Abstract
Purpose
The aim of the study was to investigate the influence of dual time point 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) positron emission tomography/x-ray computed tomography (PET/CT) on the standard uptake value (SUV) and volume-based metabolic variables of breast lesions and their relation with biological characteristics and molecular phenotypes.
Procedures
Retrospective analysis including 67 patients with locally advanced breast cancer (LABC). All patients underwent a dual time point [18F]FDG PET/CT, 1 h (PET-1) and 3 h (PET-2) after [18F]FDG administration. Tumors were segmented following a three-dimensional methodology. Semiquantitative metabolic variables (SUVmax, SUVmean, and SUVpeak) and volume-based variables (metabolic tumor volume, MTV, and total lesion glycolysis, TLG) were obtained. Biologic prognostic parameters, such as the hormone receptors status, p53, HER2 expression, proliferation rate (Ki-67), and grading were obtained. Molecular phenotypes and risk-classification [low: luminal A, intermediate: luminal B HER2 (−) or luminal B HER2 (+), and high: HER2 pure or triple negative] were established. Relations between clinical and biological variables with the metabolic parameters were studied. The relevance of each metabolic variable in the prediction of phenotype risk was assessed using a multivariate analysis.
Results
SUV-based variables and TLG obtained in the PET-1 and PET-2 showed high and significant correlations between them. MTV and SUV variables (SUVmax, SUVmean, and SUVpeak) where only marginally correlated. Significant differences were found between mean SUV variables and TLG obtained in PET-1 and PET-2. High and significant associations were found between metabolic variables obtained in PET-1 and their homonymous in PET-2. Based on that, only relations of PET-1 variables with biological tumor characteristics were explored. SUV variables showed associations with hormone receptors status (p < 0.001 and p = 0.001 for estrogen and progesterone receptor, respectively) and risk-classification according to phenotype (SUVmax, p = 0.003; SUVmean, p = 0.004; SUVpeak, p = 0.003). As to volume-based variables, only TLG showed association with hormone receptors status (estrogen, p < 0.001; progesterone, p = 0.031), risk-classification (p = 0.007), and grade (p = 0.036). Hormone receptor negative tumors, high-grade tumors, and high-risk phenotypes showed higher TLG values. No association was found between the metabolic variables and Ki-67, HER2, or p53 expression.
Conclusion
Statistical differences were found between mean SUV-based variables and TLG obtained in the dual time point PET/CT. Most of PET-derived parameters showed high association with molecular factors of breast cancer. However, dual time point PET/CT did not offer any added value to the single PET acquisition with respect to the relations with biological variables, based on PET-1 SUV, and volume-based variables were predictors of those obtained in PET-2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast tumor metabolism assessed by 2-deoxy-2-[18F]fluoro-D-glucose PET/x-ray computed tomography ([18F]FDG PET/CT) has shown multiple relations with histopathological and immunohistochemical factors [1–3]. The maximum standard uptake value (SUVmax) represents a single-pixel value, which reflects maximum intensity of [18F]FDG activity in the tumor and ignores the extent of metabolic abnormality and changes in the distribution of a tracer within a lesion. This has lead to an increase of the interest on other different tumor burden variables accounting for all metabolically active regions within the tumor mass. Remarkable examples are the metabolic total volume (MTV) and total lesion glycolysis (TLG) that could serve as better predictors of clinical outcome than semiquantitative methods. However, there is a limited amount of information about the relationships between clinical and pathological factors of breast cancer and volume-based metabolic parameters. On the other hand, it is well known that the maximum glycolytic activity of tumor tissues occurs between 3 and 5 h after the administration of [18F]FDG. This is why dual time point PET has been used in order to improve the characterization of breast lesions [4, 5].
The evolution of tumor activity metabolic variables, after [18F]FDG administration, has been studied previously as well as their relations with tumor biology. However, to our knowledge, no previous study has addressed the changes in volume-based metabolic variables, or their relations with molecular tumor characteristics, in a dual time point acquisition [6–8].
Thus, the aim of the present study was to complement the previous knowledge in two ways. Firstly, to explore the relations between the metabolic parameters SUVmax, SUVmean, SUVpeak, MTV, and TLG of the dual time point [18F]FDG PET/CT and secondly, for conventional and delayed imaging, to analyze the correlations of all metabolic variables with biological characteristics and molecular phenotypes obtained from a sample of patients with locally advanced breast cancer (LABC).
Materials and Methods
Patients
Patients reported in this work were participants of an ongoing prospective multicenter study initiated in September 2009 with the following inclusion criteria: newly diagnosed breast cancer with clinical indication of neoadjuvant chemotherapy (NC) and absence of distant metastases confirmed by other methods previously to request the PET/CT for staging purpose. For the present study, patients with PET scans with a unique or predominant breast lesion uptake higher than background and with a clinical size of at least 2 cm greatest diameter in any projection on conventional imaging techniques (ultrasonography or mammography) were selected. The study included seven hospitals and was approved by the respective institutional review boards. Written informed consent was obtained from all patients.
Pre-Treatment Histopathological Analysis
The histopathological analysis of the primary tumor was performed on specimens obtained by core aspiration biopsy. The determination of tumor type and the histopathological grading were obtained. Immunohistochemistry was performed on paraffin-embedded material using primary antibodies for estrogen and progesterone receptors (ER/PR), epidermal growth factor receptor (HER2), and the proliferation index based on the Ki-67 antibody. ER, PR, and HER2 were scored as positive (+) or negative (−) as previously was referred [9].
Final positive lymph node status (positive or negative) was established by the clinician attending to histopathological confirmation, by fine needle aspiration biopsy (FNAB)/sentinel lymph node biopsy (SLNB). In cases of multiple pathologic lymph nodes, classification was based on ultrasonography and PET/CT. N stage was established, integrating histology and PET information and M stage by PET/CT according to the classification of the American Joint Committee on Cancer (AJCC) [10].
Molecular Classification of Subgroups
Patients were categorized into five molecular phenotypes [11] [luminal A, luminal B-HER2(−), luminal B-HER2(+), HER2(+) pure, and triple negative] on the basis of the different combinations of ER, PR, and HER2 status and Ki-67 labeling index. Furthermore, patients were classified in risk categories depending on tumor phenotype: high risk [basal like or HER2(+) pure], intermediate risk [luminal B-HER2(−) or luminal B-HER2(+)], and low risk [luminal A].
[18F]FDG-PET/CT Imaging
Patients fasted for at least 6 h before the PET examination and had blood glucose levels less than 160 mg/dl at the time of injection. PET/CT was performed on the same dedicated whole-body PET/CT machine (Discovery DSTE-16 s, GE Medical Systems) following a standardized protocol in three-dimensional (3D) mode.
Fifty-seven patients underwent dual time point imaging with a mean interval of 127 min between the two phases (range between 112 and 138 min). The first examination was performed as whole-body images from head to thigh 60 min after intravenous administration of approximately 370 MBq of [18F]FDG (PET-1 or early PET). The second examination imaged only the chest, with acquisition of one or two bed positions (PET-2 or delayed PET). Both acquisitions were performed following a standardized protocol [9].
Imaging Assessment and Lesion Segmentation
Two nuclear medicine physicians performed visual assessment, independently. For the present study, PET scans with a unique or dominant breast lesion uptake higher than background and with a size of at least 2 cm of greatest diameter were selected.
For the semiquantitative assessment, the PET images in DICOM format (Digital Imaging and Communication in Medicine) files were imported into the scientific software package Matlab (R2015b, The MathWorks, Inc., Natick, MA, USA) and pre-processed using a semi-automatic image segmentation procedure. All image visualizing and processing procedures were performed using in-house software in Matlab. The tumor was manually located and automatically segmented in three dimensions to acquire the metabolic and volumetric variables for each patient. The software allows enclosing the tumor within a box. In case of multiple breast lesions (multicenter or multifocal cancer), the largest one with the highest [18F]FDG uptake was selected for the analysis.
The standard uptake values (SUV) were computed using the formula:
The parameters SV, RS, W, RTD, DF, Et, and Hf were stored value, rescale slope, patient weight, radiopharmaceutical injected dose, decay factor, elapsed time, and half-life, respectively.
Regions contained in the box with a SUV equal to or larger than 40 % the SUVmax were automatically included in the volume of interest (VOI).
Metabolic Variables Acquisition
In each VOI, semiquantitative parameters were obtained as maximum, mean, and peak standard uptake value (SUVmax, SUVmean, and SUVpeak, respectively). The metabolic tumor volume (MTV) and the total lesion glycolysis (TLG) were also computed for each patient. The variables were defined as follows:
-
SUVmax was defined as the maximum uptake in the VOI reflects the maximum tissue concentration of [18F]FDG in the tumor.
-
SUVmean was taken as the average of the SUV values contained in the VOI.
-
To compute SUVpeak, all possible averages SUV in cubes of 3 × 3 × 3 voxels included in the tumor were computed. The maximum value of those averages was assigned to SUVpeak.
-
MTV was the volume of the VOI after the segmentation.
-
TLG was taken as the product of SUVmean by the MTV, providing an estimate of the total tumor glycolytic activity.
The percentage difference in the SUVmax or retention index (RI) between PET-1 and PET-2 was calculated according to the formula: [(SUV-2) − (SUV-1)]/(SUV-1).
Statistical Analysis
Normality of the variables was analyzed using the Kolmogorov-Smirnov test. Based on the results of this analysis, Spearman’s test was used to study the correlations between all semiquantitative variables SUVmax, SUVmean, SUVpeak, MTV, TLG, and age.
First, we computed the correlations between the PET-1 and PET-2 metabolic tumor variables. Due to its non-parametric nature, Spearman’s correlation coefficient was considered. Values below 0.1 were taken as indicators of no correlation between the variables while correlation values over 0.7 (and p value < 0.05) were taken as indicators of strong correlation. Based on significant and high associations were found between any metabolic variable obtained in PET-1 with the same variable obtained in PET-2, only PET-1 variables were used for the following analysis.
The association of semiquantitative variables with clinic-pathological prognostic factors and biological subtypes (qualitative variables) and the RI with the risk categories were evaluated using the Kruskal-Wallis test. This test measures the difference of medians between groups of qualitative variables and identifies the significant groups. The Mann-Whitney test was used for categorical variables. According to the age at the diagnosis, patients were divided into two groups, ≤45 years or > 45 years. Furthermore, in order to convert Ki-67 to a categorical variable, two groups were defined (high grade with a Ki-67 ≥ 14 % and low grade Ki-67 < 14 %).
We also computed the area under the curve (AUC) of all metabolic quantitative variables using the receiver operator characteristics (ROC) analysis. The point on the curve furthest from the line of no discrimination was considered the optimum threshold and the cutoff value.
Finally, a multivariable logistic regression analysis with the most significant quantitative variables was used to obtain the odds ratio for each metabolic variable. These variables were binarized using the cutoff value threshold obtained from their respective ROC curves. Also, the dependence variable, that is the phenotype risk, was divided in two groups: high risk [basal like and HER2(+) pure] and low/intermediate risk [luminal B-HER2(−), luminal B-HER2(+), and luminal A]. Wald’s test was used for this analysis.
A significance level (p value) of p < 0.05 was used in all statistical tests. All p values obtained from multiple comparisons were corrected using Bonferroni’s method.
Results
Sixty-seven patients were included for the present study, 57 of them with a dual time point PET/CT acquisition. Disease characteristics of the studied population including among others, histopathological analysis, molecular classification, and PET information are summarized in Tables 1 and 2.
Significant differences were found between mean SUVmax, SUVmean, SUVpeak, and TLG obtained in PET-1 and PET-2 (p < 0.001 for SUV-based variables and p = 0.008 for TLG). No statistical significant differences were found for the MTV (p = 0.291).
Significant relations were observed between SUVmax and SUVpeak with other metabolic variables in PET-1 and PET-2 with the only exception of MTV. Detailed results are shown in Tables 3 and 4.
SUVmean showed significant relations with the TLG both in PET-1 (r = 0.730; p < 0.0001) and PET-2 (r = 0.786; p < 0.0001).
Integrated metabolic variables, as MTV and TLG, showed significant association in PET-1 (r = 0.825; p < 0.0001) and PET-2 (r = 0.848; p < 0.0001).
MTV showed a very weak, yet significant, association with SUVmean in PET-1 (r = 0.259; p = 0.033) and PET-2 (r = 0.370; p = 0.004).
Most metabolic variables obtained in PET-1 and PET-2 showed high and significant associations. The only exceptions were found between the MTV and the SUV variables, both in the PET-1 and PET-2. Table 5 shows all the results.
The mean ± SD RI was 14.07 ± 16.35. Although high-risk tumors had higher RI (mean ± SD of 17.68 ± 18.18) with respect to intermediate and low-risk tumors (mean ± SD of 12.32 ± 15.17 and 12.89 ± 18.84, respectively), no significant differences between the RI with the risk categories were found (p = 0.619).
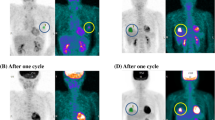
Any metabolic variable obtained in PET-1 showed high and significant association with the same variable obtained in PET-2. Based on the associations between the PET-1 and PET-2 metabolic variables, only PET-1 variables were used to study their correlations with biological characteristics and molecular phenotypes. Figs. 1 and 2 show examples of low- and high-risk phenotype lesions with their metabolic results.
Lesion segmentation and metabolic variables of a luminal A tumor (low-risk phenotype category). Maximum intensity projection PET image and axial, coronal, and sagital projections showing the location, metabolic morphology, and 3D segmented image of the breast tumor. The obtained metabolic variables were SUVmax = 9.57, SUVpeak = 7.03, SUVmean = 5.80, MTV = 3.13 cm3, and TLG = 18.16.
Lesion segmentation and metabolic variables of a basal tumor (high-risk phenotype category). Maximum intensity projection PET image and axial, coronal, and sagital projections showing the location, metabolic morphology, and 3D segmented image of the breast tumor. The obtained metabolic variables were SUVmax = 11.66, SUVpeak = 9.00, SUVmean = 7.15, MTV = 17.90 cm3, and TLG = 127.91.
SUV variables were found to be associated with hormone receptor status (ER: p < 0.001 in all cases and PR: p = 0.001 in all cases) and risk-classification attending phenotype (SUVmax, p = 0.003; SUVmean, p = 0.004; SUVpeak, p = 0.003).
With respect to volume-based variables, only TLG showed association with hormone receptors status (ER: p < 0.001 and PR: 0.031), risk-classification (p = 0.007), and grade (p = 0.036). Hormone receptors negative tumors, high-grade tumors, and high-risk phenotypes showed higher SUV and volume values compared to the others (Table 6).
No association was found between SUV or volume-based variables with the Ki-67, HER2, p53 expression, or nuclear grade. Only the TLG was associated with the molecular phenotypes (p = 0.007). Neither relations were found with the clinical T stage, except for the SUVpeak (p = 0.04), metabolic stage, N metabolic stage, metabolic foci pattern, metabolic necrosis, lymph node histopathologic involvement, and patient age (cutoff of 45 years).
No significant relations were found between Ki-67, as continuous variable, and SUV or volume-based variables. However, when we considered Ki-67 as a categorical variable (high grade with a Ki-67 ≥ 14 % and low grade with a Ki-67 < 14 %), significant relations were found for the volume-based variables (MTV, p = 0.006; TLG, p = 0.020); however, SUV variables did not show significant relations (SUVmax, p = 0.652; SUVmean, p = 0.707; SUVpeak, p = 0.598).
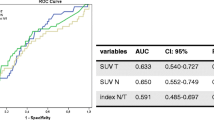
ROC curves were computed for all the metabolic variables, and the accuracy of the test was obtained depending on whether PET variables separated the patients with and without high-risk phenotype. Typically, the 95 % confidence interval was between 0.65 and 0.90. The AUC was 0.737, 0.757, 0.757, 0.752, and 0.647 for TLG, SUVmax, SUVpeak, SUVmean, and MTV, respectively. The cutoff values with the best sensitivity and specificity for the prediction of high-risk phenotype were SUVmax = 6.95, SUVmean = 4.42, SUVpeak = 5.48, MTV = 10.02, and TLG = 67.10.
In the multivariable logistic regression analysis for the prediction of high-risk phenotype, the only significant variable was the SUVmax (p < 0.001) with an odds ratio value of 10 (Table 7).
Discussion
The SUVmax is a sensitive indicator of metabolic activity and tissue proliferation in breast cancer. However, this is influenced by multiple factors, among others, tumor size [12, 13]. For this reason, we excluded tumors smaller than 2 cm of long axial axis as other authors did previously [14]. Kaida et al. [15] were less restrictive and excluded patients with tumors smaller than 10 mm based on the full-width at half-maximum of PET and those with negative [18F]FDG uptake for breast cancer.
With respect to the dual time point acquisition in [18F]FDG PET/CT, a different metabolic behavior with time has been reported depending on the tumor biology. A decrease of SUVmax over time of 50 % was found in luminal A tumors while a 25 % decrease was observed in triple negative tumors. However, no statistical differences of the retention index were found between the different groups [1]. In the present analysis, although high-risk tumors had a mean RI higher compared with intermediate- and low-risk categories, no significant differences were found.
In the present work, the relation between metabolical variables obtained in PET-1 and PET-2 was studied, finding a high and significant association between SUV variables with TLG. On the other hand, significant differences were found between mean SUV-based variables and TLG obtained in PET-1 and PET-2. Thus, tumor metabolism was higher in PET-2 compared with PET-1. However, no differences were found for the MTV, probably explained by the use of the 40 % of the SUVmax in the segmentation process, instead of a fixed value.
We have previously reported a good linear relation between SUVmax in PET-1 and SUVmax in PET-2 (R 2 = 0.948) allowing predicting SUVmax in PET-2 as a function of SUVmax in PET-1 [16]. In the present study, significant associations were found both between SUV and volume-based variables obtained in the same PET acquisition (Tables 3 and 4) and the cross-relation between PET-1 and PET-2 variables (Table 5). To our knowledge, no previous reported work has addressed this relation. Thus, dual time point PET does not seem to add any useful information with respect to the standard single acquisition since all the PET-2 metabolic variables studied could be predicted from the obtained in PET-1.
MTV is able to represent the total volume and activity of the metabolically active cancer cells and may be less sensitive to statistical noise. Even more, TLG combined from SUV and MTV represents both the degree of [18F]FDG uptake and the size of the metabolically active tumor. However, the selection of a validated optimal threshold to delineate the tumor and determine the volume-based variables seems to be a challenge taking into account the different clinical context as lesions size, tumor biology, and background breast activity that can be influenced by patient age and hormonal status.
The methods used for lesion segmentation are classified in fixed or adaptive. Some authors have used a threshold of maximum activity for each lesion, i.e., a fraction of SUVmax [17]. Others used fixed absolute values [15, 18]. In the present study, we decided to choose the 40 % of the maximum uptake for each analyzed breast lesion. Based on we did not use a fixed threshold for tumor segmentation, partial volume correction was not performed.
With respect to the metabolic variables and clinical and metabolic stages, previous works have found significant relations between volume-based metabolic variables and the presence of metabolic lymph node involvement and distant metastases [14]. Kaida et al. [15] described associations between volumetric parameters and SUVmax with pathological T stage and pathological N status. We did not find any relation of metabolic variables with clinical or metabolic stages, except for an association between SUVpeak and T3 and T4.
The value of some PET-derived parameters as biomarkers has been previously described [19]. In breast cancer, correlation between the [18F]FDG uptake and the expression of proliferation-associated antigen Ki-67 was found [20]. In the present work, a significant association was found between Ki-67 groups and volume-based metabolic variables although no relations were observed for the SUV-based variables.
Few works have explored the relations between tumor biological characteristics and volume-based variables such as MTV and TLG. These works found that TLG and/or MTV were significantly associated with high nuclear grade but no significant relations were found with hormone receptor or HER2 expression [14, 15]. In our study, TLG was found to be correlated to estrogen and progesterone receptor status and showed statistical differences between GI, GII, and GIII (with larger values in more dedifferentiated tumors) and MTV did not show any significant relation, except for the ER expression. No significant associations were found between MTV and nuclear grade. TLG was correlated with molecular tumor characteristics probably due to its combined nature (volumetric and metabolic). Based on that, our study seems to support TLG as a better indicator of clinicopathological factors of breast cancer than SUVmax or MTV [15]. In our case, TLG had the additional advantage, with respect to SUV variables, of showing association with tumor grade.
With regard to molecular phenotypes, Kajary et al. [14] observed the largest differences in TLG between the luminal A and the triple negative group, with overlapping values for the other groups. The lowest median value of MTV was also found in the luminal A group, and the highest in HER2(+) cancers. Luminal B-HER2(−) cancers have in average lower SUVmax, SUV, and TLG as compared to luminal B-HER2(+) cancers. The opposite was found for MTV. Luminal B-HER2(+) have lower SUV average, MTV, and TLG compared with HER2(+) cancers, but the medians of SUVmax were similar. Kaida et al. [15] found higher SUV and volume-based metabolic variables in triple negative tumors compared to the others. In our dataset, the more aggressive phenotypes, such as triple negative and HER2 (+) pure, had higher SUV and TLG than the intermediate- and low-risk tumors. In the present work, higher values of SUV-based variables and TLG were found in the high-risk phenotype group with respect to the others. Using a multivariable logistic regression analysis, we found that the most significant variable was the SUVmax. Thus, in our sample, patients with elevated SUVmax had a 90 % risk of belonging to the high-risk phenotype category.
About the limitations, considering that MTV and TLG of [18F]FDG PET/CT are suggested to be better indicators of whole tumor burden than SUVmax, we analyzed only the representative lesion responsible of the T staging. The fact that some patients had multifocal lesions could affect our results. However, multifocal and multicentric lesions represented only the 25 % of our sample.
Conclusion
Statistical differences were found between mean SUV-based variables and TLG obtained in the dual time point PET/CT. PET-derived parameters, especially the ones most directly related to tumor glycolysis, such as the SUV variables and TLG, could act as predictors of tumor biology based on their association with histopathological factors of breast cancer, although in the multivariable logistic regression analysis for the prediction of high-risk phenotype, the most significant variable was the SUVmax.
Dual time point PET/CT did not offer any added value to the single PET acquisition with respect to the relations with biological variables, based on PET-1 SUV and volume-based variables were predictors of those obtained in PET-2.
References
García Vicente AM, Soriano Castrejón A, León Martín A et al (2013) Molecular subtypes of breast cancer: metabolic correlation with 18F-FDG PET/CT. Eur J Nucl Med Molec Imag 40:1304–1311
Bolouri MS, Elias SG, Wisner DJ et al (2013) Triple-negative and non-triple-negative invasive breast cancer: association between MR and fluorine-18 fluorodeoxyglucose PET imaging. Radiology 269:354–361
Koo HR, Park JS, Kang KW et al (2014) 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol 24:610–618
Zytoon AA, Murakami K, El-Kholy M-R et al (2008) Dual time point FDG-PET/CT imaging. Potential tool for diagnosis of breast cancer. Clin Radiol 63:1213–1227
Mavi A, Urhan M, Yu JQ et al (2006) Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med 47:1440–1446
Basu S, Chen W, Tchou J et al (2008) Comparison of triple-negative and estrogen receptorpositive/ progesterone receptor positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters. A potentially useful method for disease characterization. Cancer 112:995–1000
Shimoda W, Hayashi M, Murakami K et al (2007) The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer 14:260–268
García Vicente AM, Soriano Castrejón A, Relea Calatayud F et al (2012) 18F-FDG retention index and biologic prognostic parameters in breast cancer. Clin Nucl Med 37:460–466
García Vicente AM, Cruz Mora MA, León Martín AA et al (2014) Glycolitic activity with 18F-FDG PET/CT predicts final neoadjuvant chemotherapy response in breast cancer. Tumour Biol 35:11613–11620
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22:1736–1747
Lu P, Yu L, Li Y et al (2010) A correlation study between maximum standardized uptake values and pathology and clinical staging in non-small cell lung cancer. Nucl Med Commun 31:646–651
Koksal D, Demiragn F, Bayiz H et al (2013) The correlation of SUVmax with pathological characteristics of primary tumor and the value of tumor/ lymph node SUVmax ratio for predicting metastasis to lymph nodes in resected NSCLC patients. J Cardiothorac Surg 8:63
Kajary K, Tokes T, Dank M et al (2015) Correlation of the value of 18F-FDG uptake, described by SUVmax, SUVavg, metabolic tumour volume and total lesion glycolysis, to clinicopathological prognostic factors and biological subtypes in breast cancer. Nucl Med Commun 36:28–37
Kaida H, Toh U, Hayakawa M et al (2013) The relationship between 18F-FDG metabolic volumetric parameters and clinicopathological factors of breast cancer. Nucl Med Commun 34:562–570
García Vicente AM, Soriano Castrejón A, Pruneda González RE et al (2016) Basal-18F-FDG PET/CT as a predictive biomarker of tumor response for neoadjuvant therapy in breast cancer. Rev Esp Med Nucl Imagen Mol 35:81-87
Kahraman D, Scheffler M, Zander T et al (2011) Quantitative analysis of response to treatment with erlotinib in advanced non-small cell lung cancer using 18F -FDG and 3′-deoxy-3′-18F-fluorothymidine PET. J Nucl Med 52:1871–1877
Lee SM, Bae SK, Kim TH et al (2014) Value of 18F -FDG PET/CT for early prediction of pathologic response (by residual cancer burden criteria) of locally advanced breast cancer to neoadjuvant chemotherapy. Clin Nucl Med 39:882–886
Groheux D, Giacchetti S, Moretti J-L et al (2011) Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging 38:426–435
Deng SM, Zhang W, Zhang B et al (2015) Correlation between the uptake of 18F-fluorodeoxyglucose (18F-FDG) and the expression of proliferation-associated antigen Ki-67 in cancer patients: a meta-analysis. PLoS One 10:e0129028
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study included seven hospitals and was approved by the respective institutional review boards. Written informed consent was obtained from all patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
All the authors have participated in the writing and revision of this article and take public responsibility for its content.
The present publication is approved by all authors and by the responsible authorities where the work was carried out.
All the authors confirm the fact that the article is not under consideration for publication elsewhere.
Rights and permissions
About this article
Cite this article
Garcia-Vicente, A.M., Pérez-Beteta, J., Pérez-García, V.M. et al. Metabolic Tumor Burden Assessed by Dual Time Point [18F]FDG PET/CT in Locally Advanced Breast Cancer: Relation with Tumor Biology. Mol Imaging Biol 19, 636–644 (2017). https://doi.org/10.1007/s11307-016-1034-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-016-1034-x