16:265–279
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837, 837a
Carnicer R, Crabtree MJ, Sivakumaran V, Casadei B, Kass DA (2013) Nitric oxide synthases in heart failure. Antioxid Redox Signal 18:1078–1099
Yamamoto K, Ohishi M, Ho C, Kurtz TW, Rakugi H (2009) Telmisartan-induced inhibition of vascular cell proliferation beyond angiotensin receptor blockade and peroxisome proliferator-activated receptor-gamma activation. Hypertension 54:1353–1359
Li L, Luo Z, Yu H, Feng X, Wang P, Chen J et al (2013) Telmisartan improves insulin resistance of skeletal muscle through peroxisome proliferator-activated receptor-delta activation. Diabetes 62:762–774
Yuen CY, Wong WT, Tian XY, Wong SL, Lau CW, Yu J et al (2011) Telmisartan inhibits vasoconstriction via PPARgamma-dependent expression and activation of endothelial nitric oxide synthase. Cardiovasc Res 90:122–129
Rinaldi B, Di Filippo C, Capuano A, Donniacuo M, Sodano L, Ferraraccio F et al (2012) Adiponectin elevation by telmisartan ameliorates ischaemic myocardium in Zucker diabetic fatty rats with metabolic syndrome. Diab Obesity Metab 14:320–328
Hui-guo L, Kui L, Yan-ning Z, Yong-jian X (2010) Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med 11:205–212
Del RR, Moya EA, Parga MJ, Madrid C, Iturriaga R (2012) Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J 39:1492–1500
Gudi T, Hong GK, Vaandrager AB, Lohmann SM, Pilz RB (1999) Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP-dependent protein kinase. Faseb J 13:2143–2152
Li CQ, Wogan GN (2005) Nitric oxide as a modulator of apoptosis. Cancer Lett 226:1–15
Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC (1999) Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A 96:657–662
Massion PB, Feron O, Dessy C, Balligand JL (2003) Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93:388–398
Walford G, Loscalzo J (2003) Nitric oxide in vascular biology. J Thromb Haemost 1:2112–2118
Damy T, Ratajczak P, Robidel E, Bendall JK, Oliviero P, Boczkowski J et al (2003) Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. Faseb J 17:1934–1936
Gonon AT, Bulhak A, Labruto F, Sjoquist PO, Pernow J (2007) Cardioprotection mediated by rosiglitazone, a peroxisome proliferator-activated receptor gamma ligand, in relation to nitric oxide. Basic Res Card 102:80–89
Ye Y, Lin Y, Manickavasagam S, Perez-Polo JR, Tieu BC, Birnbaum Y (2008) Pioglitazone protects the myocardium against ischemia-reperfusion injury in eNOS and iNOS knockout mice. Am J Physiol Heart Circ Physiol 295:H2436–H2446
Frantz S, Adamek A, Fraccarollo D, Tillmanns J, Widder JD, Dienesch C et al (2009) The eNOS enhancer AVE 9488: a novel cardioprotectant against ischemia reperfusion injury. Basic Res Card 104:773–779
Mungrue IN, Gros R, You X, Pirani A, Azad A, Csont T et al (2002) Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest 109:735–743
Fu Y, Wang Z, Chen WL, Moore PK, Zhu YZ (2007) Cardioprotective effects of nitric oxide-aspirin in myocardial ischemia-reperfused rats. Am J Physiol Heart Circ Physiol 293:H1545–H1552
Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH (1999) Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res 84:21–33
Lee JH, Yang ES, Park JW (2003) Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem 278:51360–51371
Papademetriou V (2009) Inhibition of the renin-angiotensin-aldosterone system to prevent ischemic and atherothrombotic events. Am Heart J 157:S24–S30
Saavedra JM, Sanchez-Lemus E, Benicky J (2011) Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology 36:1–18
Michel MC, Foster C, Brunner HR, Liu L (2013) A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev 65:809–848
Acknowledgments
This study was funded by the National Natural Science Foundation of PR China (grants 81370185 and 81070067).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, X., Zhu, D., Guo, Xl. et al. Telmisartan attenuates myocardial apoptosis induced by chronic intermittent hypoxia in rats: modulation of nitric oxide metabolism and inflammatory mediators. Sleep Breath 19, 703–709 (2015). https://doi.org/10.1007/s11325-014-1081-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-1081-y