Abstract
Purpose of the Report
Paediatric diffuse high-grade gliomas (PDHGG) are rare central nervous system neoplasms lacking effective therapeutic options. Molecular imaging of tumour metabolism might identify novel diagnostic/therapeutic targets. In this study, we evaluated the distribution and the dosimetry aspects of [64Cu]CuCl2 in PDHGG subjects, as copper is a key element in cellular metabolism whose turnover may be increased in tumour cells.
Material and Methods
Paediatric patients with PDHGG were prospectively recruited. [64Cu]CuCl2 PET/CT was performed 1 h after tracer injection; if the scan was positive, it was repeated 24 and 72 h later. Lesion standardised uptake value (SUV) and target-to-background ratio (TBR) were calculated. Tumour and organ dosimetry were computed using the MIRD algorithm. Each patient underwent an MRI scan, including FLAIR, T2-weighted and post-contrast T1-weighted imaging.
Results
Ten patients were enrolled (median age 9, range 6–16 years, 6 females). Diagnoses were diffuse midline gliomas (n = 8, 5 of which with H3K27 alterations) and diffuse hemispheric gliomas (n = 2). Six patients had visible tracer uptake (SUV: 1.0 ± 0.6 TBR: 5 ± 3.1). [64Cu]CuCl2 accumulation was always concordant with MRI contrast enhancement and was higher in the presence of radiological signs of necrosis. SUV and TBR progressively increased on the 24- and 72-h acquisitions (p < 0.05 and p < 0.01, respectively). The liver and the abdominal organs received the highest non-target dose.
Conclusions
[64Cu]CuCl2 is a well-tolerated radiotracer with reasonably favourable dosimetric properties, showing selective uptake in tumour areas with visible contrast enhancement and necrosis, thus suggesting that blood–brain barrier damage is a pre-requisite for its distribution to the intracranial structures. Moreover, tracer uptake showed an accumulating trend over time. These characteristics could deserve further analysis, to determine whether this radiopharmaceutical might have a possible therapeutic role as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paediatric gliomas are the most common central nervous system neoplasms in children [1]. Unlike their adult counterparts, paediatric-type diffuse high-grade gliomas (PDHGG) are rare, accounting for only 5–20% of all gliomas [2,3,4]. The prognosis of these forms is dismal, with median overall survival ranging between 9 and 16 months for PDHGG and less than 1 year for diffuse intrinsic pontine glioma (DIPG) [3, 5,6,7,8,9].
Complete surgical resection of these infiltrative tumours is virtually impossible, and surgery is typically not attempted in patients with DIPG, owing to the location of the tumour [10,11,12]. Concurrent adjuvant radiotherapy, in combination with chemotherapy, is the standard of care for newly diagnosed PDHGG, but still less than 5% of patients survive longer than 5 years post-diagnosis [13, 14]. Since no chemotherapeutic drugs have proven effective in the treatment of DIPG, radiation therapy is the current standard regimen [15,16,17,18].
Despite dramatic improvements in the genetic and epigenetic analyses of PDHGG [4], we are still in the early stages of developing gene-targeted therapies. An alternative approach is to focus on the tumour cell metabolism; in this context, molecular imaging could provide additional information on the biological behaviour of the tumour and possibly identify new targets for a tailored approach [19, 20]. Copper is a key element in cellular turnover, being a vital co-enzyme in a number of cell functions, including mitochondrial respiration [21, 22]. Unsurprisingly, this element plays a major role in tumorigenesis and in cancer metabolism; more specifically, it has been found that copper is linked with mitogen-activated kinase stimulation and, in particular, with BRAF and K-RAS [23,24,25]. Moreover, copper is involved in cancer mitochondria-dependent energy production [26], tumour invasiveness, and even chemotherapy resistance [27]. By contrast, the normal brain parenchyma has little need for this element. Imaging aggressive brain neoplasms with a copper radioisotope might provide a favourable target-to-background ratio.
In particular, 64Cu could play both diagnostic and therapeutic roles, as its decay scheme entails the production of positrons, high-energy beta particles and Auger electrons with high linear energy transfer (LET) [28,29,30]. Indeed, this high-LET radiation could play the most important role in the theranostic effect of [64Cu]CuCl2 [31]. This tracer [64Cu]CuCl2 PET/CT has been proposed as a promising procedure for identifying adult high-grade gliomas, which often display intense tracer uptake [32].
This pilot trial aimed to evaluate the potential diagnostic role of [64Cu]CuCl2 PET/CT in patients with PDHGG by comparing PET images with MRI. We aimed to estimate kinetics, tumour/background ratio (TBR) over time, the absorbed dose of [64Cu]CuCl2 in gliomas and in organs, as well as the effective dose.
Material and Methods
Radiopharmaceutical
Copper-64 dichloride ([64Cu]CuCl2, average specific activity, 3700 MBq/µg, radiochemical purity > 99%, radionuclide purity > 99%) was produced according to a procedure previously reported [32, 33]. Briefly, 64Cu was produced by bombarding an electroplated 64Ni target using a proton current of 18 µA and energy of 14.6 MeV. Following bombardment, 64Cu was purified from other contaminants by means of chromatography and an ion-exchange column (Biorad Laboratories). The radioisotope was eluted with concentrated HCl and sieved through a 0.2-µm filter (Merck Millipore). Radionuclide purity and 64Cu half-life were measured by means of an HPGe detector (Ortec), by identifying the characteristic 511, 1022, and 1345.8 keV photopeaks. A radionuclide purity ≥ 99.5% was considered acceptable. Radiochemical purity (RCP) was assessed by having [64Cu]CuCl2 react with the tetraazacyclotetradecane-N, N′, N″, N′′′-tetraacetic acid ligand; a purity ≥ 99% was deemed acceptable.
Patient Population and Diagnostic Protocol
The local ethics committee (Comitato Etico Regionale Liguria Registration Number: 076/2019) and the “Agenzia Italiana del Farmaco” (Italian Drug Agency) approved this study. All children’s legal guardians signed a written informed consent form. The trial was registered in the European Clinical Trial Database (EudraCT number 2018–004,667-30). The neuro-oncological departments of three Italian children’s hospitals (Istituto G. Gaslini in Genoa, Bambino Gesù Children’s Hospital in Rome and AORN Santobono-Pausilipon Hospital in Naples) prospectively enrolled patients with recurrent/progressive PDHGG according to the inclusion/exclusion criteria (Table 1).
[64Cu]CuCl2 was injected into a cubital vein (median activity: 179 MBq, range 113–280 MBq, corresponding to a median of 3.9 MBq/kg, range 2.4–9.4 MBq/kg). Brain PET/CT acquisition was started 60 min thereafter and lasted 30 min. Subsequently, whole-body PET acquisition was started. Further acquisitions (i.e., brain and whole-body) were performed 24 and 72 h after the injection. In all patients, an MRI examination, including fluid attenuation inversion recovery (FLAIR), T2-weighted and pre- and post-contrast (0.1 mmol/kg, macrocyclic ionic agent) T1-weighted images, was performed. All patients were acquired on a Discovery ST PET/CT device (General Electric Healthcare Technologies, Milwaukee, WI, USA) and on a 1.5 Tesla MRI scanner (Intera Achieva, Philips, Best, the Netherlands).
Image Analysis: Registration and Voi Segmentation
PET/CT images were evaluated visually and semi-quantitatively in a patient-by-patient and lesion-by-lesion analysis after fusion of the images with MRI images (T1, T2 and FLAIR). Fusion was obtained automatically by means of a commercially available image-registration software tool (AW Server, General Electric Medical Systems). As previously described [31], volumes of interest (VOIs) were constructed on the L4-L5 vertebral bodies to assess the biokinetics and dosimetry of the active bone marrow. Subsequently, the active bone marrow in the whole body was calculated as a function of body weight [34]. VOIs were also delineated at the venous access site, to estimate the net activity administered.
In our analysis, the [64Cu]CuCl2 PET detection rate (DR) was defined as the ability to detect at least one pathological finding in each individual subject. The characteristics and extent of the tumour on conventional MRI were also correlated with [64Cu]CuCl2 PET uptake. Given the diffuse nature of the disease, tumour extent was delineated on the basis of T2/FLAIR MRI signal abnormalities. Any area of contrast enhancement within each lesion was also reviewed and correlated with [64Cu]CuCl2 PET uptake.
PET/CT was rated as positive if tumours identified on MRI exhibited tracer uptake above the level of the corresponding contralateral or remote normal brain tissue.
Kinetics of Tumours and Organs
The activity concentration of [64Cu]CuCl2 (as a percentage of injected activity/ml) in VOIs of tumours and organs for all PET datasets was recorded and fitted to a mono-exponential function or with the trapezoidal methods [35]. Hence, the time-integrated activity and its coefficient were determined. The mean activity concentration inside the tumour was corrected for the partial volume effect (PVE), as previously described [31]. The protocol is based on numerical recovery coefficients that are experimentally derived by using radioactive phantoms. Finally, the average standardised uptake values (SUV) in tumours over time was calculated and expressed as mean ± standard deviation.
Tumour/Background Ratio (TBR)
The tumour-to-background ratio (TBR) of all lesions was defined as the relation between the mean activity concentration of the lesion and the one of the background tissue; it was expressed as mean ± standard deviation. The background radioactivity concentration was obtained by calculating the mean value of VOIs drawn at 1-cm distance from each detectable lesion, at the four cardinal points.
Dosimetry of Tumours and Organs
Tumour and organ dosimetry was carried out by calculating the absorbed dose coefficient (absorbed dose per administered activity) according to the Medical Internal Radiation Dose (MIRD) system [36, 37]. The OLINDA/EXM dosimetry software was used to calculate mean organ absorbed doses based on reference anatomic models, including the reference newborn, 1-year-old, 5-year-old, 10-year-old, 15-year-old, and adult female and adult male [38]. Tumour dosimetry was calculated with the dose factors of sphere-shaped phantoms. Finally, the absorbed dose coefficients were obtained by multiplying the dose factors by the time-integrated activity coefficients. The effective dose was calculated on the basis of the tissue weighting factors of the organs (ICRP protocols 60 and 103) [39, 40].
Results
Patient Population
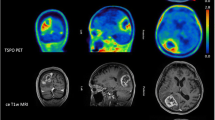
We prospectively enrolled ten patients (median age 9, range 6–16 years, 6 females), according to the inclusion criteria (Table 1). All patients had a Karnofsky performance status above 80%. Injection of the radiopharmaceutical was well tolerated, and no immediate or late side DIPG was established on the basis of clinical and MRI criteria, in accordance with RAPNO guidelines [41]. In two subjects with diffuse high-grade hemispheric gliomas, the WHO grade was determined according to histological features, owing to the lack of molecular information. Most patients (n = 8) were affected by diffuse midline gliomas (five H3K27-altered). Six of the ten patients had a positive [64Cu]CuCl2 PET/CT scan (all patients’ characteristics are reported in Table 2). In all patients, no uptake was detected in the normal parenchyma, while intense uptake was observed in the structures located outside the blood–brain barrier (e.g., choroid plexus and hypophysis) (Fig. 1).
Diagnostic Evaluation and Lesion Analysis
On MR imaging, all gliomas showed increased T2/FLAIR signal intensity and variable (iso- to hypointense) appearance on T1-weighted imaging. After administration of contrast material, six tumours displayed contrast enhancement [eight contrast-enhancing areas, five of which exhibited signs of necrosis (ring-shaped enhancement)].
Regarding [64Cu]CuCl2 PET/MR imaging fusion, increased uptake was concordant with areas of contrast enhancement in all lesions, which, in some cases, was associated with ring enhancement. Infiltrative components without contrast enhancement did not show increased uptake (Table 2, Fig. 2). The [64Cu]CuCl2 uptake pattern was heterogeneous. Specifically, the highest avidity for [64Cu]CuCl2 was displayed in tumour areas with contrast enhancement along the margins of necrotic components (Fig. 3). Three non-necrotic contrast-enhancing areas displayed low uptake.
a Post-contrast T1-weighted image. b, c [64Cu]CuCl2 PET/MR imaging fusion. Subject 4. Necrotic component displaying ring enhancement (arrow, a) with intense tracer uptake (arrow, b) in a diffuse hemispheric glioma. On later imaging 24 h after injection, the tracer intensity within the lesion had increased (arrow, c).
Five patients, with a total of seven areas with increased tracer uptake, underwent at least one further PET, 24 h after tracer injection; two of these five subjects (four lesions in total) underwent a third PET acquisition 72 h after the injection. In all these patients, the mean SUV increased over time (0.9 ± 0.5, 1.2 ± 0.7 and 1.8 ± 0.9 at the first, second and third time points, respectively). Likewise, mean TBR markedly increased over time (5.1 ± 3.5, 6.8 ± 3.7, and 11.3 ± 9.6). Subject 10’s lesion, which was located in the thoracic spinal cord, had a lower TBR than the others, although the uptake was about average, owing to the higher background uptake caused by the proximity to the liver. See Table 3 for details.
[ 64 Cu]CuCl 2 Distribution in Normal Organs and Tumours
Among the organs considered, the liver showed the highest uptake (mean percent injected activity: 38.9%). By contrast, all other organs displayed less marked tracer affinity, ranging from 0.17% (salivary glands) to 2.1% (kidneys). See Supplemental Fig. 1 for an outline. We also observed that the specific concentration depended on the patient’s body weight, as can be seen from the decreasing trend in uptake with increasing weight in all organs (Fig. 2).
In general, the tumours showed moderate copper avidity, which was lower than that of most of the normal organs, except for the active bone marrow and the cerebral parenchyma. Figure 4 depicts the specific concentration of [64Cu]CuCl2 in the organs of all patients and the tumour concentration.
Dosimetry Estimates in Organs and Tumours
The liver displayed the highest normalised absorbed dose coefficient (6.39E-1 mGy/MBq), followed by the other abdominal organs (gallbladder, pancreas, kidneys and spleen). By contrast, the tumours received only 3.44E-2 mGy/MBq. However, the tumour dose coefficient was widely variable, ranging from 2.80E-5 (in a hemispheric DPHGG, H3-wildtype and IDH-wildtype) to 7.5E-2 mGy/MBq (in two pontine DPHGG, H3-wildtype and IDH-wildtype). Supplemental Fig. 2 and Table 4 depict these data.
The mean effective dose, according to the estimates provided by the ICRP protocols 60 and 103, was 6.51E-2 mSv/MBq and 6.10E-2 mSv/MBq, respectively.
Discussion
The present data are the result of what is, to the best of our knowledge, the first attempt to evaluate the potential diagnostic role, the biodistribution and the dosimetry of [64Cu]CuCl2 PET/CT in paediatric patients with diffusely infiltrating gliomas. In PDHGG, including diffuse pontine lesion, this radiopharmaceutical shows a characteristic distribution within tumours. Increased uptake was observed in paediatric gliomas with contrast-enhancing foci on MRI, and, in some cases, correlated with radiological evidence of tumour necrosis on MR imaging. This pattern is in line with previous preclinical and clinical studies performed by Pérès et al. [42] and Tateishi K et al. [43]. However, the extent of MRI contrast-enhancing areas with concomitant [64Cu]CuCl2 increased uptake was limited in comparison with non-enhancing infiltrative components (T2/FLAIR tumour extension), and four out of ten subjects (40%) with entirely non-enhancing high-grade gliomas did not show increased tracer uptake. Indeed, this radiopharmaceutical underestimates the overall extent of PDHHG in comparison with amino-acid PET tracers such as 18F-DOPA [44,45,46]. However, the kinetic characteristics of [64Cu]CuCl2 make this element potentially suitable for late imaging, dosimetry and targeted therapy.
In positive lesions, the [64Cu]CuCl2 uptake pattern was mixed. This could be due to differences in tumour-specific biological variables, and might indicate a heterogeneous oxygen micro-environment [43[]. At the same time, since [64Cu]CuCl2 uptake was concordant with contrast enhancement on MRI, blood–brain barrier damage seems to be a potential prerequisite for the concentration of this tracer within lesions. Nevertheless, the significant increase in mean SUV over time in positive lesions suggests that a simple passive mechanism of uptake due to loss of integrity of the blood–brain barrier is unlikely. The contrast-enhancing MRI pattern seen in our population is in line with the findings of previous multicentre studies of both paediatric non-brainstem high-grade gliomas (HERBY study) [47] and DIPG [48], in which imaging showed little or no enhancement in 33% and 36% of lesions, respectively.
Negative [64Cu]CuCl2 PET/CT lesions were all located in the midline. According to the 2016 and 2021 WHO classifications, these lesions are categorised as WHO grade IV, based on their molecular profile, including histologically low-grade H3K27-altered diffuse astrocytomas. In this context, accelerated cell proliferation could be variably present [49, 50]. Therefore, midline tumours with mild-to-moderate proliferation indices may not exhibit copper avidity, even in the presence of a diffusely infiltrating indicating a dire prognosis. On the other hand, the highest uptake was shown by a hemispheric PDHGG with a histological diagnosis of glioblastoma, which normally exhibits characteristics linked with accelerated proliferation [51].
Our study adds further insight into copper kinetics, in that the standardised uptake values increased over time, up to 3 days after administration. This pattern suggests that once incorporated, [64Cu]CuCl2 does not leave the tumour cells; such a prolonged accumulation is probably typical of gliomas since our previous experience with prostate cancer documented rapid clearance in the first hour after the maximum uptake [31].
Overall, on the basis of this pilot study, we suggest that [64Cu]CuCl2 PET/CT might be selectively used in paediatric gliomas showing contrast enhancement in the whole tumour volume; in such lesions, the prolonged accumulation might constitute the rationale for potential theranostic use. However, further larger studies are needed to test this concept. Specifically, in paediatric patients with BRAF V600E-mutant gliomas—a distinctive clinical-biological group of paediatric gliomas which typically present a contrast-enhancing MRI pattern—the role of [64Cu]CuCl2 in patients who do not respond to current treatment regimens [52] could be explored, given that copper is required for oncogenic BRAF signalling and tumorigenesis [23]. As demonstrated in a previous multicentre study of BRAF V600E-mutant paediatric high-grade gliomas, new strategies beyond BRAF-inhibitor monotherapy are needed [52].
From the dosimetry point of view, should a theranostic approach be considered, the liver is the limiting organ. Indeed, our analysis showed that, on average, the liver received about twenty times more energy than the tumour per MBq employed. However, previous evidence suggests that the 64Cu-mediated cell damage is dependent on the emission of Auger electrons, with this specific component delivering up to a 25-fold higher dose than beta radiation [31, 53, 54]. Given the low range of Auger electrons, the dose actually absorbed by the cell depends on whether the radiopharmaceutical is absorbed into the cell nucleus or not [55]. Should the tracer remain in the hepatic cell cytoplasm, then the dose absorbed by the liver cell would be determined by the beta components mainly, which could be less limiting in the therapeutic use of the radiopharmaceutical, especially if we also consider the relative resilience of the liver [56]. An ideal scenario for the theranostic application of [64Cu]CuCl2 would be one of intra-nuclear absorption in the tumour cells and a cytoplasmatic distribution in non-target tissue. Such an evaluation, however, requires the set-up of targeted experiments.
Finally, the dosimetry information provided by this study could be carried over to other disease settings. Particularly, the potential of this tracer could be evaluated in neuroblastoma, which is the most frequent extracranial neoplasm and whose cells express a copper transporter, playing a role in chemotherapy sensitivity [57,58,59].
Some limitations of this study must be borne in mind. Being a pilot, project aimed mainly at assessing the feasibility, tolerability and dosimetry of [64Cu]CuCl2 in children, it involved only a small number of patients. Moreover, as many of these patients had a midline glioma, the amount of data available on hemispheric glial tumours was limited. Finally, we did not have information on the late phase of all lesions in all patients, since ethics considerations prevented us from carrying out repeated imaging in cases without visible tracer uptake on the first examination.
Conclusions
[64Cu]CuCl2 is a safe tracer in the imaging of paediatric gliomas. It is selectively taken up by MRI contrast-enhancing/necrotic tumours, suggesting that blood–brain barrier damage is a prerequisite for tracer uptake. Moreover, this radiopharmaceutical shows excellent target-to-background contrast and an accumulating pattern over time. The possibility of employing this radiopharmaceutical for therapeutic applications could represent the subject of further research.
Supplementary Information.
References
Blionas A, Giakoumettis D, Klonou A et al (2018) Paediatric gliomas: diagnosis, molecular biology and management. Ann Transl Med 6:251
Immanuel V, Kingsley PA, Negi P et al (2017) Variegated colors of pediatric glioblastoma multiforme: what to expect? Rare Tumors 9:6552
Rashed WM, Maher E, Adel M et al (2019) Pediatric diffuse intrinsic pontine glioma: where do we stand? Cancer Metastasis Rev 38:759–770
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251
Ansari M, Nasrolahi H, Kani AA et al (2012) Pediatric glioblastoma multiforme: a single-institution experience. Indian J Med Paediatr Oncol 33:155–160
Konar SK, Bir SC, Maiti TK et al (2017) A systematic review of overall survival in pediatric primary glioblastoma multiforme of the spinal cord. J Neurosurg Pediatr 19:239–248
Boudaouara O, Charfi S, Bahri M et al (2019) Pediatric high grade gliomas: clinico-pathological profile, therapeutic approaches and factors affecting overall survival. Neurochirurgie 65:63–68
Grimm SA, Chamberlain MC (2013) Brainstem glioma: a review. Curr Neurol Neurosci Rep 13:346
Felker J, Broniscer A (2020) Improving long-term survival in diffuse intrinsic pontine glioma. Expert Rev Neurother 20:647–658
Covarrubias G, Johansen ML, Vincent J et al (2020) PTPmu-targeted nanoparticles label invasive pediatric and adult glioblastoma. Nanomedicine 28:102216
Lieberman NAP, DeGolier K, Kovar HM et al (2019) Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol 21:83–94
Shabason JE, Sutton D, Kenton O et al (2016) Patterns of failure for pediatric glioblastoma multiforme following radiation therapy. Pediatr Blood Cancer 63:1465–1467
Fangusaro J (2012) Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front Oncol 2:105
Cohen KJ, Pollack IF, Zhou T et al (2011) Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol 13:317–323
Janssens GO, Jansen MH, Lauwers SJ et al (2013) Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys 85:315–320
Janssens GO, Gandola L, Bolle S et al (2017) Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 73:38–47
Janjua MB, Ban VS, El Ahmadieh TY et al (2020) Diffuse intrinsic pontine gliomas: diagnostic approach and treatment strategies. J Clin Neurosci 72:15–19
Katagi H, Louis N, Unruh D et al (2019) Radiosensitization by Histone H3 demethylase inhibition in diffuse intrinsic pontine glioma. Clin Cancer Res 25:5572–5583
Christensen M, Kamson DO, Snyder M et al (2014) Tryptophan PET-defined gross tumor volume offers better coverage of initial progression than standard MRI-based planning in glioblastoma patients. J Radiat Oncol 3:131–138
Law I, Albert NL, Arbizu J et al (2019) Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging 46:540–557
Huskisson E, Maggini S, Ruf M (2007) The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res 35:277–289
Horn D, Barrientos A (2008) Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life 60:421–429
Brady DC, Crowe MS, Turski ML et al (2014) Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509:492–496
Ishida S, Andreux P, Poitry-Yamate C et al (2013) Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci U S A 110:19507–19512
Shanbhag VC, Gudekar N, Jasmer K et al (2021) Copper metabolism as a unique vulnerability in cancer. Biochim Biophys Acta Mol Cell Res 1868:118893
Cui L, Gouw AM, LaGory EL et al (2021) Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat Biotechnol 39:357–367
Petruzzelli R, Polishchuk RS (2019) Activity and trafficking of copper-transporting ATPases in tumor development and defense against platinum-based drugs. Cells 8(9):1080
Williams HA, Robinson S, Julyan P et al (2005) A comparison of PET imaging characteristics of various copper radioisotopes. Eur J Nucl Med Mol Imaging 32:1473–1480
Bolzati C, Duatti A (2020) The emerging value of 64Cu for molecular imaging and therapy. Q J Nucl Med Mol Imaging 64:329–337
Bé MM, Cassette P, Lépy MC et al (2012) Standardization, decay data measurements and evaluation of 64Cu. Appl Radiat Isot 70:1894–1899
Righi S, Ugolini M, Bottoni G et al (2018) Biokinetic and dosimetric aspects of (64)CuCl(2) in human prostate cancer: possible theranostic implications. EJNMMI Res 8:18
Panichelli P, Villano C, Cistaro A et al (2016) Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother Radiopharm 31:159–167
McCarthy DW, Shefer RE, Klinkowstein RE et al (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–43
ICRP (1995) Basic anatomical & physiological data for use in radiological protection: the skeleton: the Skeleton. ICRP Publication 70. Ann ICRP 25:1–80
Siegel JA, Thomas SR, Stubbs JB et al (1999) MIRD pamphlet no. 16: techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med 40:37s–61s
Loeevinger R, Berman M (1968) A schema for absorbed-dose calculations for biologically-distributed radionuclides. J Nucl Med. Suppl 1:9–14
Snyder W, Ford, Warner GG, et al (1975) MIRD Pamphlet #11: S, absorbed dose per unit cumulated activity for selected radionuclides and organs
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
ICRP (2007) The 2007 Recommendations of the International Commission on Radiological Protection (2007) ICRP publication 103. Ann ICRP 37:1–332
ICRP (1991) 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP 21:1–3
Cooney TM, Cohen KJ, Guimaraes CV et al (2020) Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21:e330–e336
Pérès EA, Toutain J, Paty LP et al (2019) (64)Cu-ATSM/(64)Cu-Cl(2) and their relationship to hypoxia in glioblastoma: a preclinical study. EJNMMI Res 9:114
Tateishi K, Tateishi U, Nakanowatari S et al (2014) (62)Cu-diacetyl-bis (N(4)-methylthiosemicarbazone) PET in human gliomas: comparative study with [(18)F]fluorodeoxyglucose and L-methyl-[(11)C]methionine PET. AJNR Am J Neuroradiol 35:278–284
Morana G, Piccardo A, Tortora D et al (2017) Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur J Nucl Med Mol Imaging 44:2084–2093
Morana G, Tortora D, Bottoni G et al (2020) Correlation of multimodal (18)F-DOPA PET and conventional MRI with treatment response and survival in children with diffuse intrinsic pontine gliomas. Theranostics 10:11881–11891
Piccardo A, Tortora D, Mascelli S et al (2019) Advanced MR imaging and (18)F-DOPA PET characteristics of H3K27M-mutant and wild-type pediatric diffuse midline gliomas. Eur J Nucl Med Mol Imaging 46:1685–1694
Grill J, Massimino M, Bouffet E et al (2018) Phase II, Open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol 36:951–958
Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E et al (2015) Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol 17:160–166
Thust S, Micallef C, Okuchi S et al (2021) Imaging characteristics of H3 K27M histone-mutant diffuse midline glioma in teenagers and adults. Quant Imaging Med Surg 11:43–56
Leach JL, Roebker J, Schafer A et al (2020) MR imaging features of diffuse intrinsic pontine glioma and relationship to overall survival: report from the International DIPG Registry. Neuro Oncol 22:1647–1657
Colwell N, Larion M, Giles AJ et al (2017) Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol 19:887–896
Nobre L, Zapotocky M, Ramaswamy V, et al (2020) Outcomes of BRAF V600E pediatric gliomas treated with targeted BRAF inhibition. JCO Precis Oncol 4:PO.19.00298
Chan PC, Lisco E, Lisco H et al (1976) The radiotoxicity of iodine-125 in mammalian cells II. A comparative study on cell survival and cytogenetic responses to 125IUdR, 131TUdR, and 3HTdR. Radiat Res 67:332–343
Kassis AI, Adelstein SJ, Haydock C et al (1982) Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res 90:362–373
Kassis AI, Sastry KS, Adelstein SJ (1985) Intracellular distribution and radiotoxicity of chromium-51 in mammalian cells: Auger-electron dosimetry. J Nucl Med 26:59–67
Piccardo A, Paparo F, Puntoni M et al (2018) (64)CuCl(2) PET/CT in prostate cancer relapse. J Nucl Med 59:444–451
Bohlken A, Cheung BB, Bell JL et al (2009) ATP7A is a novel target of retinoic acid receptor β2 in neuroblastoma cells. Br J Cancer 100:96–105
Parmar A, Pascali G, Voli F et al (2018) In vivo [64 Cu]CuCl 2 PET imaging reveals activity of Dextran-Catechin on tumor copper homeostasis. Theranostics 8:5645–5659
Kilari D, Icykowski KA, Pandya C et al (2016) copper transporter-CTR1 expression and pathological outcomes in platinum-treated muscle-invasive bladder cancer patients. Anticancer Res 36:495–501
Funding
This work was supported by the Italian Ministry of Health and by Regione Liguria “Ricerca Finalizzata di Rete NET-2019–12371188 GLI-HOPE”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giovanni Morana and Arnoldo Piccardo share senior co-authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fiz, F., Bottoni, G., Ugolini, M. et al. Diagnostic and Dosimetry Features of [64Cu]CuCl2 in High-Grade Paediatric Infiltrative Gliomas. Mol Imaging Biol 25, 391–400 (2023). https://doi.org/10.1007/s11307-022-01769-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01769-3