Abstract
Purpose
Triple-negative breast cancer has a poor prognosis. We evaluated several metabolic and volumetric parameters from preoperative 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in the prognosis of triple-negative breast cancer and compared them with current clinicopathologic parameters.
Methods
A total of 228 patients with triple-negative breast cancer (mean age 47.0 ± 10.8 years, all women) who had undergone preoperative PET/CT were included. The PET/CT metabolic parameters evaluated included maximum, peak, and mean standardized uptake values (SUVmax, SUVpeak, and SUVmean, respectively). The volumetric parameters evaluated included metabolic tumor volume (MTV) and total lesion glycolysis (TLG). Metabolic and volumetric parameters were evaluated separately for tumor (T) and lymph nodes (N). The prognostic value of these parameters was compared with that of clinicopathologic parameters.
Results
All lymph node metabolic and volumetric parameters showed significant differences between patients with and without recurrence. However, tumor metabolic and volumetric parameters showed no significant differences. In a univariate survival analysis, all lymph node metabolic and volumetric parameters (SUVmax-N, SUVpeak-N, SUVmean-N, MTV-N, and TLG-N; all P < 0.001), T stage (P = 0.010), N stage (P < 0.001), and TNM stage (P < 0.001) were significant parameters. In a multivariate survival analysis, SUVmax-N (P = 0.005), MTV (P = 0.008), and TLG (P = 0.006) with TNM stage (all P < 0.001) were significant parameters.
Conclusions
Lymph node metabolic and volumetric parameters were significant predictors of recurrence in patients with triple-negative breast cancer after surgery. Lymph node metabolic and volumetric parameters were useful parameters for evaluating prognosis in patients with triple-negative breast cancer by 18F-FDG PET/CT, rather than tumor parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common female malignancy and second leading cause of cancer-related death in women [1]. Breast cancer is recognized as a heterogeneous disease with a high degree of diversity in response to therapy and in patterns of metastasis, which are important for patient outcome and prognosis [2]. Among several types of breast cancer, triple-negative breast cancer is a heterogeneous group of tumors characterized by a poor prognosis [3]. Stratification of patients with triple-negative cancer in relation to prognosis would be highly desired, because patients with a better prognosis could benefit from appropriately targeted treatment(s) [4].
Currently, 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) is widely used in clinical evaluation of cancers. In particular, 18F-FDG PET/CT has been used for breast cancer characterization, staging, predicting treatment response, and detecting recurrence [5,6,7]. It has also been reported to be effective in predicting tumor recurrence in breast cancer using visual and metabolic parameters, including standardized uptake values (SUV) [8, 9]. Previous studies have shown that tumor uptake of 18F-FDG in triple-negative breast cancer is significantly higher than in other phenotypes [10,11,12].
18F-FDG PET/CT volumetric parameters are also being increasingly studied because they can reflect tumor burden as well as metabolic activity. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) from 18F-FDG PET/CT are useful parameters, particularly for prognosis prediction and treatment response monitoring [13, 14]. These volumetric parameters have also been shown to be valuable in the prognosis of breast cancer, but previous studies were performed in patients with phenotypically heterogeneous various cancers [15, 16]. As far as we are aware, there has been no 18F-FDG PET/CT study to determine the prognostic value of volumetric parameters in patients with triple-negative breast cancer. Most previous 18F-FDG PET/CT studies in breast cancer were limited to tumor characteristics. However, lymph node uptake of 18F-FDG can also have prognostic value [17]. Therefore, we hypothesized that volumetric parameters relating to the tumor and the lymph nodes could also have prognostic value.
In this study, we investigated the value of 18F-FDG PET/CT volumetric parameters in predicting recurrence of surgically resected triple-negative breast cancer. Tumor and lymph node volumetric parameters were compared with metabolic parameters, and their prognostic value was compared with that of clinicopathologic prognostic factors.
Materials and methods
Patients
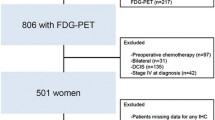
From January 2008 to December 2013, 5,231 breast cancer patients underwent 18F-FDG PET/CT in our institution. Among these patients, those who underwent surgery for their breast cancer were retrospectively enrolled in the current study with the following inclusion criteria:
-
1.
Pathologically confirmed triple-negative infiltrating ductal carcinoma [estrogen receptor (ER) negative/progesterone receptor (PR) negative/human epidermal growth factor receptor-2 (HER2) negative]
-
2.
Preoperative 18F-FDG PET/CT performed within 6 months without any previous treatment
-
3.
Measurable tumor (longest diameter >1 cm on CT) with FDG avidity (maximum SUV >2.5)
-
4.
No evidence of distant metastasis before breast cancer surgery
-
5.
Follow-up after breast cancer surgery of more than 36 months in case of no recurrence
Patients were routinely checked using imaging studies including mammography, ultrasonography, contrast-enhanced CT, and/or bone scintigraphy every 4–6 months for 5 years after surgery, and every year thereafter. Imaging studies were also performed when tumor markers (routine check-up at intervals of 4–6 months) increased or other suspicious symptoms or signs presented. Recurrence of a lesion was confirmed based on the follow-up imaging studies. The study design was approved by the Institutional Review Board (IRB) of our institution and informed consent was waived.
A total of 228 patients with triple-negative breast cancer were included in the study (all women, mean age 47.0 ± 10.8 years). Tumor pathology was infiltrating ductal carcinoma in all patients, and patients were followed up at our institution after surgery (mean follow-up 55.9 ± 15.9 months). Tumor recurrence was found in 72 patients (31.6%) 14.8 ± 9.4 months after surgery. Patient TNM stages were I–III (I 12.7%, II 55.3%, III 32.0%). Patients underwent breast cancer surgery in accordance with their surgeon’s decision (breast-conserving surgery in 72.8%, modified radical mastectomy in 6.6%, total mastectomy in 20.6%). Chemotherapy was performed in 96.9% of patients, and radiation therapy in 78.9% of patients in accordance with their oncologist’s decision (Table 1).
18F-FDG PET/CT protocol
After fasting for at least 6 h, 18F-FDG (5.18 MBq/kg) was injected intravenously, and imaging was performed 1 h later using a hybrid PET/CT scanner (Biograph 40 Truepoint; Siemens Healthcare, Knoxville, TN, USA). CT images were acquired from the skull base to the upper thigh area for the attenuation map and lesion localization (50 mA, 120 kVp, 5-mm section width, 4 mm collimation). PET images of the same area were acquired after the CT scans in three-dimensional mode for six or seven bed positions (1 min per bed position, 21.6 cm increments). Images were reconstructed on 128 × 128 matrices using an iterative algorithm. The images were analyzed using a dedicated workstation and analysis software (Syngo.via, Siemens Healthcare, Knoxville, TN, USA).
18F-FDG PET/CT image analysis
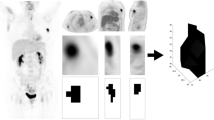
All of the following metabolic and volumetric PET/CT parameters were used to define a volume of interest (VOI) on the workstation. The VOI for metabolic and volumetric parameters was defined by nuclear medicine physicians to be large enough to cover the lesions. Metabolic and volumetric parameters were evaluated separately for tumor (T) and lymph nodes (N). The VOI was defined on the breast area for the tumor, and on the axillary/supraclavicular area for the lymph nodes. An additional VOI was defined when internal mammary lymph node uptake was found. In this case, higher values for the metabolic parameters were selected by comparing the axillary/supraclavicular VOIs with the internal mammary VOIs. For volumetric parameters, two axillary/supraclavicular and internal mammary VOIs were added (Fig. 1).
Measurement of 18F-FDG PET/CT metabolic and volumetric parameters. a For tumor measurement, a volume-of-interest (VOI) was drawn (red dashed circle) to include the whole breast cancer and an isoactivity contour was automatically drawn (red solid line) by setting a margin SUV threshold of 2.5. b For lymph node (LN) measurements, VOIs were drawn to include all metastatic axillary and supraclavicular LNs (blue dashed circle), and internal mammary LN metastases (green dashed circle). Similar to tumor measurements, an isoactivity contour was automatically drawn by setting a margin SUV threshold of 2.5 (blue and green solid lines, respectively)
SUVs were calculated using the following equation: SUV = (tissue radioactivity [Bq]/tissue weight [g])/(injected activity [Bq]/body weight [g]). The metabolic parameters maximum SUV (SUVmax), measured as the SUV of the pixel with the highest uptake, and peak SUV (SUVpeak) and mean SUV (SUVmean), measured as the SUVs of the pixels with the peak uptake and mean uptake, respectively) were recorded. Volumetric parameters included MTV, estimated from VOI SUV isoactivity contours automatically drawn by setting a margin SUV threshold of 2.5, and TLG, calculated as SUVmean × MTV.
Clinicopathologic parameters and pathologic evaluation
Clinicopathologic information was obtained from patient medical records. Age, tumor location, pathologic T stage, pathologic N stage, pathologic TNM stage, operation method, chemotherapy, and radiation therapy were included.
Histologic type and tumor grade based on the Elston-Ellis system were evaluated during routine histopathologic examination of surgical specimens [18]. The immunohistochemistry (IHC) parameters evaluated were the expression levels of ER, PR, and HER2. ER and PR expression levels were evaluated semiquantitatively as the percentages of cells with positive nuclear staining (range 0–100%). A 1% cut-off value was used for the expression of ER and PR [19]. Expression levels of HER2 were evaluated by combined IHC and/or fluorescent in situ hybridization (FISH). To assess HER2 expression by IHC, the following semiquantitative scoring system was used: 0 no membrane staining, 1+ weak and inhomogeneous membrane staining in some tumor cells, 2+ weak to moderate membrane staining in a large number of tumor cells, 3+ strong and homogeneous membrane staining in most tumor cells. A score of 2+ was considered equivocal and additional FISH analysis was conducted to confirm HER2 gene amplification. Either a score of 3+ indicating strong positive expression or HER2 gene amplification by FISH was classified as positive [20].
Statistical analysis
Clinicopathologic and 18F-FDG PET/CT parameters were compared between patients with and without recurrence using the chi-squared test for categorical data (Fisher’s exact test for chemotherapy), and the independent samples Student’s t test for continuous data. In survival analyses, optimal cut-off values for quantitative factors that maximized the significance in the survival analysis were determined using an algorithm [21]. Kaplan-Meier analysis and a log-rank test were performed to evaluate disease-free survival (DFS) according to the cut-off values applied. Finally, univariate and multivariate Cox regression analyses were performed to assess the effect of clinicopathologic and PET/CT parameters, where P < 0.05 was considered significant. All statistical analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA) and MedCalc, version 12.2 (MedCalc Inc., Mariakerke, Belgium).
Results
Clinicopathologic parameters and recurrence
Among clinicopathologic parameters, T stage (P = 0.009), N stage (P < 0.001), and TNM stage (P < 0.001) showed significant differences between patients with and without recurrence. However, patient age, tumor location, operative method, chemotherapy, and radiation therapy showed no significant differences (Table 2).
18F-FDG PET/CT parameters and recurrence
All the lymph node PET/CT metabolic and volumetric parameters showed significant differences between patients with and without recurrence (SUVmax-N, SUVpeak-N, SUVmean-N and MTV-N, P < 0.001; TLG-N, P = 0.013), whereas tumor metabolic and volumetric parameters showed no significant differences (Table 3).
Univariate and multivariable analysis
In the univariate analysis, T stage (P = 0.010), N stage (P < 0.001), TNM stage (P < 0.001), SUVmax-N (P < 0.001), SUVpeak-N (P < 0.001), SUVmean-N (P < 0.001), MTV-N (P < 0.001), and TLG-N (P < 0.001) were significant parameters. Among the clinicopathologic and PET/CT parameters, the volumetric parameters showed the highest hazard ratios (HR) (HR of MTV-N = 4.89, HR of TLG-N = 4.75). Among the metabolic parameters, SUVmax-N showed the highest HR (HR of SUVmax-N = 4.23) (Table 4 and Fig. 2).
Kaplan-Meier analysis of clinical and 18F-FDG PET/CT parameters. a Patients with TNM stage I/II showed significantly longer disease-free survival (DFS) than those with TNM stage III (5-year DFS 82.5% vs. 37.0%; P < 0.001). b Patients with low LN SUVmax (SUVmax-N ≤9.83) showed significantly longer DFS than those with high SUVmax-N (>9.83; 5-year DFS 75.6% vs. 28.8%; P < 0.001). c Patients with low LN metabolic tumor volume (MTV-N ≤11.04) showed significantly longer DFS than those with high MTV-N (>11.04; 5-year DFS 75.1% vs. 21.5%; P < 0.001)
For the multivariate analysis, TNM stage was selected from among clinicopathologic parameters as it showed a higher hazard ratio than both T stage and N stage, and interaction was found between them. In the multivariate analysis of SUVmax-N and TNM stage, SUVmax-N (HR 2.16, P = 0.005) and TNM stage (HR 1.97, P < 0.001) were the significant parameters. In the multivariate analysis of the volumetric parameters and TNM stage, MTV-N (HR 2.17, P = 0.008) and TLG-N (HR 2.20, P = 0.006) were significantly associated with TNM stage (HR 1.98 and 1.96, respectively; all P < 0.001; Table 5, Figs. 3 and 4).
Preoperative PET/CT imaging in a 35-year-old woman with stage III triple-negative breast cancer as an example of recurrence prediction using 18F-FDG PET/CT lymph node (LN) metabolic and volumetric parameters. a, b Transaxial PET/CT and PET images of the LNs show significantly increased uptake (SUVmax-N 14.29, MTV-N 30.60 cm3, TLG-N 153.92). c, d However, transaxial PET/CT and PET images of the tumor show only moderately increased uptake (SUVmax-T 5.81, MTV-T 1.19 cm3, TLG-T 4.14). The patient had tumor recurrence in the brain pons 9 months after breast cancer surgery
Preoperative PET/CT imaging in a 24-year-old woman with stage III triple-negative breast cancer as an example of prediction of no recurrence using 18F-FDG PET/CT lymph node (LN) metabolic and volumetric parameters. a, b Transaxial PET/CT and PET images of the LNs show mildly increased uptake (SUVmax-N 2.54, MTV-N 0.82 cm3, TLG-N 2.41). c, d However, transaxial PET/CT and PET images of the tumor show significantly increased uptake (SUVmax-T 39.29, MTV-T 53.87 cm3, TLG-T 846.30). The patient had no tumor recurrence 42 months after breast cancer surgery
Discussion
The results of the current study suggest that 18F-FDG PET/CT can provide effective prediction of recurrence in patients with surgically resected triple-negative breast cancer. Several 18F-FDG PET/CT and clinicopathologic parameters were compared, and lymph node metabolic and volumetric parameters along with TNM stage showed significant prognostic value. Thus, 18F-FDG PET/CT lymph node volumetric parameters (MTV-N and TLG-N) could be prognostic factors.
The main advantage of the current study is that the studied group was homogeneous. All patients had triple-negative infiltrating ductal carcinoma and had undergone breast cancer surgery. Furthermore, the usefulness of 18F-FDG PET/CT lymph node volumetric parameters has not been previously reported. Song et al. [17] investigated the prognostic value of 18F-FDG PET/CT lymph node metabolic parameters in patients with operable breast cancer, and found that they provided better prognostic value than tumor metabolic parameters. However, the study was limited to metabolic parameters and phenotypically heterogeneous tumors. Kim et al. [16] evaluated 18F-FDG PET/CT lymph node metabolic and volumetric parameters in patients with operable phenotypically heterogeneous breast cancer. Lymph node metabolic volumetric parameters showed significant correlations with the histologic grade of tumor, but showed no significant prognostic value. Our study showed that lymph node volumetric parameters have significant prognostic value and higher HRs than lymph node metabolic parameters in patients with operable triple-negative infiltrating ductal carcinoma.
18F-FDG PET/CT has been used for initial staging and stratification of various phenotypes of breast cancer. In particular, 18F-FDG PET/CT has been used to identify occult distant metastases and involvement of extra-axillary lymph nodes, which serve as critical indices in initial staging of breast cancer [22]. Previous studies investigating FDG uptake in breast cancers have indicated that triple-negative breast cancer typically has higher metabolic activity than other phenotypes, and that FDG uptake could potentially be used to predict the outcome of neoadjuvant chemotherapy [23,24,25]. Recently, the correlation between 18F-FDG uptake and prognostic markers in triple-negative breast cancer has been investigated [26]. The prognostic value of 18F-FDG uptake was further studied in one histologic type of breast cancer to provide more specific information. Previous studies have also shown that tumor SUVmax predicts survival outcome in patients with luminal type [27] and triple-negative [28] breast cancer.
The current study demonstrated that volumetric parameters (MTV and TLG) have higher HRs than metabolic parameters (SUVmax, SUVpeak, and SUVmean). Among PET/CT parameters, metabolic parameters are the most commonly used for quantitative analysis in clinical situations [29]. However, there has been some debate as to the use of SUV. Heterogeneity of the tumor, partial volume effects, and the timing of SUV evaluation may limit the ability of SUV to represent the exact metabolism of the lesion and to reflect accurately the tumor characteristics [30]. In addition to metabolic parameters, volumetric parameters have recently become widely used for analyzing FDG PET/CT in various cancers [31,32,33]. MTV is the volume of tissue that exhibits higher metabolism over a certain threshold, and TLG = MTV × SUVmean. Thus, while SUV reflects only metabolic activity of the tumor cell component, volumetric parameters reflect both metabolic activity and tumor burden from the whole tumor lesion. In previous studies, MTV has been shown to be a better prognostic factor in triple-negative metastatic breast cancer [13] and phenotypically heterogeneous metastatic breast cancer [16] than metabolic parameters. These results support those of our study showing that volumetric parameters have better prognostic value than metabolic parameters.
Lymph node metabolic and volumetric parameters showed significant prognostic value, whereas tumor parameters did not. Lymph node involvement in breast cancer is well known to be the most important prognostic factor [34]. Since triple-negative breast cancer is the most aggressive form [35], lymph node metastasis could be a more important factor for recurrence. We defined large VOIs for the evaluation of supraclavicular lymph nodes, and separate VOIs for more accurate evaluation of internal mammary lymph nodes. Including all possible metastatic lesions provides more exact evaluation of the lymph nodes. Recent studies have strongly indicated that high 18F-FDG uptake by lymph nodes predicts a worse outcome in breast cancer [17], and head and neck cancer [36]. The current study outcomes are consistent with lymph node parameters showing a poor prognosis in triple-negative breast cancer.
The current study had several limitations. First, this was a retrospective study and the study protocol was not strictly controlled. There was some variation in the intervals between 18F-FDG PET/CT acquisition and breast cancer surgery, which may have led to bias may not have been a critical factor. The status of other mutations, such as epidermal growth factor receptor, which could have influence FDG uptake was not studied. Second, combined chemotherapy and radiation therapy could have interfered with evaluation of the results. Finally, follow-up was relatively short. Patient follow-up was more than 36 months with a mean 55.9 months. A larger scale study with a longer follow-up is required to confirm our results.
Conclusion
18F-FDG PET/CT volumetric lymph node parameters are effective predictors of recurrence in patients with surgically resected triple-negative breast cancer. We expect that prediction of recurrence in triple-negative breast cancer can be optimized using lymph node metabolic/volumetric parameters and TNM stage, which will assist in the choice of therapeutic strategies.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18:123–33.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67.
Groheux D, Cochet A, Humbert O, Alberini J-L, Hindié E, Mankoff D. 18F-FDG PET/CT for staging and restaging of breast cancer. J Nucl Med. 2016;57:17S–26S.
Groheux D, Mankoff D, Espié M, Hindié E. 18F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging. 2016;43:983–93.
Jo I, Zeon SK, Kim SH, Kim HW, Kang SH, Kwon SY, et al. Correlation of primary tumor FDG uptake with clinicopathologic prognostic factors in invasive ductal carcinoma of the breast. Nucl Med Mol Imaging. 2015;49:19–25.
Groheux D, Hindié E, Delord M, Giacchetti S, Hamy A-S, de Bazelaire C, et al. Prognostic impact of 18FDG-PET-CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst. 2012;19:1879–87.
Chang JS, Lee J, Kim HJ, Kim KH, Yun M, Kim SI, et al. 18F-FDG/PET may help to identify a subgroup of patients with T1-T2 breast cancer and 1–3 positive lymph nodes who are at a high risk of recurrence after mastectomy. Cancer Res Treat. 2016;48:508–17.
Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters. Cancer. 2008;112:995–1000.
Groheux D, Giacchetti S, Moretti J-L, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Vicente AMG, Castrejón ÁS, Martín AL, López-Muñiz IC, Madero VM, Sánchez MMM, et al. Molecular subtypes of breast cancer: metabolic correlation with 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2013;40:1304–11.
Marinelli B, Espinet-Col C, Ulaner GA, McArthur HL, Gonen M, Jochelson M, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am J Nucl Med Mol Imaging. 2016;6:120–7.
Hyun SH, Ahn HK, Park YH, Im Y-H, Kil WH, Lee JE, et al. Volume-based metabolic tumor response to neoadjuvant chemotherapy is associated with an increased risk of recurrence in breast cancer. Radiology. 2014;275:235–44.
Son SH, Lee S-W, Jeong SY, Song B-I, Chae YS, Ahn B-C, et al. Whole-body metabolic tumor volume, as determined by 18F-FDG PET/CT, as a prognostic factor of outcome for patients with breast cancer who have distant metastasis. AJR Am J Roentgenol. 2015;205:878–85.
Kim J, Yoo SW, Kang S-R, Cho S-G, Oh J-R, Chong A, et al. Prognostic significance of metabolic tumor volume measured by 18F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–85.
Song B-I, Lee S-W, Jeong SY, Chae YS, Lee WK, Ahn B-C, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med. 2012;53:1337–44.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–72.
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43.
Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862.
Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, et al. 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med. 2013;54:5–11.
Groheux D, Hindié E, Giacchetti S, Delord M, Hamy A-S, de Roquancourt A, et al. Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med. 2012;53:249–54.
Groheux D, Giacchetti S, Delord M, de Roquancourt A, Merlet P, Hamy A, et al. Prognostic impact of 18F-FDG PET/CT staging and of pathological response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:377–85.
Humbert O, Riedinger J-M, Charon-Barra C, Berriolo-Riedinger A, Desmoulins I, Lorgis V, et al. Identification of biomarkers including 18FDG-PET/CT for early prediction of response to neoadjuvant chemotherapy in triple-negative breast cancer. Clin Cancer Res. 2015;21:5460–8.
Koo HR, Park JS, Kang KW, Han W, Park IA, Moon WK. Correlation between 18F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur Radiol. 2015;25:3314–21.
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, et al. Utility of 18F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150:209–17.
Yue Y, Cui X, Bose S, Audeh W, Zhang X, Fraass B. Stratifying triple-negative breast cancer prognosis using 18F-FDG-PET/CT imaging. Breast Cancer Res Treat. 2015;153:607–16.
Lucignani G. SUV and segmentation: pressing challenges in tumour assessment and treatment. Eur J Nucl Med Mol Imaging. 2009;36:715–20.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–4.
Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–22.
Chen HH, Chiu N-T, Su W-C, Guo H-R, Lee B-F. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non–small cell lung cancer. Radiology. 2012;264:559–66.
Pak K, Cheon GJ, Nam H-Y, Kim S-J, Kang KW, Chung J-K, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–90.
Ferguson DJ, Meier P, Karrison T, Dawson PJ, Straus FH, Lowenstein FE. Staging of breast cancer and survival rates: an assessment based on 50 years of experience with radical mastectomy. JAMA. 1982;248:1337–41.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Liao C-T, Chang JT-C, Wang H-M, Ng S-H, Hsueh C, Lee L-Y, et al. Preoperative [18F]fluorodeoxyglucose positron emission tomography standardized uptake value of neck lymph nodes predicts neck cancer control and survival rates in patients with oral cavity squamous cell carcinoma and pathologically positive lymph nodes. Int J Radiat Oncol Biol Phys. 2009;74:1054–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2009-0093820), and by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1072).
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kim, Yi., Kim, Y.J., Paeng, J.C. et al. Prediction of breast cancer recurrence using lymph node metabolic and volumetric parameters from 18F-FDG PET/CT in operable triple-negative breast cancer. Eur J Nucl Med Mol Imaging 44, 1787–1795 (2017). https://doi.org/10.1007/s00259-017-3748-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3748-7