Abstract
Purpose
Recently brown adipose tissue (BAT) activation has been proposed to have a possible role in breast cancer. The aim of this study was to evaluate BAT activation in patients with breast cancer and its relationship with molecular characteristics of tumor.
Procedures
The study group comprised 79 patients with histologically proven ductal breast carcinoma (51 ± 13 years). Data on distribution, intensity (SUVmax), and total metabolic activity (TMA) of BAT were obtained from [18F] FDG-PET/CT. Clinical and biochemical data were obtained from the database.
Results
BAT activation was present in 12 of the 79 patients (15.2 %). Patients with BAT activation were younger and had a lower body mass index (BMI) (p < 0.05 and p < 0.0005, respectively) and showed less frequently metastasis (p < 0.05). No significant differences were found in estrogen receptor (ER), progesterone receptor (PgR), Ki67, grade, and in molecular subtypes. In patients younger than 55 years and with a BMI < 26, no significant differences were observed between patients with and without BAT activation. In the 12 patients with BAT activation, a significant inverse correlation was observed between TMA and BMI (r = − 0.64, p < 0.05). TMA and SUVmax were higher in grade 2 than in grade 3 patients. No significant differences were found in both TMA and SUVmax between patients with and without lymph node metastases. A significant difference in both TMA and SUVmax was observed among different molecular types, with luminal B patients showing higher values.
Conclusions
In conclusion, the present study suggests a relation between BAT activation and positive known prognostic factor in breast cancer, such as intermediate tumor grade and luminal B cancer type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown adipose tissue (BAT) is a thermoregulatory organ involved in non-shivering thermogenesis and plays a role in metabolic processes from the clearance of lipids to glucose tolerance [1]. BAT could be present in the cervical, supraclavicular, and thoracic paravertebral regions, and less frequently in the subphrenic areas. Since the first report by Hany et al. in 2002 [2], 2-deoxy-2-[18F]fluoro-d-glucose PET/CT ([18F] FDG-PET/CT) has been extensively used to study BAT in humans. In particular, it has been definitely confirmed the relatively high incidence of active BAT in adult humans and proven histologically that [18F] FDG uptake is related to the presence of BAT [3,4,5]. Presence and activity of BAT are related to several factors [1]. Among them, the most relevant are age, body mass index (BMI), and outdoor temperature, all showing an inverse correlation, and gender, with a prevalence in women. Besides these physiological factors, BAT is involved in human disease: obesity, diabetes, cachexia, atherosclerosis, and leanness [1, 6]. Recently, an intriguing relationship of BAT with certain mutated tumor suppressor genes has been reported [7, 8]. A few human studies with [18F] FDG-PET/CT dealing with BAT and cancer have been published suggesting a role of BAT in cancer [9,10,11,12]. In a large population (N = 1740) of patient with oncological and non-oncological disease studied with [18F] FDG-PET/CT, Huang et al. found both a wider BAT distribution and a larger volume of active BAT in oncological patients [9]. Cao et al. [10] found a 3-fold higher frequency of BAT in breast cancer patients as compared to controls with other cancers. In a study involving only breast cancer patients [11], the intensity of [18F] FDG uptake in BAT had a positive correlation with human epidermal growth factor receptor 2 (HER2) expression and a negative one with progesterone receptor expression, and a higher recurrence rate was observed in patients with lower [18F] FDG uptake in BAT. More recently, Bos et al. [12] observed a greater BAT activity in patients with active cancer compared with age-, sex-, and BMI-matched BAT-positive patients without active cancer.
The aim of this study was to evaluate BAT in patients with breast cancer and its relationship with molecular characteristics of tumor.
Materials and Methods
Patients
Among all patients with newly diagnosed breast cancer studied with [18F] FDG-PET/CT in our laboratory during 2018, those with histological data were retrospectively selected. The study group comprised 79 patients with histologically proven ductal breast carcinoma (mean age 51 ± 13 years, range 31–82). Information obtained from the database were the following: age, histological type, grade, lymph node metastasis, distant metastasis, estrogen receptor (ER) and progesterone receptor (PgR) status, human epidermal growth factor receptor 2 (HER2), Ki67, breast cancer subtypes [13], and body mass index (BMI). Outdoor temperature of the day of each [18F] FDG-PET/CT scan for Naples, Italy, were obtained from the weather service of the University of Naples (Osservatorio Metereologico, Università degli Studi di Napoli Federico II).
[18F] FDG-PET/CT Acquisition and Analysis
After a fasting period of 8 h, all patients underwent a PET/CT scan on a commercial scanner (Discovery IQ, GE Healthcare; Discovery PET/TC 710, GE Healthcare); PET/CT scan was acquired 60 min after intravenous administration of 3.5 MBq/kg of [18F] FDG with 3 min per bed position. PET data were reconstructed with and without attenuation correction into transverse, sagittal, and coronal images. MDCT (pitch 0.94; mAs 80; kVp 120; FoV 70 cm) was performed without intravenous and/or oral contrast medium as part of the PET/CT scan, and CT data were reconstructed into transverse images with a 4.25-mm section thickness while sagittal and coronal images were obtained by reconstruction of the transverse data. In all patients’ blood, glucose level was obtained before the radiotracer administration, and a cut-off value of less than 140 mg/dL was adopted to perform the examination. In both injection and waiting room, temperature was maintained at a constant 24 °C by an air conditioning/heating and thermostats system. [18F] FDG-PET/CT image analysis was performed as previously described [14]. Briefly, all images were reviewed at a workstation (Volumetrix MI, version n. 3.0562, GE Healthcare). Total body [18F] FDG-PET/CT scans were assessed for the presence of lymph node and distant metastases. [18F] FDG uptake in BAT was considered to be present when the uptake in characteristic areas of brown fat localization, showing the CT density of adipose tissue (− 250/− 50 Hounsfield units), was higher than background soft-tissue activity. The site of uptake was determined as: cervical, supraclavicular, mediastinal, thoracic paravertebral, and subphrenic. Maximum body weight–corrected standardized uptake values were determined for each area by using the vendor-provided software (syngo.via, version VB30A_HF03 Siemens Healthcare GmbH 2009–2018) on PET scan using a region of interest diameter of 1 cc. For each patient showing [18F] FDG BAT uptake, the maximum SUVmax was recorded. In addition, the volume of activated BAT for each region was computed and the total metabolic activity (TMA) was defined as the evaluation of all regions using total tumor load (syngo.via Frontier, version n. 1.3.3 Siemens Healthcare GmbH 2009–2018).
Statistical Analysis
Data are expressed as mean ± one standard deviation or as proportion, as appropriate. Differences between continuous data were assessed using unpaired Student’s t test. Differences among more than 2 groups were assessed by analysis of variance followed by post hoc comparison. Categorical data were evaluated by chi-square analysis. Relationships between variables were assessed by Pearson or Spearman analysis, as appropriate. A p value < 0.05 was considered significant. A commercial statistical software was used (MedCalc®).
Results
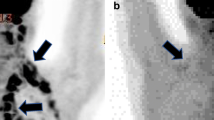
BAT was present in 12 of the 79 patients (15.2 %). None of these 12 patients showed BAT uptake in the subphrenic region, while 8 had uptake in 4 regions (cervical, supraclavicular, mediastinal, thoracic paravertebral), 1 in 3 regions (cervical, supraclavicular, thoracic paravertebral), and 3 in 2 regions (2 patients: cervical and supraclavicular; 1 patient: supraclavicular and thoracic paravertebral). In these 12 patients, mean value of SUVmax was 7.6 ± 2.1 (range 4.9–10.6), and the mean value of TMA was 52.5 ± 36.3 (range 7–128).
Table 1 shows the clinical and PET finding difference between patients with BAT activation and those without. Patients with BAT activation were younger and had a lower BMI than patients without (p < 0.05 and p < 0.0005, respectively), and outdoor temperature was lower at time of [18F] FDG-PET/CT of patients with BAT activation (p < 0.005). Moreover, a significantly (p < 0.05) lower percentage of patients with BAT activation had lymph node and distant metastasis at [18F] FDG-PET/CT. No significant differences were found in ER, PgR, Ki67, and grade. Of the 12 patients with BAT activation, 8 (66 %) were considered luminal A, 2 (17 %) luminal B, 2 (17 %) triple negative, and none HER-like; on the other hand, of the 67 patients without BAT activation, 28 (42 %) were considered luminal A, 29 (43 %) luminal B, 6 (9 %) triple negative, and 4 (6 %) HER-like (p = n. s. vs patients with BAT activation). Thereafter, the whole analysis was repeated only in patients younger than 55 years and with a BMI < 26. Table 2 shows the results obtained in this subgroup of patients. No significant differences were observed between patients with and without BAT activation, although a slightly lower percentage of patients with BAT activation showed lymph node and distant metastases.
In the 12 patients with BAT activation, a significant inverse correlation was observed between TMA and BMI (r = − 0.64, p < 0.05), while no significant correlations were found between TMA or SUVmax and all the other clinical characteristics of patients (Table 3). TMA was higher in grade 2 patients than in grade 3 (70.1 ± 18.5 vs 42.7 ± 15.1, respectively, p < 0.05) as well as SUVmax (8.4 ± 0.8 vs 7.2 ± 0.9, respectively, p < 0.05). No significant differences were found in both TMA and SUVmax between patients with and without lymph node metastases (TMA: 51.0 ± 42.51 vs 54.4 ± 30.1; SUVmax: 7.89 ± 2.35 vs 7.57 ± 1.9; both p = n. s.). A significant difference in both TMA and SUVmax was observed among different molecular types, with luminal B patients showing higher values (Table 4).
Discussion
The frequency of active BAT among breast cancer patients in the present study was 15.2 %, and patients with active BAT showed a lower prevalence of both lymph node and distant metastasis when compared with patients without active BAT. However, since patients showing active BAT were younger and had a lower BMI than patients without, the whole analysis was repeated in a subgroup matched for age and BMI. In this subgroup, the frequency of active BAT was 27.9 % (12/43), and the prevalence of both lymph node and distant metastasis in patients with active BAT was lower, although not significantly, than in patients without. When considering only patients with active BAT, clear and significant differences in both volume and activity of BAT were observed among molecular types of breast cancer.
The incidence of BAT activation detection by [18F] FDG-PET/CT has been reported to range from 1.7 to 9.3 % [1], while relatively higher prevalence of activated BAT has been observed in patients with lymphoma (17 %) or breast cancer (16.7–80 %) [9, 10, 15, 16]. In the present study, 15.2 % of breast cancer patients showed active BAT, which is close to the 16.7 % incidence found by Cao et al. [10]. When only younger patients were analyzed, 27.9 % of them had active BAT, again similar to the 25.6 % frequency previously found [10].
A possible role of BAT activation in cancer has been hypothesized [9,10,11,12, 15, 16], and neoplastic status has been advocated to be a critical determinant of BAT activity [9, 10]. The results of published studies are somewhat conflicting. Actually, while some of them suggest that BAT activity is greater in patients with active cancer [9,10,11], a better prognosis in breast cancer patients is associated with the presence of BAT activation [11]. In the group of breast cancer patients included in this study, lower incidence of lymph node and distant metastases have been found in those showing active BAT. Moreover, in patients with active BAT, significantly higher volume and activity of BAT have been found in grade 2 than in grade 3 patients. In addition, TMA and SUVmax of BAT activation were higher in luminal B than in triple negative. These findings suggest that the presence of BAT activation may be considered an indicator of a lower level of biological aggressiveness in accordance with a previous survival study [17].
The possible association between active BAT and breast cancer and its mechanisms are not fully understood. Several factors have been demonstrated to be associated with activation of BAT: age, BMI, outdoor temperature, gender, anxiety, and rest metabolic rate [1]. However, recent studies in animals and humans suggest that BAT activation could be involved in cancer [7,8,9,10,11,12, 15, 16]. In animal studies, BAT has been associated with increased tumor growth and neovascularization and reduced hypoxia [7, 18]. Moreover, a possible link between BAT and systemic cytokine-mediated response to cancer has been postulated [19, 20]. Two possible alternate hypotheses have been proposed to explain the role of active BAT in breast cancer [10]. First, active BAT could accelerate the progression of cancer due to induction of neovascularization and growth rate [18, 21], or BAT can be activated by molecules secreted by cancer cells [22]. However, evidences from the literature are not conclusive, and more data on the molecular implications of active BAT in patients affected by cancer are needed to refine the role of [18F] FDG [23].
The present study has some limitations. It is retrospective and data indices of the stresses associated with cancer were not assessed. The actual menopause status, the timing of prior medication and treatment history of every patient, were not available. Since only patients with a definite histological diagnosis were included, a selection bias could be present. In addition, the number of patients for the analysis of the associations between the total metabolic volume and the activity of BAT and biological factors was relatively small.
Conclusion
In conclusion, the present study does not support the hypothesis of an association with active BAT and more aggressive breast cancer; on the contrary, our data suggest a relation between total volume of activated BAT and positive known prognostic factor. However, the small number of patients does not allow definite conclusions and larger studies are needed.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bauwens M, Wierts R, van Royen B, Bucerius J, Backes W, Mottaghy F, Bran B (2014) Molecular imaging of brown adipose tissue in health and disease. Eur J Nucl Med Mol Imaging 41:776–791
Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK (2002) Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 29:1393–1398
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
Pasanisi F, Pace L, Fonti R, Marra M, Sgambati D, De Caprio C, De Filippo E, Vaccaro A, Salvatore M, Contaldo F (2013) Evidence of brown fat activity in constitutional leanness. J Clin Endocrinol Metab 98:1214–1218
Berstein LM (2012) Cancer and heterogeneity of obesity: a potential contribution of brown fat. Future Oncol 8:1537–1548
Jones LP, Buelto D, Tago E, Owusu-Boaitey KE (2011) Abnormal mammary adipose tissue environment of Brca1 mutant mice show a persistent deposition of highly vascularized multilocular adipocytes. J Cancer Sci Ther 8(Suppl 2). https://doi.org/10.4172/1948-5956.s2-004
Huang YC, Chen TB, Hsu CC, Li SH, Wang PW, Lee BF, Kuo CY, Chiu NT (2011) The relationship between brown adipose tissue activity and neoplastic status: an (18)F-FDG PET/CT study in the tropics. Lipids Health Dis 10:238–244
Cao Q, Hersl J, La H, Smith M, Jenkins J, Goloubeva O, Dilsizian V, Tkaczuk K, Chen W, Jones L (2014) A pilot study of FDG PET/CT detects a link between brown adipose tissue and breast cancer. BMC Cancer 14:126–131
Fujii T, Yajima R, Tatsuki H, Oosone K, Kuwano H (2017) Implication of atypical supraclavicular F18-fluorodeoxyglucose uptake in patients with breast cancer: association between brown adipose tissue and breast cancer. Oncol Lett 14(6):7025–7030
Bos SA, Gill CM, Martinez-Salazar EL, Torriani M, Bredella MA (2019) Preliminary investigation of brown adipose tissue assessed by PET/CT and cancer activity. Skelet Radiol 48(3):413–419
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24:2206–2223
Pace L, Nicolai E, D’Amico D, Ibello F, Della Morte AM, Salvatore B, Pizzuti LM, Salvatore M, Soricelli A (2011) Determinants of physiologic 18F-FDG uptake in brown adipose tissue in sequential PET/CT examinations. Mol Imaging Biol 13:1029–1035
Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji Bm Chatal JF, Resche I, Campone M (2006) Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging 33:785–791
Dobert N, Menzel C, Hamscho N, Wordehoff N, Kranert WT, Grünwald F (2004) Atypical thoracic and supraclavicular FDG-uptake in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Q J Nucl Med Mol Imaging 48:33–38
Howlader N, Cronin KA, Kurian AW, Andridge R (2018) Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev 27(6):619–626
Lim S, Hosaka K, Nakamura M, Cao Y (2016) Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget 7(25):38282–38291
Chan XHD, Balasundaram G, Attia ABE, Goggi JL, Ramasamy B, Han W et al (2018) Multimodal imaging approach to monitor browning of adipose tissue in vivo. J Lipid Res 59:1071–1078
Wang H, Willershäuser M, Karlas A, Gorpas D, Reber J, Ntziachristos V et al (2019) A dual Ucp1 reporter mouse model for imaging and quantitation of brown and brite fat recruitment. Mol Metab 20:14–27
Lim S, Honek J, Xue Y, Seki T, Cao Z, Andersson P, Yang X, Hosaka K, Cao Y (2012) Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat Protoc 7:606–615
Gao D, Vahdat LT, Wong S, Chang JC, Mittal V (2012) Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res 72:4883–4889
Gennaro N, Pepe G, Antunovic L (2019) 18F-FDG uptake of brown fat and cancer: casualty or causality? Eur J Nucl Med Mol Imaging 46:1395–1396
Author information
Authors and Affiliations
Contributions
LP conceived the study, performed the statistical analysis, and wrote the manuscript. EN participated in its design and coordination and helped to draft the manuscript. LB acquired the scans and elaborated them. NG acquired the scans and elaborated them. AS participated in the study design and coordination. MS was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the Institutional Ethics Committee (Prot 2-11, IRCCS Fondazione SDN).
Informed Consent
For this type of study, retrospective, formal consent is not required.
Consent for Publication
The manuscript does not contain individual person’s data in any form.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pace, L., Nicolai, E., Basso, L. et al. Brown Adipose Tissue in Breast Cancer Evaluated by [18F] FDG-PET/CT. Mol Imaging Biol 22, 1111–1115 (2020). https://doi.org/10.1007/s11307-020-01482-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01482-z